Synthesis and Evaluation of Chitosan Derivatives for Large Size DNA Delivery
Sankov V1,2, Shagdarova B3, Chudakov D3, Reshetov P1, Grechikhina M1, Zubareva A3, Varlamov V3, Esipov R1, Zubov V1 and Svirshchevskaya E1*
1Shemyakin-Ovchinnikov Institute of Bioorganic Chemistry RAS, Russia
2Lomonosov Moscow State University, Russia
3Institute of Bioengineering, Research Center of Biotechnology of the Russian, Academy of Sciences, Russia
Submission: March 02, 2017; Published: March 07, 2018
*Corresponding author: Elena Svirshchevskaya, Shemyakin-Ovchinnikov Institute of organic Chemistry RAS, Russia, Institute of Bioorganic Chemistry of the Russian Academy of Sciences, Russia, Tel: +74953304011; Email: esvir@mx.ibch.ru
How to cite this article: Sankov V, Shagdarova B, Chudakov D, Reshetov P, Grechikhina, et, al. Synthesis and Evaluation of Chitosan Derivatives for Large Size DNA Delivery. Organic & Medicinal Chem IJ. 2017; 1(5): 555572. DOI: 10.19080/OMCIJ.2017.01.555572.
Abstract
Chitosan (Chi) is a natural inexpensive renewable biodegradable cationic polymer with promising potency for different applications including gene delivery. Multiple studies demonstrate that unmodified Chi shows poor cellular uptake leading to low transfection efficiency. Chi possesses multiple reactive groups used to obtain derivatives with modified properties. Numerous attempts in Chi modification demonstrated improved transfection efficacy. However a direct comparison of different Chi derivative was not done. This paper analyses the efficacy of large size DNA delivery by 24 Chi. samples. A pRFP pmKate2 reporter plasmid with MW 3065 kDa was used as a model DNA to transfect HEK293 cells. Among all only a set of hexanoyl-chitosan with molecular weight 20 kDa and substitution degree 10/15/30% was able to deliver the plasmid into cells at a significant level comparable with the efficacy of liposomal reagent Metafectene Pro.
Keywords: Chitosan Derivatives; DNA Delivery; Hydrophobic Groups; pRFP pmKate2 Reporter Plasmid
Abbreviations: Chi - chitosan, MW - molecular weight, DD - deacetylation degree, SD - substitution degree.
Introduction
Transfection efficacy of Chi with plasmid DNA (polyplexes) primarily depends on the properties of Chi such as its MW and degree of deacetylation (DD). It was shown that polyplexes of low (7 to 18 kDa) to medium (40-60 kDa) MW Chi were more effective in comparison with high MW Chi (>150 kDa) [1-4]. Several groups demonstrated that the increase in DD improve s Chi transfection activity [1-3]. Good results were shown after introduction of hydrophobic substituents such as short-chain fatty acids hexanoic, octanoic, decanoic [5], dodecanoic [6], stearic [7-8], or linoleic [9] ones. For the same purpose Chi grafted with hydrophobic amino acids - valine, leucine, or isoleucine were used [10]. Hydrophobic modifications were designed to increase the affinity of polyplexes to the cell membrane and to decrease the affinity of Chi to DNA. Other authors introduced additional positively charged groups such as arginine [11], oligoamine [12], betaine [13], or even polyethylenimine [12]. One more approach to increase transfection efficacy is to modify Chi with penetrating peptides such as Tat-peptide from HIV [14-15]. These peptides have the ability to penetrate lysosomal membranes due to high content of arginine and lysine residues.
At the same time, in another work the opposite approach - modification of Chi with 5-10% of negatively charged groups (succinic acid) was also effective [16]. Other approaches included Chi modification with sulfhydryl groups [17] or EDTA [18]. Many groups demonstrated that that transfection efficacy of Chi derivatives was less than of liposomal commercial agents,but still comparable with them [5-8,16], while in other cases the result was very modest and the activity was more than an order of magnitude [19] and even more than two orders of magnitude [20] lower than of commercial agent. In spite of significant efforts devoted to the development of Chi based DNA delivery vehicle, new research is required to select the most perspective derivatives [21-23]. A direct comparison of Chi derivatives could provide the information which physicochemical characteristics of Chi derivatives predispose to a better transfection efficacy
Materials
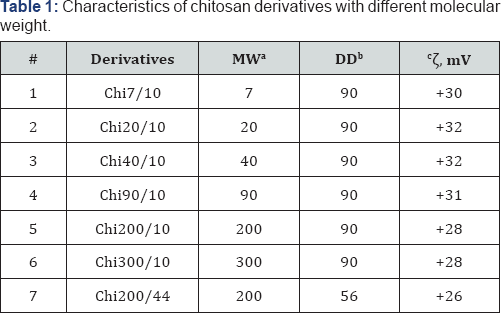
aMW: Molecular Weight, kDa; bDD: Deacetylation Degree, %; : Zeta-potential, mV.
Chitosan with MW 200 kDa and DD 90% (Table 1) sample 5 and chitosan 1000 kDa, DD 90% were purchased from OAO «Bioprogress», Russia. Chitosan 1000 kDa was used to obtain low MW samples. Glacial acetic acid, sodium hydroxide, hydrochloric acid, acetic anhydride, caproic (C6), lauric (C12) anhydrides, ammonium hydroxide, methanol (Sigma-Aldrich, USA) was used as received.
Synthesis
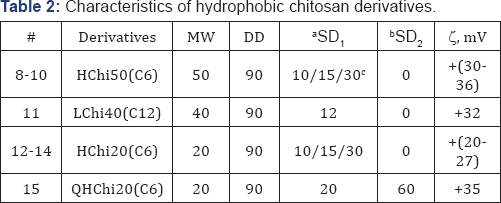
aSD1: Substitution Degree for carbonic acid groups, %;
bSD2: Substitution Degree for quaternary ammonium groups, %;
cSD: Substitution Degree of samples 8-10 and 13-15 were 10, 15, or 30 %% accordingly.
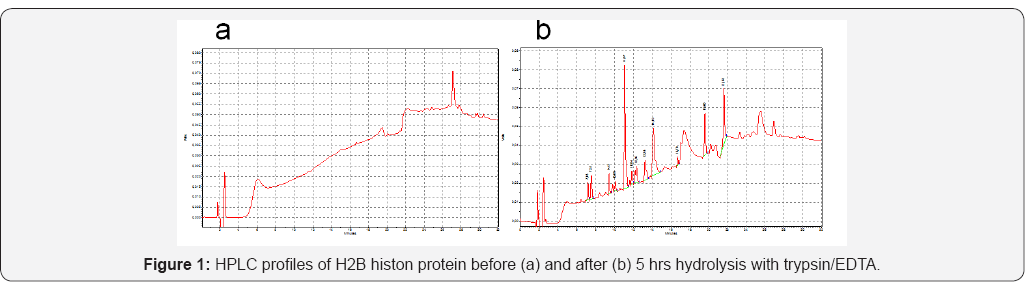
Low MW Chi Samples 1-4, 6, (Table 1) were obtained from Chi 1000 kDa by acidic hydrolysis as described [24]. Chi with low DD Sample 7, (Table 1) was reactivated as described [25]. Hydrophobic derivatives Samples 8-15, (Table 2) were synthesized by carbodiimide mediated coupling reaction as described in [26,27] using anhydrides of different carbonic acids. Succinyl-chitosan derivatives Samples 16-18, (Table 3) were obtained using succinic anhydride as described [28]. Quaternized derivatives Samples 19-22 and 15, (Tables 2 & 3) were synthesized as described in [29]. Hydrophobic quaternized derivative 15 (Table 2) was synthesized sequentially [26,29]. Samples 23 and 24 were obtained by coupling of SChi40/30 to arginine hydrochloride or histone H2B peptides replacing succinic groups for the peptides. Major histone protein H2B (a gift from Dr. I. Boni, Shemyakin-Ovchinnikov Institute of Bioorganic Chemistry, RAS, Moscow) was hydrolyzed to peptides by trypsin/EDTA. Peptide quality and content were analyzed by HPLC, (Figure 1). DD and SD of Chi derivatives were determined by 1H-NMR.
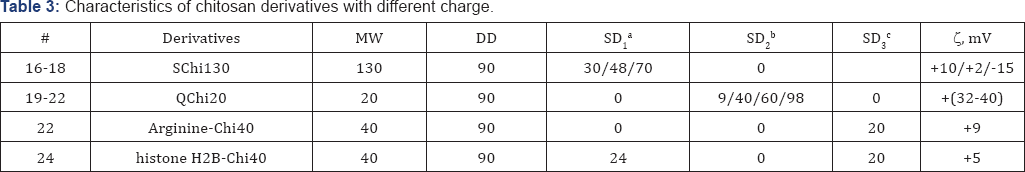
aSD1: Substitution Degree for succinic acid groups, %; bSD2: Substitution Degree for quaternary ammonium groups, %; CSD3 : Substitution Degree for arginine or histone H2B peptides, %.
Cells
Human embryonic kidney cell line HEK293 was grown in DMEM supplemented with 10% fetal calf serum (FCS) and pen- strep-glut (all from PanEco, Moscow, Russian Federation). Cells were passaged using Trypsin/EDTA solution (PanEco, Moscow, Russian Federation) twice a week. Twenty four hours before assays, cells were seeded in the appropriate plates (6- or 24-well plates) adjusted to 3x105 cells/mL and incubated overnight to achieve standardized growth conditions.
Transfection
Bright far-red fluorescent protein coded by reporter plasmid pRFP pmKate2 [30] (Euro gene, Moscow, Russian Federation), MW 3065 kDa, was used to analyze Chi transfection efficacy Experiments with Histone-H2B-Chi40 were conducted using GFP cloned in pOptiVEC, MW 3570 kDa (a gift of V. Toporova, Shemyakin-Ovchinnikov Institute of Bioorganic Chemistry, RAS, Moscow). All positively charged Chi samples were dissolved in 0.1% acetic acid at 5 mg/ml, negatively charged sample 1818 was dissolved in PBS at the same concentration. To form polyplexes 10 μl of Chi derivatives were mixed with 2-3 μg of plasmid in 50 μl of serum-free medium and incubated 20 min. Commercial liposomal transfection reagent Metafectene Pro (MF) (Biontex, Germany) was used as a control. To prepare polyplex 3 μl Metafectene Pro were dissolved in 50 μl serumfree medium and mixed with 1 μg of pRFP dissolved in 50 μl. Polyplexes were formed during 20 min and added drop-wise to cells.
Confocal Analysis
For confocal analysis cells were grown overnight on sterile cover slips in 200 μl of complete culture medium in 6-well plates (Costar). Warm serum- and antibiotic-free 1 ml medium was added to polyplexes and transferred to the wells. Cells were incubated for 1 hr and then 2 ml of complete medium was added. Before the analysis cells were fixed with 1% paraformaldehyde, washed, and polymerized with Mowiol 4.88 medium (Calbiochem, Germany). Slides were analyzed using Eclipse TE2000 confocal microscope (Nikon, Japan).
Flow cytometry
Total level of transfection was estimated by flow cytometry For this, HEK293 cells were seared in 24 well plates overnight; transfected as described above, and incubated for 72 hrs. Before the analysis cells were trypsinized, washed in pre-warmed complete culture medium, and analyzed using FACScan device (BD, USA).
Dynamic light scattering
The diameters of polyplexes were characterized by dynamic light scattering (DLS) (90 Plus Particle Size Analyzer Brookhaven Instruments Corporation, Vernon Hills, IL, USA). All measurements were performed using 661 nm laser light at room temperature with 90o angle of detection. The zeta potential of Chi was determined in 10 mM KCl using identical equipment with an additional Zeta PALS apparatus. Measurement was performed at 25.0 ± 0.1°C.
Laser interference microscopy
Polyplex visualization was fulfilled by MIM-321 laser interference microscope (LIM) (AMPHORA Laboratories LLC, Moscow, Russian Federation). The spatial resolution up to 0.1 nm vertical (Z) and 10 nm lateral (XY) was achieved with Olympus MPLFLN 100x/0.9 dry objective. The 10 ml of polyplexes were placed on the mirror glass and dried. The gradient filtering was applied to visualize the shape and the internal structure of polyp lexes.
Results

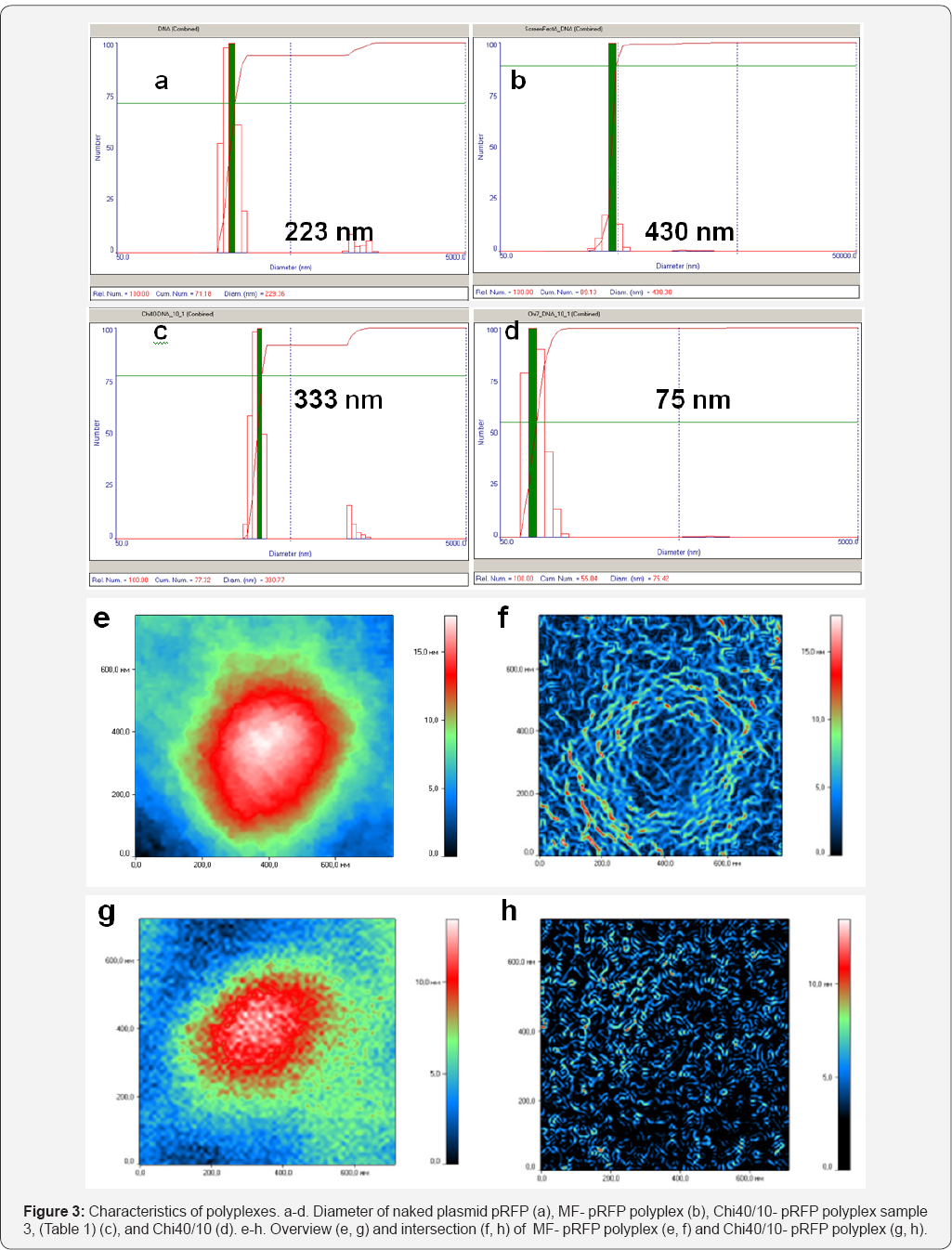
Physicochemical characteristics of Chi derivatives are shown in Tables 1-3. All synthesized Chi samples were selected basing on the published data which demonstrated some efficacy of transfection. Formation of complexed was verified by gel-electrophoresis retardation of polyplexes. The results of retardation analysis are shown for some samples (Figure S2) while the same was found for all Chi derivatives including SChi130 with SD 30 and 48 %%. The mean diameter of polyplexes determined by DLS varied depending on Chi derivative from 190 to 450 nm (Figures 3a-3d) which corresponds to earlier published data [2,5]. We also analyzed polyplex morphology and their internal structure using LIM. Representative structures of polyplexes formed by MF and Chi40/10 are shown in Figures 3e-3h. Lipid reagent formed more homogenous nanoparticles than Chi and both have spherical structure with empty center The other types of Chi polyplexes demonstrated the same heterogeneous nanoparticles as Chi40/10 (data not shown).
Transfection of HEK293 cells using unmodified Chi with MW 7, 20, 40, 90, 200, and 300 kDa was completely ineffective. A representative confocal image for Chi 20/10 is shown in Figure 4b. The same was found for all other unmodified Chi samples (data not shown). Decrease in DD (Chi200/44) did not enhance transfection rate (Figure 4c). Neither quaternized Chi samples were effective; a representative image for QChi20/98 with the highest quaternization degree is shown in Figure 4d. Significant however low transfection was registered in hydrophobic 50 kDa Chi samples LChi50/12% and HChi50/30% (Figures 4d-4f). HChi50 with SD 10 and 15 %% transfected HEK293 cells slightly weaker than HChi50/30 (data not shown). Interesting that hydrophobic ally modified quaternized Chi 15 (QHChi20) was unable to deliver pRFP (data not shown).
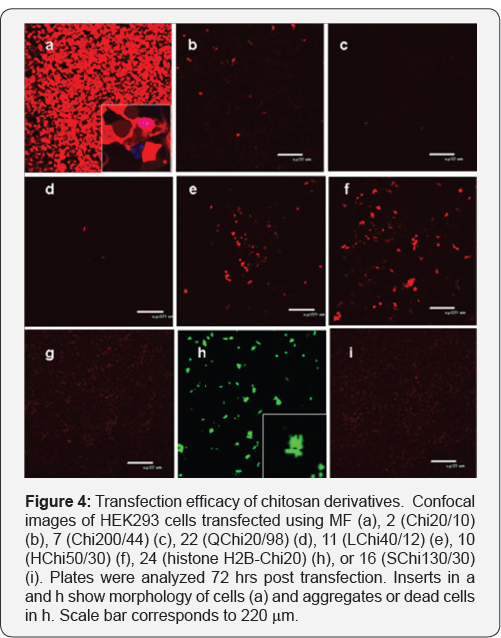
Transfection of cells with histone H2B-Chi20 induced some level of protein expression, at the same time, all transfected cells were dead (Figure 4, compare inserts in a and h). SChi50/30% also did not deliver pRFP into cells (Figure 4i). Taken collectively we concluded that only hydrophobically modified nonquaternized Chi were able to deliver pRFP into cells. Among two carbonic acids better results were obtained with hexanoic Chi derivatives.
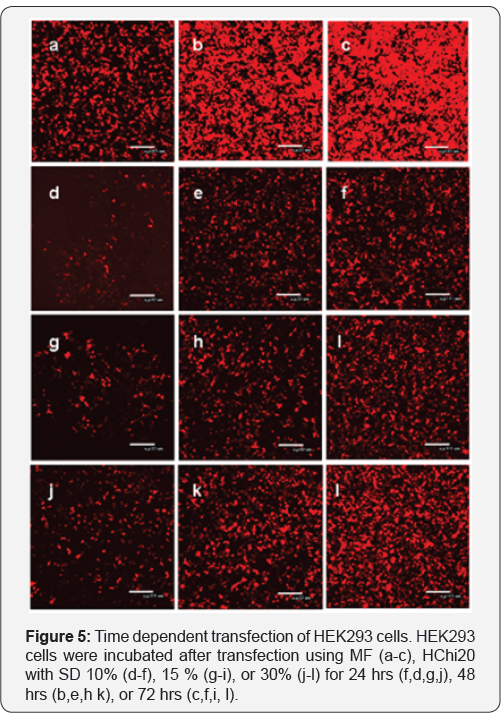
To follow this line we hypothesized that MW of HChi can affect transfection activity of Chi. A new set of HChi samples 12-14 was synthesized using 20 kDa Chi. It appeared that transfection activity of all HChi20 samples was much higher (Figure 5). A dynamic of RFP protein expression in the cells transfected either with MF or HChi was comparable and increase from 24 to 72 hrs. When analyzed by flow cytometry at 72 hrs, percentages of
Discussion
In spite of numerous studies there are many inconsistencies in the literature concerning the effectiveness of Chi systems for gene transfer [31]. Chi is an attractive positively charged biopolymer with abundant reactive amino groups which can be used to obtain functional conjugates. However flexible Chi structure constrains regular packaging of DNA. LiM analysis demonstrated that liposomal polyplexes form dense regularly packed DNA while Chi condenses it less efficiently. Analysis of gene transfer requires a robust detection system. Reporter genes such as GFP allow flow cytometry and confocal microscopy based quantitative assessment. Other reporter proteins such as luciferase and galactosidases are also often used [32]. Multiple groups develop Chi based delivery systems for si RNA transfer [2] . It is likely that not only Chi MW but also the size of DNA plays a role in the resultant transfection efficacy MW of GFP, luciferase, and galactosidases are around 3000, 4500, and 4800 kDa accordingly while siRNA MW is only 15kDa.
This difference can significantly affect polyplex formation and can explain the difference in the results obtained. Oliveira et al. [33] tried to develop a universal Chi based vector for the delivery of small and large plasmids however it resulted in much more sophisticated system. In this work we used a new reporter plasmid pRFP pmKate2 of MW 3065 kDa which encodes far-red fluorescent protein. For this plasmid almost none Chi derivatives, earlier shown to be effective in gene transfer, were able to transfect HEK293 cells. The only exclusion was hydrophobic Chi derivatives. The most efficient for this particular pRFP plasmid were Chi derivatives of 20 kDa modified by caproic acid anhydride. Our data closely reproduce the results obtained by Layek et al. [5] and Zhang et al. [34]. Recently Layek group and others published amphiphilic Chi derivatives which demonstrated even better efficacy [35,36].
Acknowledgement
Chemical synthesis was supported by the grant from the Russian Scientific Foundation (#16-14-00046); molecular biology and cell culture work was supported by Russian Foundation for Basic Research (#17-34-80027).
References
- Jiang HL, Cui PF, Xie RL, Cho CS (2014) Chemical modification of chitosan for efficient gene therapy. Adv Food Nutr Res 73: 83-101.
- Fernandes JC, Qiu X, Winnik FM, Benderdour M, Zhang X, et al. (2012) Low molecular weight chitosan conjugated with folate for siRNA delivery in vitro: optimization studies. Int J Nanomedicine 7: 58335845.
- Thibault M, Nimesh S, Lavertu M, Buschmann MD (2010) Intracellular trafficking and decondensation kinetics of chitosan-pDNA polyplexes. Mol Ther 18(10): 1787-1795.
- Köping-Höggard M, Varum KM, Issa M, Danielsen S, Christensen BE, et al. (2004) Improved chitosan-mediated gene delivery based on easily dissociated chitosan polyplexes of highly defined chitosan oligomers. Gene Ther 11(19): 1441-1452.
- Layek B, Singh J (2012) N-hexanoyl, N-octanoyl and N-decanoyl chitosans: Binding affinity, cell uptake, and transfection. Carbohydr Polym 89(2): 403-410.
- Zhu D, Jin X, Leng X, Wang H, Bao J, et al. (2010) Local gene delivery via endovascular stents coated with dodecylated chitosan-plasmid DNA nanoparticles. Int J Nanomedicine 5: 1095-1102.
- Du YZ, Lu P, Yuan H, Zhou JP, Hu FQ (2011) Quaternary complexes composed of plasmid DNA/protamine/fish sperm DNA/stearic acid grafted chitosan oligosaccharide micelles for gene delivery. Int J Biol Macromol 48(1):153-159.
- Hu FQ, Zhao MD, Yuan H, You J, Du YZ, et al. (2006) A novel chitosan oligosaccharide-stearic acid micelles for gene delivery: properties and in vitro transfection studies. Int J Pharm 315(1-2): 158-166.
- Wang B, He C, Tang C, Yin C (2011) Effects of hydrophobic and hydrophilic modifications on gene delivery of amphiphilic chitosan based nanocarriers. Biomaterials 32(20): 4630-4638.
- Layek B, Singh J (2013) Amino acid grafted chitosan for high performance gene delivery: comparison of amino acid hydrophobicity on vector and polyplex characteristics. Biomacromolecules 14(2): 48594.
- Gao Y, Xu Z, Chen S, Gu W, Chen L, et al. (2008) Arginine-chitosan/DNA self-assemble nanoparticles for gene delivery: In vitro characteristics and transfection efficiency. Int J Pharm 359(1-2): 241-246.
- Lu B, Wang CF, Wu DQ, Li C, Zhang XZ, et al. (2009) Chitosan based oligoamine polymers: synthesis, characterization, and gene delivery. J Control Release 137(1): 54-62.
- Gao Y, Zhang Z, Chen L, Gu W, Li Y (2009) Chitosan N-betainates/DNA self-assembly nanoparticles for gene delivery: in vitro uptake and transfection efficiency. Int J Pharm 371(1-2): 156-162.
- Deshayes S, Morris MC, Divita G, Heitz F (2005) Cell-penetrating peptides: tools for intracellular delivery of therapeutics. Cell Mol Life Sci 62(16): 1839-1849.
- Rahmat D, Khan MI, Shahnaz G, Sakloetsakun D, Perera G, et al.(2012) Synergistic effects of conjugating cell penetrating peptides and thiomers on non-viral transfection efficiency. Biomaterials 33(7): 2321-2326.
- Toh EK, Chen HY, Lo YL, Huang SJ, Wang LF (2011) Succinated chitosan as a gene carrier for improved chitosan solubility and gene transfection. Nanomedicine 7(2): 174-183.
- Schmitz T, Bravo-Osuna I, Vauthier C, Ponchel G, Loretz B, et al. (2007) Development and in vitro evaluation of a thiomer-based nanoparticulate gene delivery system. Biomaterials 28(3): 524-531.
- Loretz B, Bernkop-Schnürch A (2006) In vitro evaluation of chitosan- EDTA conjugate polyplexes as a nanoparticulate gene delivery system. AAPS J 8(4): E756-E764.
- Alatorre-Meda M, Taboada P, Hartl F, Wagner T, Freis M, et al. (2011) The influence of chitosan valence on the complexation and transfection of DNA: the weaker the DNA-chitosan binding the higher the transfection efficiency. Colloids Surf B Biointerfaces 182(1): 54-62.
- MacLaughlin FC, Mumper RJ, Wang J, Tagliaferri JM, Gill I, et al. (1998) Chitosan and depolymerized chitosan oligomers as condensing carriers for in vivo plasmid delivery. J Control Release 56(1-3): 259-272.
- Thibault M, Lavertu M, Astolfi M, Buschmann MD (2016) Structure Dependence of Lysosomal Transit of Chitosan-Based Polyplexes for Gene Delivery. Mol Biotechnol 58(10): 648-656.
- Lee YH, Park HI, Choi JS (2016) Novel glycol chitosan-based polymeric gene carrier synthesized by a Michael addition reaction with low molecular weight polyethylenimine. Carbohydr Polym 137: 669-677.
- Nam JP, Nah JW (2016) Target gene delivery from targeting ligand conjugated chitosan-PEI copolymer for cancer therapy. Carbohydr Polym 135: 153-161.
- Nemtsev SV, Gamzazade AI, Rogozhin SV, Bykova VM, Bykov VP (2002) [Deacetylation of chitin under homogeneity]. Prikl Biokhim Mikrobiol 38(6): 609-615.
- Hirano S, Kondo Y, Fujii K (1985) Preparation of acetylated derivatives of modified chito-oligosaccharides by the depolymerisation of partially ^-acetylated chitosan with nitrous acid. Carbohydr Res 144(2): 338341.
- Zhu A, Yuan L, Jin W, Dai S, Wang Q, et al. (2009) Polysaccharide surface modified Fe3O4 nanoparticles for camptothecin loading and release. Acta Biomater 5(5): 1489-1498.
- Zubareva AA, Shcherbinina TS, Varlamov VP, Svirshchevskaya EV (2015) Intracellular sorting of differently charged chitosan derivatives and chitosan-based nanoparticles. Nanoscale 7(17): 7942-7952.
- Kato Y, Onishi H, Machida Y (2000) Evaluation of N-succinyl-chitosan as a systemic long-circulating polymer. Biomaterials 21(15): 1579-1585.
- Shagdarova BTs, Ifina AV, Varlamov VP (2016) Antibacterial Activity of Alkylated and Acylated Derivatives of Low-Molecular Weight Chitosan. Appl Biochem Microbiol 52(2): 222-225.
- Shcherbo D, Merzlyak EM, Chepurnykh TV, Fradkov AF, Ermakova GV, et al. (2007) Bright far-red fluorescent protein for whole-body imaging. Nat Methods 4(9): 741-746.
- Buschmann MD, Merzouki A, Lavertu M, Thibault M, Jean M, et al.(2013) Chitosans for delivery of nucleic acids. Adv Drug Deliv Rev 65(9): 1234-1270.?
- Welsh S, SA Kay (1997) Reporter gene expression for monitoring gene transfer. Curr Opin Biotechnol 8 (5): 617-622.
- Oliveira AV, Silva GA, Chung DC (2015) Enhancement of chitosan- mediated gene delivery through combination with phiC31 integrase. Acta Biomater 17: 89-97.
- Zhang X, Ercelen S, Duportail G, Schaub E, Tikhonov V, et al. (2008) Hydrophobically modified low molecular weight chitosans as efficient and nontoxic gene delivery vectors. J Gene Med 10(5): 527-539.
- Layek B, Haldar MK, Sharma G, Lipp L, Mallik S, et al. (2014) Hexanoic acid and polyethylene glycol double grafted amphiphilic chitosan for enhanced gene delivery: influence of hydrophobic and hydrophilic substitution degree. Mol Pharm 11(3): 982-994.
- Zhu D, Yao K, Bo J, Zhang H, Liu L, et al. (2010) Hydrophilic/lipophilic N-methylene phosphonic chitosan as a promising non-viral vector for gene delivery. J Mater Sci Mater Med 21(1): 223-229.






