Efficient Room Temperature Synthesis of 2-Aryl benzimidazoles using ZnO Nanoparticles as Reusable Catalyst
Shyam Kumar Banjare, Soumen Payra, Arijit Saha and Subhash Banerjee*
Department of Chemistry, Guru Ghasidas Vishwavidyalaya, India
Submission: February 09, 2017; Published: February 27, 2017
*Corresponding author: Subhash Banerjee, Department of Chemistry, Guru Ghasidas Vishwavidyalaya, Bilaspur 495009, Chhattisgarh, India.
How to cite this article: Shyam K B, Soumen P, Arijit S, Subhash B. Efficient Room Temperature Synthesis of 2-Aryl benzimidazoles Using ZnO Nanoparticles as Reusable Catalyst. Organic & Medicinal Chem IJ. 2017; 1(4): 555568. DOI: 10.19080/OMCIJ.2016.01.555568
Abstract
A facile and green protocol has been developed for the synthesis of 2-aryl-1,3-benzimidazole derivatives using ZnO nanoparticles as reusable catalyst. The reactions are very fast, high yielding and the nano-catalyst was recycled for ten times. In this paper we have reported the synthesis of 2-aryl-1,3-benzimidazole derivatives from aromatic aldehydes (containing electron donating group like, -OMe, -Me etc. and electron withdrawing group like, -NO2 etc.) and 1,2-phenyl-diamine.We have synthesized a series of 2-aryl-1,3-benzimidazoles with various 5- and 6-position substituent (-H, -OCH3, -CH3, -OH, -Cl, -Br, -NO2, ) derivatives.
Keywords: Nano-catalysis; ZnO nanoparticles; 2-Aryl-1,3-benzimidazoles; Reusable catalyst; Green synthesis
Introduction
Synthesis of biologically potent 2-substituted benzimidazole derivatives is important because of their wide applications in medicinal chemistry and reported to act as antiviral [1], antiulcer [2] antihypertension [3] and anticancer agent [4]. Thus, development of environmentally benign synthetic methodology for the construction of 2-substituted benzimidazole derivatives is appreciated in the context of green chemistry. Generally, benzimidazole derivatives have been synthesized by the condensation of 1,2-phenylenediamine with aldehydes using various catalysts. Many of the methods utilized homogeneous catalysts such as FeCl3,6H2O [5a] I2/KI/K2CO3/H2O [5b], sodium metabisulfite [5c] Na2S2O5 [5d] iodobenzenediacetate(IBD) [5e], CAN/H2O2 [5f] HCl/H2O2 [5g], Co(OH)2/CoO(II) [5h], Pd(OAc)2/ O2 [5i], TiCl3OTf [5j], B(C6F5)3 [5k] VOSO4 [5l], Ru(byp)3Cl2 [5m] Na2S2O4 or Na2S2O5 [5n], Pd(dppf)Cl2 [5o] and few protocols used solid supported catalysts such as SiO2/sulphuric acid [6a], AlKIT-5 [6b], FeCl3/Montmorillonite K-10 [6c], polymer- supported hypervalent iodine [6d] and KHSO4/SiO2 [6e].
However, most of the methods involves use of toxic catalysts, acid reaction conditions, salts of heavy metal and expensive Pd-catalyst, poor catalytic efficiency due to the low surface area supporting materials and finally these catalysts were not environment-friendly in nature. Thus, introduction of an efficient heterogeneous catalyst for the synthesis of 2-substituted benzimidazole derivatives is important.
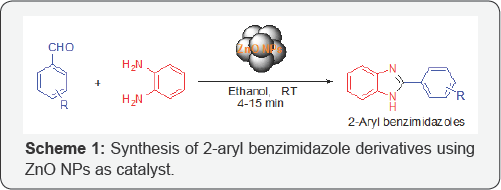
Recently, catalysis by nanoparticles (NPs) has become attractive area of research due to their large and reactive surface areas, chemo-selectivity and ability to perform the reaction under mild reaction conditions. In addition, NPs have considered as bridge between homogeneous and heterogeneous catalysis and advantages of both disciplines can be achieved by using a nano-catalyst. Among them, oxide NPs were snatched attractive attention because these NPs are relatively more stable compare to metal NPs due to presence of oxide (O-) and hydroxyl (-OH) groups in the surface. Moreover, surface of oxide NPs has exhibit redox and Lewis acid-Lewis basic properties [7]. Recently, few NPs (NPs (CuO NPs [8a]. nano-ZSM-5 [8b], CeO2NPs) [8c], have also been applied as catalyst for the synthesis of 2-substituted benzimidazole derivatives. However, these NPs were toxic in nature. As a part of our research interest in catalysis by oxide NPs [9], here we have demonstrated synthesis of 2-aryl benzimidazole derivatives using environment-friendly free-ZnO NPs as reusable catalyst at room temperature (Scheme 1).
Materials and Methods
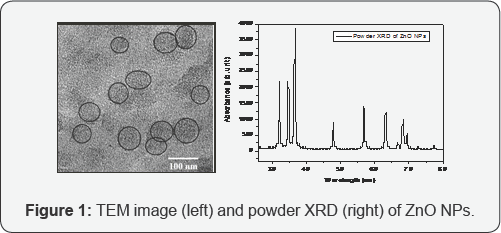
Initially, ZnO NPs have been synthesized following our previously reported method [8], simply by condensing Zn(OAc)2. 2H2O in a basic medium 70oC temperature by sol-gel method and stored this nano-catalyst in ethanol medium. The detail experimental procedure for the synthesis of ZnO NPs has been given in reference section. The formation of ZnO NPs was confirmed by transmission electron microscopy (TEM) study. The TEM image indicates the formation of nearly spherical shaped particles with average size of 50 nm (Figure 1). The formation of crystalline ZnO NPs was evident from powder X-ray diffraction (XRD) study (Figure 1). The average particle size of ZnO NPs was determined to be 30 nm from the XRD study from the full-width at half wave full maxima (FWHM) via Scherrer formula.
Results and Discussion
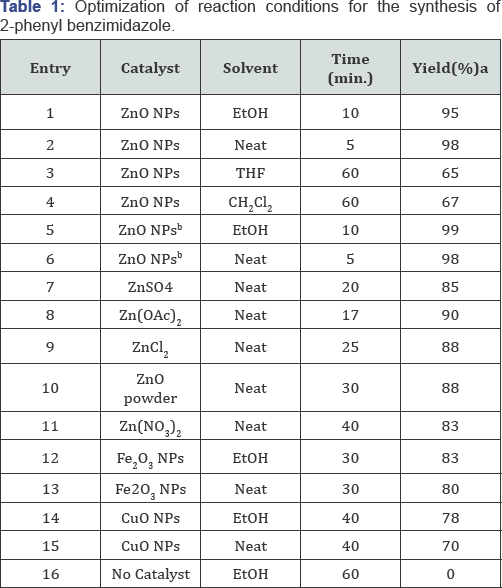
Reaction conditions: 1,2-phenyl-diamine(1 mmol), benzaldehyde (1 mmol), catalyst (5 mg), room temparature, continuous stirring. alsolated yield. bCatalyst (10 mg).
Initially by the freshly prepared ZnO NPs has tested for the synthesis of 2-phenyl-1,3-benzimidazole via the condensation of 1,2-phenyl-diamine with benzaldehyde in ethanol medium. When a mixture 1,2-phenyl-diamine (1 mmol), benzaldehyde (1 mmol) and ZnO NPs (5 mg) was stirred at room temperature in ethanol (2 ml) an excellent yield of product (95%) was obtained after 10 minutes. Next, we have optimized the experimental conditions by changing the reaction parameters such as solvent, reaction time and also the amount of catalyst etc. The results were summarized in Table 1. The high yield of product was obtained by using ZnO NPs (5 mg) as catalyst Entry 2, (Table 1) and the reaction was not initiated in absence of ZnO NPs even after 1 hour Entry 16, (Table 1).

Screening of different solvents revealed that ZnO NPs were more efficient ethanol compared to non-polar solvent like tetrahydrofuran (THF), dichloromethane (CH2Cl2) etc. However, a best result was obtained in the basis of time and yield under solvent free reaction condition. In the solvent free condition 5 mg of ZnO NPs is sufficient to produced excellent yield of product (98%; Table 1, Entry 2). ZnO NPs showed superior catalytic activity compared to other Zn-catalyst, Fe3O4 NPs and CuO NPs as listed in (Table 1) (Entry 12-15). No such changes have been observed in increasing the amount of the catalyst 5 mg to 10 mg (99 %; Table 1, Entry 5). We have also performed the reaction under solvent free condition using 10 mg of the catalyst for five minutes but no such changes have been observed in the formation of yield with respect to the time 10 minutes. To investigate the scope of this protocol, next using the optimized reaction conditions i.e., taking ZnO NPs (5 mg) catalyst and solvent-free condition (solid aldehyde required solvent; EtOH) we have tried to synthesize series of 2-aryl benzimidazole derivatives (Figures 2 & 3), 1a-i using variety of aryl aldehydes.
In a general experimental procedure, the ZnO NPs were very efficiently catalyzed the condensation of 1,2-diaminobenzene with several aromatic aldehydes at room temperature. Aryl aldehydes bearing both electron withdrawing (e.g. -NO2) and electron donating (e.g. -OMe, -Cl, -OH, -Br) group underwent condensation smoothly within very short time period (4-15 minutes).
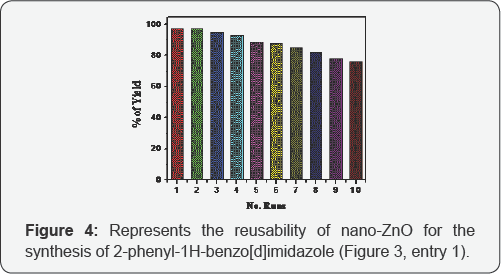
All the reactions tested here are fast and high yielding (9299%). The completions of reactions were checked by monitoring TLC. After the reaction the products were separated from catalyst simply by filtration and purified by recrystallization from hot ethanol. The formation of products was initially confirmed by checking their melting points. All the 2-aryl benzimidazole derivatives prepared here are known to literature (Figure 3) and the observed melting points of each benzimidazole derivative were in well accordance to the literature values. The corresponding references are given in Figure 3. Then the products were further characterized by spectroscopic data. The ZnO NPs were recycled and reused for at least 10 times without significant loss of catalytic activity (Figure 4).

Conclusion
In conclusion, an efficient protocol has been developed for the synthesis of 2-aryl benzimidazole derivatives at room temperature using uncapped ZnO NPs as catalyst. All the reactions tested here were very fast (4-15 minutes) and high yielding (9299%). The protocol is very mild (room temperature) and neutral in nature, the products were purified by recrystallization and thus use of volatile and hazardous solvents has been avoided here. Finally, this present ZnO NPs catalyzed synthesis of biologically important 2-aryl-benzoimidazole derivatives fulfills the criteria for green synthesis.
Representative method
A mixture of 1,2-diphenyl amine (1 mmol; 108 mg) benzaldehyde (1 mmol; 106 mg) and ZnO NPs (10 mg) in ethanol (1 ml)was stirred at room temperature for 8 minutes (TLC monitored). After that solid product was dissolved in hot ethanol and separated the ZnO NP by simple filtration (Whatman 41 filter paper) and recrystallized from ethanol. Pure 2-phenyl benzimidazole (Figure 3), entry 1; 97%, 188.10 mg) was obtained from the filtrate.
The formation of the product was confirmed by melting point and spectroscopic (1H NMR and 13C NMR) studies. (Figure 3), entry 1): Melting point- 291-293OC; 1H NMR (400 MHz, CDCl3): d = 8.18 (d, J = 7.3 Hz, 2H), 7.65-7.58 (m, 1H), 7.51-7.39 (m, 4H), 7.21-7.12 (m, 2H); 13C-NMR (100 MHz, CDCl3): d = 151.4, 143.5, 135.1, 130.0, 129.7, 129.3, 126.9, 122.5, 121.6, 118.4, 111.5. The same protocol was used for the synthesis of all compounds listed in Figure 3. The ZnO NPs were dried and recycled for subsequent reactions.
Preparation of ZnO NPs 2M solution of NaOH in ethanol (100 mL) was slowly added drop by drop to 50 ml of 1M stirred solution of Zn(CH3COO)2^2H2O in ethanol at 70°C and the pH was maintained at 11. The final solution was stirred at 70°C for 2 h. After 2 h the solution were cooled to room temperature and settled down the solution. After that the solids were separated by centrifugation and washed from de-ionized water (3x15 ml) and ethanol (2x5 ml), and finally dried in air. To allow formation of ZnO nanoparticles and prevent precipitation of hydroxides, it is critical to maintain the temperature above 60°C. The formation of nanoparticles was confirmed from TEM studies (Figure 1b in main text) and powder XRD studies (Figure 1b in main text). The average particle sizes of the NPs were found to ~ 30 nm.
Acknowledgement
We are pleased to acknowledge funding agencies Department of Science and Technology, New Delhi, Govt. of India (NO.SB/FT/ CS-023/2012).
References
- G L Gravatt, B C Baguley, W R Wilson, W A Denny (1994) DNA- Directed Alkylating Agents. 6. Synthesis and Antitumor Activity of DNA Minor Groove-Targeted Aniline Mustard Analogs of Pibenzimol. J Med Chem 37(25):4338-4345.
- J S Kim, B Gatto, C Yu, A Liu, L F Liu, et al. (1996) Substituted 2,5'-Bi-1H- benzimidazoles: Topoisomerase I Inhibition and Cytotoxicity. J Med Chem 39(4): 992-998.
- T Roth, M L Morningstar, P L Boyer, S H Hughes, R W J Buckheit, et al. (1997) Synthesis and Biological Activity of Novel Non-nucleoside Inhibitors of HIV-1 Reverse Transcriptase. 2-Aryl-Substituted Benzimidazoles. J Med Chem 40(26): 4199-4207.
- D A Horton, G T Bourne, M L Smythe (2003) The Combinatorial Synthesis of Bicyclic Privileged structures or Privileged Substructures. Chem Rev 103(3): 893-930.
- A. B Das, H Holla, Y Srinivas (2007) Efficient (bromodimethyl)sulfonium bromide mediated synthesis of benzimidazoles. Tetrahedron Lett 48(1): 61-64.
B. P Gogoi, D Konwar (2006) An efficient and one-pot synthesis of imidazolines and benzimidazoles via anaerobic oxidation of carbon- nitrogen bonds in water. Tetrahedron Lett 47(1): 79-82.
C. H Goker, C Ku, D W Boykin, S Yildiz, N Altanar (2002) Synthesis of some new 2-substituted-phenyl-1H-benzimidazole-5 carbonitriles and their potent activity against candida species. Bioorg Med Chem 10(8): 25892596.
D. G N Vazquez, H M Diaz, F A Crespo, I L Rivera, R V Molina, et al. (2006) Design, microwave-assisted synthesis, and spasmolytic activity of 2-(alkyloxyaryl)-1H-benzimidazole derivatives as constrained stilbenebioisosteres. Bioorg Med Chem Lett 16(16): 4169-1673.
E. L H Du, Y G Wang (2007) A rapid and efficient synthesis of benzimidazoles using hypervalent iodine as oxidant. Synthesis 5(1): 675-678.
F. K Bahrami, M M Khodaei, F Naali (2008) Mild and Highly Efficient Method for the Synthesis of 2-Arylbenzimidazoles and 2-Arylbenzothiazoles. J Org Chem 73(17): 6835-6837.
G. K Bahrami, M M Khodaei, I Kavianinia (2007) A simple and efficient one-pot synthesis of 2-substituted benzimidazoles. Synthesis 4(1): 417-427.
H. M A Chari, D Shobha, T Sasaki (2011) Room temperature synthesis of benzimidazole derivatives using reusable cobalt hydroxide (II) and cobalt oxide (II) as efficient solid catalysts. Tetrahedron Lett 52(43): 5575-5580.
I. W H Chen, Y Pang (2009) Efficient synthesis of 2-(2'-hydroxyphenyl) benzoxazole by palladium(II)-catalyzed oxidative cyclization. Tetrahedron Lett 50(48): 6680-6683.
J. J Azizian, P Torabi, J Noei (2016) Synthesis of benzimidazoles and benzoxazoles using TiCl3OTf in ethanol at room temperature. Tetrahedron Lett 57(2): 185-188.
K. S K Prajapati, A Nagarsenkar, S D Guggilapu, N B Bathini (2015) B(C6F5)3 as versatile catalyst: an efficient and mild protocol for the one-pot synthesis of functionalized piperidines and 2-substituted benzimidazole derivatives. Tetrahedron Lett 56(48): 6795-6799.
L. C S Digwal, U Yadav, A P Sakla, P V S Ramya, S Aaghaz, et al. (2016) VOSO4 catalyzed highly efficient synthesis of benzimidazoles, benzothiazoles, and quinoxalines. Tetrahedron Lett 57(36): 4012-4016.
M. I R Siddiqui, F Ibad, A Ibad, M A Waseem, G Watal (2016) Eco-friendly strategy: design and synthesis of biologically potent benzimidazole- amine hybrids via visible-light generated oxidative C-H arylamylation of analenic amidines. Tetrahedron Lett 57(1): 5-10.
N. H T B Bui, Q T K Ha, W K Oh, D D Vo, Y N T Chau, et al. (2016) Microwave assisted synthesis and cytotoxic activity evaluations of new benzimidazole derivatives. Tetrahedron Letters 57(8): 887-891.
O. X Li, R Hua, Y Tong, Q Pan, D Miao, S Han (2016) An efficient route for the synthesis of benzimidazoles via a hydrogentransfer strategy between o-nitroanilines and alcohols. Tetrahedron Lett 57(41): 46454649. - A. P Salehi, M Dabiri, M A Zolfigol, S Otokesh, M Baghbanzadeh (2006) Selective synthesis of 2-aryl-1-arylmethyl-1H-1,3-benzimidazoles in water at ambient temperature. Tetrahedron Lett 47(15): 2557-2560.
B. M A Chari, D Shobha, E R Kenawy, S S A Deyab, B V S Reddy, et al. (2010) Nanoporousaluminosilicate catalyst with 3D cage-type porous structure as an efficient catalyst for the synthesis of benzimidazole derivatives. Tetrahedron Lett 51(39): 5195-5199.
C. G F Chen, H M Jia, L Y Zhang, B H Chen, J T Li (2013) An efficient synthesis of 2-substituted benzothiazoles in the presence of FeCl3/ Montmorillonite K-10 under ultrasound irradiation. Ultrasonics Sonochemistry 20(2): 627-632.
D. A Kumar, R A Maurya, P Ahmad (2009) Diversity Oriented Synthesis of Benzimidazole and Benzoxa/(thia)zole Libraries through Polymer- Supported Hypervalent Iodine Reagent. J Comb Chem 11(2): 198-201.
E. H Q Ma, Y L Wang, J Y Wang (2006) A simple KHSO4 promoted synthesis of 2-arylsubstituted benzimidazoles by oxidative condensation of aldehydes with o-phenylenediamine. Heterocycles 68(8): 1669-1673. - A. AT Bell (2003) The Impact of Nanoscience on Heterogeneous Catalysis. Science 299(5613): 1688-1691.
B. H Kung (1989) Transition metal oxides: Surface chemistry and catalysis. Books google 45: 1-281.
C. S Ibrahim (2003) Recent Studies on the Catalytic Activity of Titanium, Zirconium, and Hafnium Oxides. Catal Rev 45(2): 205-296. - A. S M Inamdar, V K More, S K Mandal (2013) CuO nano-particles supported on silica, a new catalyst for facile synthesis of benzimidazoles, benzothiazoles and benzoxazoles. Tetrahedron Lett 54(6): 579-583.
B. A Teimouria, A N Chermahinib, H Salavatia, L Ghorbanian (2013) An efficient and one-pot synthesis of benzimidazoles, benzoxazoles, benzothiazoles and quinoxalines catalyzed via nano-solid acid catalysts. Journal of Molecular Catalysis A: Chemical 373(1): 38-45.
C. R Shelkar, S Sarode, J Nagarkar (2013) Nano ceria catalyzed synthesis of substituted benzimidazole, benzothiazole, and benzoxazole in aqueous media. Tetrahedron Lett 54(51): 6986-6990. - A. S Banerjee, J Das, S Santra (2009) Native silica nanoparticle catalyzed anti-Markovnikov addition of thiols to inactivated alkenes and alkynes: a new route to linear and vinyl thioethers. Tetrahedron Lett 50(1): 124-127.
B. S Banerjee, S Santra (2009) Remarkable catalytic activity of silica nanoparticle in the bis-Michael addition of active methylene compounds to conjugated alkenes. Tetrahedron Lett 50(18): 20372040.
C. S Banerjee, G Sereda (2009) One-step, three-component synthesis of highly substituted pyridines using silica nanoparticle as reusable catalyst. Tetrahedron Lett 50(50): 6959-6962.
D. S Banerjee, J Das, R Alverez, S Santra (2010) Silica nanoparticles as a reusable catalyst: a straightforward route for the synthesis of thioethers, thioesters, vinyl thioethers andthio-Michael adducts under neutral reaction conditions. New J Chem 34(1): 302-306.
E. V Rajpara, S Banerjee, G Sereda (2010) Iron Oxide Nanoparticles Grown on Carboxy-Functionalized Graphite: An Efficient Reusable Catalyst for Alkylation of Arenes. Synthesis 16(1): 2835-2840.
F. S Banerjee, V Balasanthiran, R Koodali, G Sereda (2010) Pd- MCM-48: a novel recyclable heterogeneous catalyst for chemo- and regioselective hydrogenation of olefins and coupling reactions.Org Biomol Chem 8(1): 4316-4321.
G. S Banerjee, A Horn, H Khatri, G Sereda (2011) A green one-pot multicomponent synthesis of 4H-pyrans and polysubstituted aniline derivatives of biological, pharmacological, and optical applications using silica nanoparticles as reusable catalyst. Tetrahedron Lett 52(16): 1878-1881.
H. S Banerjee, A Saha (2013) Free-ZnO nanoparticles: a mild, efficient and reusable catalyst for the one-pot multicomponent synthesis of tetrahydrobenzo [b] pyran and dihydropyrimidone derivatives. New J Chem 37(1): 4170-4175.
I. S Banerjee, SPayra, A Saha, G Sereda (2014) ZnO nanoparticles: a green efficient catalyst for the room temperature synthesis of biologically active 2-aryl-1,3-benzothiazole and 1,3-benzoxazole derivatives. Tetrahedron Lett 55(40): 5515-5520.






























