Synthesis and Antimalarial Properties of Three Substituted Trinitro-Tribenzylamine
Junior Kindala1,4*, Ezekiel Afolabi1, Noel Wannang2, Ukpe Ajima1, Francis Agwom1, Titilayo Johnson3, Jean Kayembe4, Pius Mpiana4 and Kalulu Taba4
1Department of Pharmaceutical Chemistry, Faculty of Pharmaceutical Sciences, University of Jos, Nigeria
2Department of Pharmacology, Faculty of Pharmaceutical Sciences, University of Jos, Nigeria
3Department of Biochemistry, Faculty of Medical Sciences, University of Jos, Nigeria
4Department of Chemistry, Faculty of Sciences, University of Kinshasa, Congo-DR
Submission: September 29, 2016; Published: October 26, 2016
*Corresponding author: Junior Kindala, Department of Pharmaceutical Chemistry, Faculty of Pharmaceutical Sciences, University of Jos, Nigeria
How to cite this article: Junior K, Ezekiel A, Noel W, Ukpe A, Francis A, et al. Synthesis and Antimalarial Properties of Three Substituted Trinitro- Tribenzylamine. Organic & Medicinal Chem IJ. 2016; 1(3): 555561. DOI: 10.19080/OMCIJ.2016.01.555561
Abstract
Malaria infection is a major health burdens in Tropical Africa and this is made worst with the emergence of Plasmodium falciparum resistance to the commonly used antimalarial drugs. There is therefore the need for new potent antimalarial drugs. Syntheses of o-trinitro tribenzylamine (2TBN), m-trinitro tribenzylamine (3TBN) and p-trinitro tribenzylamine (4TBN) were carried and yields of 38.49%, 41.01% and 39.67% respectively were obtained on reacting the respective o-, m-, and p-nitro benzaldehyde with formamide. The three synthesized trinitro-tribenzylamine derivatives were tested in vitro against Plasmodium falciparum isolates (3D7 strain) using parasite lactate dehydrogenase assay (pLDH). The result showed significant activity with IC50 ranging from 18 to 30 nM. The meta derivative (IC50:18.823 nM) showed better antiplasmodial activity than ortho (IC50: 30.287 nM) and para (IC50: 22.297 nM) derivatives, and even chloroquine phosphate (IC50: 22.777 nM).
Keywords: Antimalarial drugs; Malaria infection; Plasmodium falciparum; Trinitro tribenzylamine
Abbreviations: 2TBN: Ortho Trinitro-Tribenzylamine; 3TBN: Meta Trinitro-Tribenzylamine; 4TBN: Para Trinitro-Tribenzylamine; DHA: Dihydroartemisinin; CQ: Chloroquine phosphate; PIP-DHA: Piperaquine Dihydroartemisinin
Introduction
Malaria remains one of the major causes of death in several parts of the world. Among the species of plasmodium that commonly infect humans, Plasmodium falciparum and plasmodium vivax are the most prevalent and plasmodium falciparum is the most dangerous [1-3]. The emergence of parasite resistance to commonly used antimalarial drugs, particularly in Plasmodium falciparum case, has increased the burden of malaria [4]. According to WHO, the burden is heaviest in African Region, where an estimated 90% of all malaria deaths occur [5]. The need for new antimalarial drug candidates is therefore very urgent.
Amine compounds are recognized for their polarity and basicity; they are usually incorporated into several drugs to enhance binding to different drug targets as well as to facilitate their absorption. Many antimalarial drugs such as quinine, chloroquine, mefloquine, amodiaquine, etc. are alkaloid, and their mechanism of action has been shown to also depend on the amine functional group present in their structures. The molecules are transported into the acidic food vacuole (pH 4.7) down the pH gradient and the amine gets protonated prior to inhibition of haem polymerization. Accumulation of the haem generated from the degradation of haemoglobin is lethal to the parasite due to the high toxicity of this waste product [6].
Despite decades of effort to eradicate malaria, it remains endemic in several parts of the world. The challenges associated with the treatment of malaria have led Medicinal chemists to keep working to discover potent antimalarial agents. This work aimed to synthesize, characterize and screen the antimalarial activity of some trinitro-tribenzylamine analogues. Also, to evaluate the effect of varying the position of the nitro group between the ortho, meta and para positions of the tribenzylamine moiety on the antimalarial activity.
Materials and Methods
Synthesis of compounds
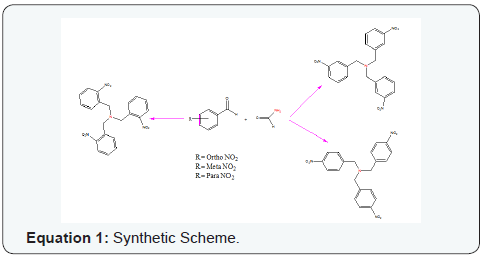
Three trinitro-tribenzylamine analogues were obtained in full synthesis using Leuckart reaction (Equation 1). In a 250 ml-round bottom flask fitted with reflux condenser is placed 0.05 Moles of nitro benzaldehyde derivative (o-, m-, and p-nitro benzaldehyde) and 0.5 Moles of formamide. The contents of the flask were heated and stirred to reflux in acetic acid; after eight hours of refluxing and stirring, the solution was cooled to room temperature. The solution was then neutralized with sodium bicarbonate and extracted with diethyl ether, the ether extracts were concentrated in vacuo to give a product. The structures of synthetized compounds were determined by nuclear magnetic resonance (1H-NMR and 13C-NMR), and FTIR techniques.
Plasmodium falciparum culture and maintenance
The human parasite Plasmodium falciparum strain used in this study was Chloroquine sensitive (3D7), it was obtained from Hematology Department of Jos University Teaching Hospital, Plateau State-Nigeria. The O+ infected erythrocyte (at 4% of hematocrit and 0.5% of parasitemia) was cultured in vitro according to the method described by Trager and Jensen with some modifications [7], in RPMI 1640 medium (Catalog #: ABI-111-02), containing 2g/L of glucose, 1mg/L of L-glutamine, 2g/L of NaHCO3, Amino Acids, Vitamins, Inorganic Salts, Phenol Red and supplemented with 0.01 mg/mL of gentamicin (Sigma-aldrich), 25 of mMN-2-hydroxyethylpiperazine-N-2- ethanesulfonic acid (HEPES) buffer (Sigma-aldrich), and 5% of lipid-enriched bovine albumin preparation (Albumax®).
Determination of in vitro antiplasmodial activity
The synthesized compounds (o-, m-, and p-nitro tribenzylamine) and standards drug: Dihydroartemisinin (Sigma-Aldrich), chloroquine phosphate (Sigma-Aldrich) and Piperaquine-dihydroartemisinin) were dissolved in DMSO 0.5% to produce a stock solution of 100 nM. The infected erythrocyte was exposed to synthesized compounds and standards drug in various concentrations (from 0.2 to 47 nM for the synthesized compounds (2TBN, 3TBN and 4TBN), 0.1 to 24 nM for piperaquine-dihydroartemisinin (PIP-DHA), 0.2 to 48 nM for chloroquine phosphate (CQ), and 0.3 to 70 nM for dihydroartemisinin (DHA) in triplicate in 96-well culture plates and incubated for 72 h at 37°C in an atmosphere of 92% N2, 5% CO2 and 3% O2. For negative control wells, the infected Erythrocytes were devoid of trinitro-tribenzylamine analogues or standards drug. Synthesized compounds and standard drugs were assessed for antiplasmodial activity in vitro using parasite lactate dehydrogenase assay (pLDH).
Parasite lactate dehydrogenase assay
After 72 h of incubation, 20 μL were removed and added to 100 μL of the Malstat reagent (Prepared by dissolving 0.11% of Triton X -100, 115.7mM of L-lactate, 30.27mM of Tris buffer, and 0.62mM of 3-acetyl pyridine adenine dinucleotide in deionized water, the pH was adjusted to 9.2 with hydrochloric acid) present in a new flat-bottomed 96-well micro titer plate in triplicate. 25 μL of NTB/PES (prepared by dissolving 1.96 mM of Nitro Blue Tetrazolium and 0.24mM of Phenazine Ethosulfate in deionized water) were then added to each well, thereby initiating the lactate dehydrogenase reaction. Absorbance was then measured at 630nm using an ELISA plate reader.
Analysis of test results from the pLDH assay
The optical density values from negative control wells were referred to as having 100% pLDH activity. The IC50-values of each tribenzylamine analogues or standard drugs were obtained by regression analysis, using a non-linear dose-response curvefitting equation in Graph Pad Prism 7.0 software.
Results
Characterization of synthesized compounds
O-trinitro tribenzylamine (2TBN): Yield 38.49%, the Infra- Red spectrum (KBr) of this compound showed characteristic peaks at 2924.18, 1527.67, 1381.08 and at 756.12 cm-1. 1H-NMR (400 MHz, DMSO-d6) δ ppm: 7.99 (d, J = 7.2 Hz, 3H), 7.66 (t, J = 6.4 Hz, 3H), 7.29 (t, J = 7.6 Hz, 3H), 6.76 (d, J = 10.4 Hz, 3H), 1.79 (s, 6H). 13C-NMR (101 MHz, DMSO-d6) δ ppm: 152.52, 148.16, 134.68, 131.80, 128.74, 124.59 and23.10.
M-trinitro tribenzylamine (3TBN): Yield 41.01%, the Infra- Red spectrum (KBr) of this compound showed characteristic peaks at 2924.18, 2800.73, 2877.89, 1527.67, 1388.79, 671.25, 725.26, and at 810.13 cm-1. 1H-NMR (600 MHz, DMSO-d6) δ ppm: 8.71 (d, J = 15.4 Hz, 3H), 8.44 (s,3H), 7.88 (t, J = 8.2 Hz, 3H), 7.46 (d, J = 4.4 Hz, 3H), 1.76 (s, 6H).13C-NMR (101 MHz, DMSO-d6) δ ppm: 148.45, 145.71, 140.09, 137.18, 128.86, 125.13 and 22.56.
P-trinitro tribenzylamine (4TBN): Yield 39.67%, the Infra- Red spectrum (KBr) of this compound showed characteristic peaks at 2924.18, 2854.74, 1527.67, 1388.79 and at 802.41 cm-1. 1H-NMR (600 MHz, DMSO-d6) δ ppm: 8.34 (d, J = 8.2 Hz, 6H), 7.74 (d, J = 11.6 Hz, 6H), 1.77 (s, 6H). 13C-NMR (101 MHz, DMSO-d6) δ ppm: 144.82, 141.60, 129.45, 127.48 and 22.95.
Biological Screening
In vitro anti-malarial activity
Parasite % survivals were plotted versus the decimal logarithm of concentration in Graph Pad Prism 7.0 software to determine the concentration inhibiting 50% of parasite growth (IC50 value), and are presented in (Figures 1- 6). The IC50 presented in Table 1, were obtained from three independent triplicate experiments and were expressed as mean ± SEM.
Discussion
A series of trinitro-tribenzylamine derivatives (ortho-, meta- and para) were prepared in good yields. The structures of the synthetized compounds were confirmed based on the data from IR, 1H-NMR and 13C-NMR spectral analysis and the signals obtained were all in a good agreement with the proposed structures. From the graph presented to the (Figures 1-6), showed the decrease in parasite survival as signify by the negative curve in all the synthesize compounds and standard drugs. The results of this study showed that the three synthesized compounds possess Plasmodium falciparum suppressive effect, with IC50 between 18 to 30 nM. From literature, a drug is potentially interesting as antimalarial if its IC50 is below 10μM, 5μM is used as a cut-off level to reduce the number of false positives [8]. Therefore the trinitro-tribenzylamine derivatives synthesized in this study can be added to the growing list of compounds with antiplasmodial activity against plasmodium falciparum, and hence serves as new lead compounds for the development of new antimalarial drugs.
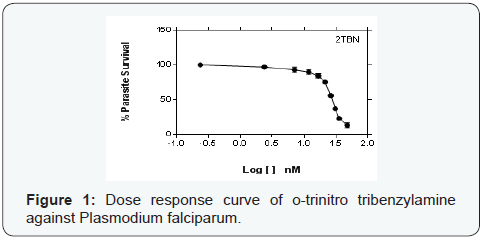
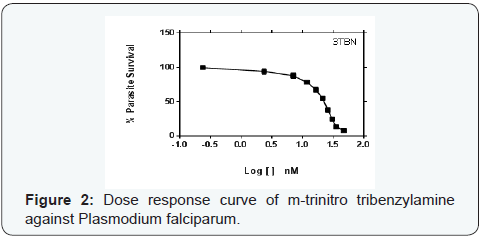
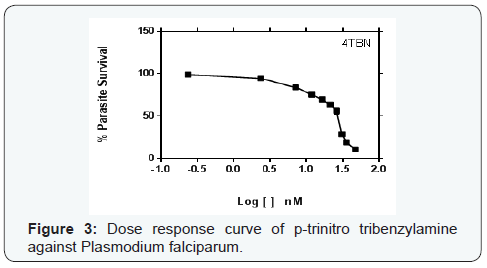
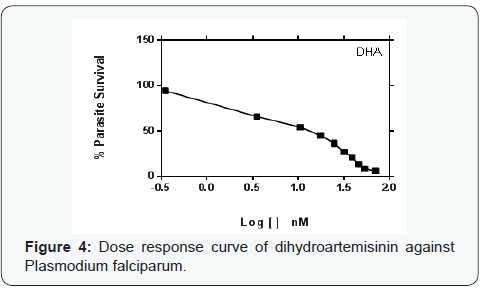

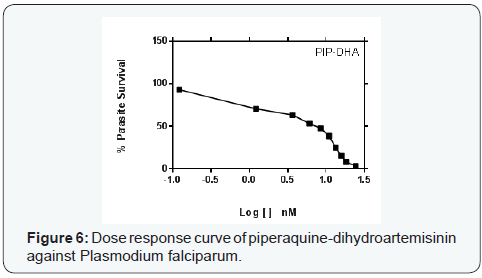
The meta-trinitro tribenzylamine had a lower IC50 than chloroquine phosphate indicating a better antimalarial activity; the para substituted analogue is comparable to chloroquine phosphate, while the ortho has lesser activity to chloroquine phosphate. All the synthesized compounds showed lesser activity compared to DHA, and dihydroartemisinin-piperaquine.
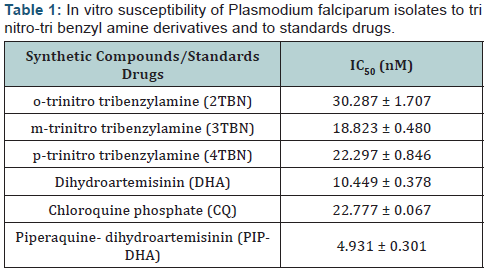
The IC50 results, summarized in (Table 1), indicated that the introduction of nitro group into tribenzylamine skeletal in metaposition enhanced bioactivity against Plasmodium falciparum isolates by almost one point six-fold factor compared to the ortho-derivative and one point two-fold factor compared to the para-derivative.
Conclusion
Ortho-, meta- and para-trinitro tribenzylamine derivatives have been synthesized successfully through a simple one-step reaction (Leuckart reaction). All the three compounds showed good antiplasmodial activity against chloroquine sensitive strain of Plasmodium falciparum (3D7). The substitution of nitro group in meta-position into tribenzylamine moiety has shown to be the most potent derivatives. These derivatives can represent a new template for antimalarial agent development.
Acknowledgments
The authors are grateful to Africa Center of Excellence in Phytomedicine Research and Development (ACEPRD), Jos, Nigeria for their financial supports.
References
- Bjorkman Anders, Bhattarai (2005) Public health impact of drug resistant plasmodium falciparum malaria. Acta Trop 94(3):163-169.
- Shahinas D, Liang M, Datti A, Pillai DR (2010) A Repurposing Strategy Identifies Novel Synergistic Inhibitors of Plasmodium falciparum Heat Shock Protein 90. J Med Chem 53(9): 3552-3557.
- World Health Organization (WHO) (2015) Global Technical Strategy for Malaria 2016-2030. Geneva pp. 32.
- White NJ (2004) Antimalarial drug resistance. J Clin Invest 113(8): 1084-1092.
- World Health Organization (WHO) (2014) World Malaria Report. Geneva.
- Dennis AS (2010) Metabolism, Pharmacokinetics, and Toxicity of Functional Groups: Impact of functional groups, impact of chemical building blocks on ADMET. Royal Society of Chemistry.
- Trager W, Jensen JB (1976) Human malaria parasites in continuous culture. Science 193(4254): 673-675.
- Nassira M, Jesus V, Liliane C, Jorge G, Dominique M, et al. (2006) Identification of new antimalarial drugs by linear discriminant analysis and topological virtual screening. J Antimicrobial Chemotherapy 57(3): 489-497.






























