Dihydroquinazolinones as Potential Antiproliferative and Tumor Inhibiting agents
Kereyagalahally H Narasimhamurthy, Hanumappa Ananda, Kothanahally S SharathKumar, Kanchugarakoppal S Rangappa*
Department of Studies in Chemistry, Manasagangotri, University of Mysore, India
Submission: July 11, 2016; Published: July 27, 2016
*Corresponding author: Kanchugarakoppal S Rangappa, Department of Studies in Chemistry, Mnasagangotri, University of Mysore, Mysuru-570006, India, Tel:+91-821-2419661; Fax: +91-821-2500846 Email:rangappaks@chemistry.uni-mysore.ac.in
How to cite this article: Kereyagalahally H N, Hanumappa A, Kothanahally S S, Kanchugarakoppal SR. Dihydroquinazolinones as Potential Antiproliferative and Tumor Inhibiting agents. Organic & Medicinal Chem IJ. 2016; 1(2): 555560. DOI: 10.19080/OMCIJ.2015.01.555560
Abstract
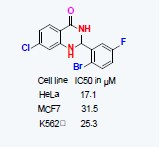
2,3dihydroquinazolinone derivatives were screened for their antiproliferative activity, among all the synthesized molecules compound 3j with electron withdrawing substituents on phenyl ring displayed considerable cytotoxicity against HeLa, MCF7 and K562 cell lines with an IC50 value of 17.1, 31.5 and 25.3 μM, respectively. Further we carried out live dead fluorescence dye assay using calcein-AM and ethidium homodimer staining results further validate the capacity of compound 3j it induces apoptosis in HeLa cells. In addition, the potent compound 3j tested for tumor regression studies in Swiss albino mouse. Both in vitro and in vivo results revealed the significant antiproliferative activity of compound 3j in tumor cell lines and tumor tissue showed multifocal areas of necrosis and numerous number of apoptotic cells.
Graphical Abstract
Keywords: 2,3dihydroquinazolinone; Apoptosis; HeLa
Abbreviations: HeLa: Human Cervical Adeno Carcinoma Cell Line; ESI: Electrospray Ionization; NMR: Nuclear Magnetic Resonance; TLC: Thin Layer Chromatography; M.P: Melting Point; CML: chronic myelogenous leukemia; PBS: Phosphate Buffer Saline
Introduction
Quinazolinones are nitrogen containing heterocycles and found in various naturally occuring alkaloid with wide range of biological activity [1,2]among different substituted quinazolinones, dihydroquinazolinones have been identified as potential anticancer agents, major reports are concerned with dihydroquinazolinones as inhibitors of tubulin depolymerisation [3-5] and as poly(ADP-ribose) polymerase-1 inhibitor [6]. Apart from this, dihydroquinazolinones exhibited different biological activities like antibacterial, [7] anticonvaluscent, [8] antiemetic and gastrointestinal motility enhancing agents, [9] IMPDH inhibitors, [10] antifibrillatory activity, [11] antihistaminic action, [12] anti-inflammatory, [13,14] antitubercular activities, [15] potential inhibitors of SIRT1, [16] Antileishmanial agents [17] and antitumor agents [18].
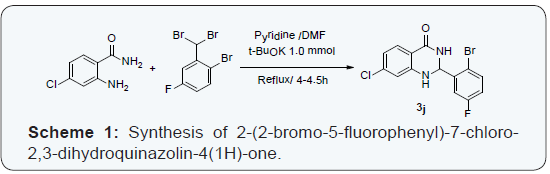
From the past few years we are engaged in developing new synthetic routes [19-21] for the synthesis of different heterocycles and recently we have reported novel method for the synthesis of structurally diversified 2,3-dihydroquinazolinones derivatives by using gem-dibromomethylarenes as synthetic benzaldehyde equivalent (Scheme 1). [22] Staying on our efforts to identify the new target molecules [23,24] and considering the pharmacological activities of dihydroquinazolinones, we have screened most of those derivatives against different cancer cell lines.
All the synthesized compounds (3a-3j) were characterized by IR, 1H NMR, 13C NMR and GCMS. The IR spectra showed a characteristic peak at 2964-2969cm-1 for C-H stretching & 1650-1654 cm-1 corresponds to carbonyl group of 2,3 dihydroquinazolinones. Further, the 1H NMR spectra of all the compounds showed singlet around δ 7.88-8.05 & 5.5-6.2 due to the NH protons of 2,3 dihydroquinazolinones moiety and in the 13C NMR the carbonyl carbon C4 of the 2,3 dihydroquinazolinones ring appeared at 162.9- 163.8 delta.
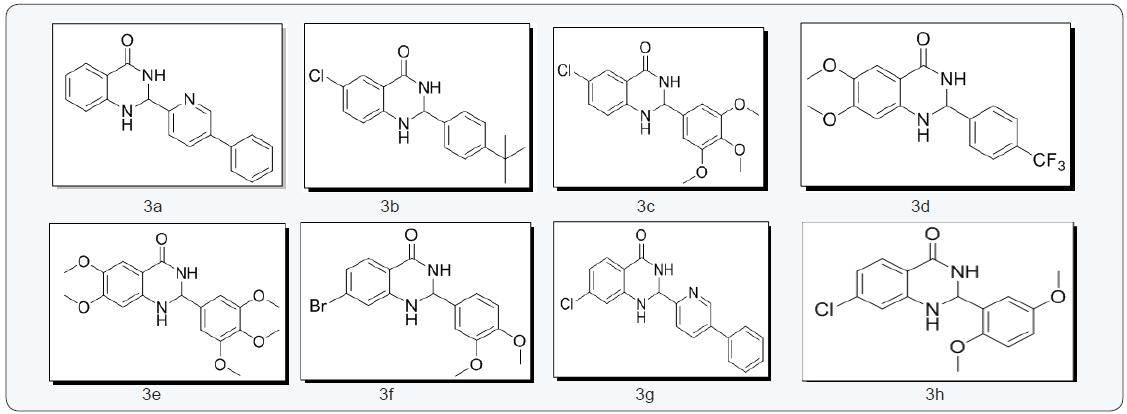
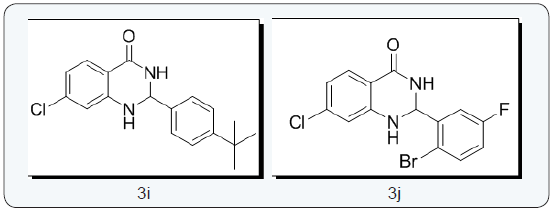
Structures of molecules
Supplementary
Experimental Section
The human chronic myelogenous leukemia (CML) cell line K562, human cervical adeno carcinoma cell line HeLa and breast adeno carcinoma cell line MCF7 used for the screening of newly synthesized compounds. To evaluate the anti proliferation MTT and trypan blue dye exclusion assays, MTT assay were employed as described earlier. Further, live dead assay and in vivo tumor regression analysis were also performed in order to understand the method of apoptosis after treatment with compound 3j.
Cell lines and culture
Human cell lines K562, HeLa and MCF7 cells were purchased from National Center for Cell Science, Pune, India. Cells were grown in RPMI 1640 & DMEM supplemented with 10% heat-inactivated fetal bovine serum (FBS), 100 U/mL of Penicillin, and 100 mg of streptomycin/mLand incubated at 37°C in a humidified atmosphere containing 5% CO2.
Trypan blue dye exclusion assay
The effect of 3(a-j) on cell viability of K562, HeLa cells was determined by Trypan blue dye exclusion assay. Cells were seeded at a density of 1x105 cells/ml cultured for 24 h and synthesized compounds were added at a concentration of 10, 20, 40 and 80 μM DMSO (Sigma Aldrich, USA) treated cells were used as vehicle control. Cells were harvested after 48 h and suspended in 0.4% Trypan blue (Sigma Aldrich, USA) and the viable cells were counted using haemocytometer.
Cytotoxicity assay
Cell multiplying was further assessed by 3-(4,5-dimethylthiazol- 2-yl)-2,5-diphenyl tetrazolium bromide (MTT) assay which is based on the ability of viable cells to metabolize a yellow tetrazolium salt to violet formazan. Exponentially growing K562, HeLa and MCF7 cells (5x104 cells/well) were plated 24 well plates and incubated with 10, 20,40 and 80 μM of compounds 3a-3j, cells were harvested after 48 h of treatment and incubated with MTT (0.5 mg/mL) at 37 ºC in 96 well plate. The blue MTT formazan precipitate was then solubilized in detergent (50% final concentration of N, N-dimethyl formamide and 10% of sodium dodecyl sulfate). Absorbance was measured at 570 nm using ELISA plate reader. The mean absorbance of culture medium was used as the blank and was subtracted. All measurements were performed in triplicate.
Live –dead viability assay
The determination of live –dead assay allowed to assess the viable and nonviable cells. Calcein-AM indicates intracellular esterase activity and the ethidiumhomodimer indicates membrane integrity. The tested compound 3j was exposed for 48h, the test compound was removed and the wells washed with phosphate buffer saline. For live-dead staining add 200 μl of 2 μM Ethidiumhomodimer, 0.5 μM Calcein–AM for 45 minutes in a moist dark chamber at room temperature. After incubation cells were immediately viewed with a fluorescence microscope at 485nm excitation and 515nm emission wavelength. The non fluorescent Calcein-AM is converted into green in colour by intracellular esterase predicts active cell metabolism. Ethidium homodimer is excludes viable cells but permeates broken cell membranes, binds to DNA and results red in colour.
In vivo studies
Animal models and in vivo
Swiss albino female mice weighing 22 to 25 g were housed under standard laboratory conditions with food and water ad libitum. All procedures for animal experimentation used were approved by the Institutional Animal Ethics Committee (IAEC), DOS in Zoology, University of Mysore, India in accordance with the CPCSEA guidelines for laboratory animal facility (Approval no UOM/IAEC/10/1012.Dated 10/11/2012).
EAC induced tumor treated with compound 3j
The antitumor activity and efficacy of the compound 3j tested against EAC cells in vivo. EAC cells were the generous gift from Dr. Shankar, Department of Biotechnology, Terrisian College, Mysuru. Tumor cell suspensions were dissolved in phosphate buffer saline (PBS), counted and re-injected (1×106 cells/animal) to the right thigh of experimental animal for development of solid tumor as described. Control and treated groups consisted of six mice each.
Group 1: Tumor control (Vehicle Control).
Group 2: Tumor treated with 30 mg/kg of compound 3j dissolved in 5% methylcellulose and diluted with distilled water.
After 9 days of tumor induction started compound 3j treatment every alternative day (8 doses) and continued maintained animals up to 30 days, at the end of the 30h day from tumor implantation animals from each group were sacrificed and the tumor tissue was collected and fixed with neutral buffer saline for histopathology studies.
Histopathology
The animals sacrificed on 30th day of the experiment. The tissue samples were collected in 10 per cent neutral buffered formalin for histopathological examination. The tissues were processed by the routine paraffin embedding technique and sections of 4 micron thicknesses were cut using a microtome and subjected to routine hematoxylin and eosin (H & E) staining. Each section was evaluated by inverted microscopy and images were captured (Carl Zeiss, Germany).
Results and Discussion
The 2-aryl- 2,3-dihydro quinazolin-4(1H)-ones (3a-3j) molecules were screened for their antiproliferative activity against various human cancer cell lines such as HeLa (Human cervical adeno carcinoma cell line), MCF7 (Breast adeno carcinoma cell line) and K562 (Chronic myelogenous leukemia) it induces cytotoxicity upon treatment with various concentration(1, 25, 50 and 100 μΜ) of the compounds (Table 1). MTT assay [25] showed the considerable amount of inhibition in HeLa, MCF7 and K562 is 17.1, 31.5 and 25.3 μM, respectively of tested compound 3j.
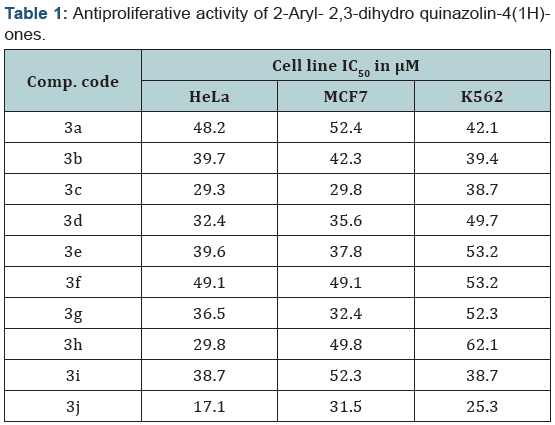
The compound 3j induces cell death in HeLa cells, we carried out livedead fluorescent dye assay using calcein-AM and ethidium homodimer staining. This assay is very useful to determine to check cytotoxicity resulting from interaction of the cells with cytotoxic agents. Calcein-AM stains live cells with an intact membrane in which esterase activity is occurring and gives green fluorescence and ethidiumhomodimer stains dead cells or cells whose membrane intergrity is damaged are distinguished by a bright red fluorescence, which indicated the amount of cell death caused by compound 3j in HeLa cells (Figure 1A and B). These results further validate the capacity of compound 3j induces cell death in HeLa (Human cervical cancer) cells.
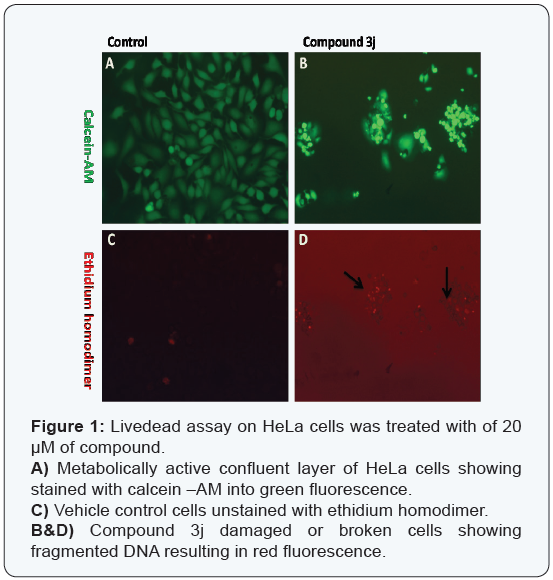
Hence further we carried out in vivo tumor regression studies for the potent compound 3j (30 mg/kg) on mice bearing mouse breast adeno carcinoma tumor cells (EAC). The histopathology studies showed that morphology and cellular construction of the tissues (Figure 2A and B) was unaltered by the compound 3j treatment. Cell proliferations as well as multifocal area of necrosis and enumerable number of apoptotic cells were observed following treatment with compound 3j.
Experimental Details
Reactions were monitored by TLC using pre coated sheets of silica gel G/UV-254 of 0.25 mm thickness (Merck 60F254) using UV light for visualization. The melting points were determined on Selaco melting point apparatus and are uncorrected. 1H and 13C NMR spectra were recorded on an NMR spectrometer operating at 400 and 100 MHz, respectively, using the residual solvent peaks as reference relative to SiMe4. Mass spectra were recorded using electrospray ionization (ESI) mass spectrometry. The C, H and N analysis were performed using CE-400 CHN analyzer. Infrared spectra were recorded on Shimadzu FT-IR model 8300 spectrophotometer.
2-(5-phenylpyridin-2-yl)-2,3-dihydroquinazolin-4(1H)- one (3a) [22] : Light yellow solid; M.P: 185-186 0C; (KBr) vmax 3184.26, 3066.61, 2929.67, 1666.38, 1610.45 cm-1. 1H NMR (400 MHz, DMSO- d6): δ 8.66-8.64 (d,J=4.8Hz, 1H), 8.32 (s 1H), 8.09-8.07 (d J=8.4Hz, 2H), 7.95-7.93 (d, J=8Hz, 1H), 7.88-7.84 (tt, J=1.6Hz, 1H), 7.62-7.58 (m 3H), 7.35-7.32 1H), 7.26-7.22 (tt, J= 8.4Hz,1H), 7.15 (s, 1H), 6.76-6.74 (d, J= 8Hz,1H), 6.69-6.52 (tt, J=8Hz,1H), 5.8 (s, 1H) ppm; 13C NMR (100 MHz, DMSO-d6): δ 163.52, 155.57, 149.54,147.76, 142.41, 138.8, 137.2, 133.3, 127.34, 127.22, 126.45, 122.68, 120.29, 117.13, 114.97,114.42, 66.18 ppm; MS(ESI): m/z = 301.122, HRMS (ESI) calcd for [C19H15N3O+H+] 302.3498, found 302.3495.
2-(4-tert-butylphenyl)-6-chloro-2,3-dihydroquinazolin- 4(1H)-one (3b) [22]: Light yellow solid; M.P: 180-182 0C; IR (KBr) vmax 3328.91, 3257.55, 2929.67, 1741.60, 1612.38 cm1.1H NMR (400MHz, DMSO-d6): δ 8.38 (s, 1H), 7.53-7.52 (d, J=2.4Hz, 1H), 7.42-7.38 (m, 4H), 7.27-7.24 (m, 2H), 6.75-6.73 (d, J=8.4Hz, 1H), 5.73 (s, 1H), 1.26 (S, 9H) ppm; 13C NMR (100 MHz, DMSO-d6): δ 162.40, 151.13, 146.62, 138.18, 132.96, 126.59, 126.35, 126.20, 125.11, 120.61, 116.31, 116, 66.27, 34.28, 31.04 ppm; MS(ESI): m/z = 314.119. HRMS (ESI) calcd for [C18H20ClN2O+H+] 315.8172 found 315.8170.
6 - c h l o r o - 2 - ( 3 , 4 , 5 - t r i m e t h ox y p h e ny l ) - 2 , 3 - dihydroquinazolin-4(1H)-one(3c)[22]: White solid; Mp: 158- 160 0C. IR (KBr) vmax 3274.90, 3197.76, 2964.39, 2929.67, 1654, 1612.33 cm-1. 1H NMR (400 MHz, DMSO-d6): δ 8.36 (s, 1H), 7.54- 7.53 (d, J=2.4Hz, 1H), 7.29-7.24 ((m, 2H),6.82-6.77 (m, 4H), 5.72 (S, 1H), 3.76 (s, 6H), 3.64 (s, 3H) ppm; 13C NMR (100 MHz, DMSO-d6): δ 162.49, 152.76, 146.73, 137.69, 136.17, 133.02, 126.40, 120.86, 116.42, 116.12, 104.45, 66.73, 59.95, 59.71, 55.92 ppm; MS (ESI): m/z = 348.088, HRMS (ESI) calcd for [C17H18ClN2O4+H+] 349.7888 found 349.7885.
6,7-dimethoxy-2-(4-(trifluoromethyl)phenyl)-2,3- dihydroquinazolin-4(1H)-one(3d) [22]: Light yellow solid; M.P: 188-189 0C; IR (KBr) vmax 3301.91, 3197.76, 2925.81, 2852.52, 1649.02, 1618.17 cm-1; 1H NMR (400 MHz, DMSO-d6): δ 8.18 (s, 1H), 7.75-7.67 (m,4H), 7.08 (s, 1H), 6.91 (s, 1H), 6.35 (s, 1H), 5.77 (s, 1H), 3.72 (s, 3H), 3.65 (S, 3H) ppm; 13C NMR (100 MHz, DMSO-d6): δ 163.44, 153.93, 146.60, 143.07, 141.58, 127.74, 127.54, 125.18, 125.15, 125.04, 109.74, 106.62, 97.99, 65.92, 55.74, 55.34 ppm; MS(ESI): m/z =352.307, HRMS (ESI) calcd for [C17H16F3N2O3+H+] 353.3157 found 353.3155.
6,7-dimethoxy-2-(3,4,5-trimethoxyphenyl)-2,3- dihydroquinazolin-4(1H)-one(3e) [22]: Light red solid; M.P: 242-244 0C. IR (KBr)vmax 3353.98, 3197.76, 2937.38, 2837.09, 1654.81, 1620.09, cm-1. 1H NMR (400 MHz, DMSO-d6): δ 7.49 (s, 1H), 7.13 (s,1H), 6.85 (s, 2H), 6.7 (s, 1H), 6.4 (s, 1H), 5.64 (s, 1H), 3.78 (s, 3H) ppm; 13C NMR (100 MHz, DMSO-d6): δ 163.82, 153.78, 152.83, 152.64, 143.75, 141.55, 137.48, 136.52, 109.79, 108.24, 104.96, 104.80, 97.99, 67.30, 59.91, 55.87, 55.77, 55.69, 55.35 ppm; MS(ESI): m/z = 374.387, HRMS (ESI) calcd for [C19H23N2O6+H+] 375.3957, found 375.3954.
7 - b r o m o - 2 - ( 3 , 4 - d i m e t h o x y p h e n y l ) - 2 , 3 - dihydroquinazolin-4(1H)-one (3f) [22]: White solid; M.P: 137- 138 0C; IR (KBr) vmax 3298.26, 3182.33, 3070.46, 2956.67, 2923.67, 2923.88, 2852.52, 1700, 1610 cm-1; 1H NMR(400 MHz, DMSO-d6): δ 8.38 (s, 1H), 7.52-7.49 (m, 3H), 7.33 (s,1H), 7.24- 7.2 (m, 1H), 6.94-6.93 (d, J=2Hz, 1H), 6.83-6.80 (dd, J=1.8Hz,1H), 5.81 (s, 1H), 3.68 (s, 6H) ppm; 13C NMR (100 MHz, DMSO-d6): δ 162.65, 148.42, 141.06, 134.75, 129.42, 127, 120.44, 118.16, 117.94, 116.63, 115.98, 115.74, 113.57, 66.20, 55.42, 55.36 ppm; MS(ESI): m/z = 363.205, HRMS (ESI) calcd for [C16H16BrN2O3+H+] 364.2138, found 364.2135.
7-chloro-2-(5-phenylpyridin-2-yl)-2,3-dihydroquinazolin- 4(1H)-one (3g) [22]:Brown solid; M.P: 208-210 0C; IR (KBr) vmax 3193.9, 3068.53, 2923.88,2854.45, 2813.95, 1666.38, 1610.45 cm-1; 1H NMR (400 MHz, DMSO-d6): δ 8.66-8.65 (d, J=4.4Hz, 1H),8.45 (s, 1H), 8.11-8.09 (d, J=8.4, 1H), 7.95-7.93 (d, J=8Hz, 1H), 7.89- 7.84 (tt, J=1.46, 1H), 7.62-7.56 (m, 4H), 7.43 (s, 1H), 7.36-7.33 (m, 1H), 6.8-6.79 (d, J=2Hz, 1H), 6.69-6.67 (dd, J=2Hz, 1H), 5.86 (s, 1H) ppm; 13C NMR (100 MHz, DMSO-d6): δ 162.63, 155.48, 149.53, 148.66, 142.04, 138.94, 137.81, 137.37, 137.21, 129.32, 128.35, 127.1, 126.54, 122.69, 120.29, 117.04, 113.62, 113.42, 66.07 ppm; MS(ESI): m/z= 335.787, HRMS (ESI) calcd for [C19H15ClN3O+H+] 336.7949, found 336.7945.
7-chloro-2-(2,5-dimethoxyphenyl)-2,3-dihydroquinazolin- 4(1H)-one (3h) [22]:Light yellow solid; M.P: 208-210 0C; IR (KBr) vmax 3330.84, 3298.05, 3234.4, 3060.82, 2962.46, 2867.95, 1643.24, 1610.45 cm-1; 1H NMR (400 MHz, DMSO-d6): δ 8.11 (s, 1H), 7.61- 7.59 (d, J=8.4Hz, 1H), 7.04 (s, 1H), 6.99-6.96 (m, 1H), 6.9-6.87 (m, 2H), 6.817-6.813 (d, J=1.6Hz,1H), 6.67-6.65 (dd, J=2Hz, 1H), 5.99 (s, 1H), 3.77 (s, 3H), 3.66 (s, 3H) ppm; 13C NMR (100 MHz, DMSO-d6): δ 162.91, 152.85, 150.36, 148.71, 137.74, 129.58, 129.21, 124.74, 116.92, 113.61, 113.5, 113.34, 112.24, 61.07, 55.99, 55.36 ppm; MS(ESI): m/z = 318.754. HRMS (ESI) calcd for [C16H16ClN2O3+H+] 319.7628, found 319.7628.
2-(4-tert-butylphenyl)-7-chloro-2,3-dihydroquinazolin- 4(1H)-one (3i) [22]: Light yellow solid; M.P: 110-112 0C; IR (KBr) vmax 3332.76, 3170.76, 3031.89, 2925.81, 2831.31, 1656.74, 1608.52 cm-1; 1H NMR (400 MHz, DMSO-d6): δ 8.31 (s, 1H), 8.14- 8.1 (dd, J=2.6Hz, 1H), 7.6-7.38 (m, 4H), 7.3 (s, 1H), 6.757-6.752 (d, J=2 Hz, 1H), 6.67-6.65 (dd, J=2Hz, 1H), 5.75 (s, 1H) ppm; 13C NMR (100 MHz, DMSO-d6): δ 162.71,151.17, 148.8, 138.24, 137.72, 129.13, 127.68, 126.55, 125.43, 125.3, 116.9, 113.6, 113.31, 66.33, 34.29, 31.04, 30.84 ppm; MS(ESI): m/z = 314.809, HRMS (ESI) calcd for [C18H20ClN2O+H+] 315.8172, found 315.8170.
2 - ( 2 - b r o m o - 5 - f l u r o p h e n y l ) - 7 - c h l o r o - 2 , 3 - dihydroquinazolin-4(1H)-one (3j) [22]: Potassium tertiary butoxide (0.323 g, 0.00288 mol) was added to a suspension of 1-bromo-2-(dibromomethyl)-4-fluorobenzene (1g, 0.00288mol) and 2-amino-5-chlorobenzamide (0.541g, 0.00317mol) in pyridine: dimethyl formamide (6.0: 2.0 mL) solvent mixture. The resulting mixture was refluxed at reflux for 4 h. Progress of the reaction was monitored by TLC. The reaction mass was mixed with water then extracted with ethyl acetate (2 x 20 mL), organic phase was washed with brine solution and dried over anhydrous sodium sulphate. The organic phase was evaporated and the crude product was purified by column chromatography using silica gel mesh 100-200 (30 % EtOAc in hexane) 2-(2-bromo-5-flurophenyl)-7-chloro-2,3- dihydroquinazolin-4(1H)-one.
White solid M.P : 197-198 0C; IR (KBr) vmax 3353.98, 3288.4,3182.33, 3051.18, 2921.96, 2854.45, 1694.02, 1610.45 cm-1; 1H NMR (400 MHz, DMSO-d6): δ 8.33 (s, 1H), 7.74-7.7 (m, 1H), 7.63 (s, 1H), 7.41-7.38 (dd,, J=2.6Hz,1H), 7.29-7.22 (m, 2H), 6.81- 6.8 (d, J=1.2Hz,1H), 6.75-6.72 (dd, J=1.8Hz,1H), 6.1 (s, 1H) ppm; 13C NMR (100 MHz, DMSO-d6): δ 162.57, 160.13, 148.35, 141.02, 138.01, 134.65, 129.35, 117.92, 117.6, 116.58, 115.99, 115.75, 113.27, 66.24 ppm; MS(ESI):m/z =355.589, HRMS(ESI) calcd for [C14H10BrClFN2O+H+] 356.5974, found 356.5971.
Conclusion
In conclusion, the cytotoxicity assay was used for preliminary screening of 2-aryl- 2,3-dihydro quinazolin-4(1H)-ones. Among all the screened compounds, compound 3j was found to be active against human cancer cell lines at lower concentrations. Further livedead fluorescent cell assay showed more red fluorescence scored as dead or apoptotic cells. Hence the compound2-(2-bromo- 5-flurophenyl)-7-chloro-2,3-dihydroquinazolin-4(1H)-one 3j was subjected to in vivo studies EAC tumor models. In control, tumor section showed solid arrangement of neoplastic cells infiltrating between adjacent tissues. Tumor tissue treated with compound 3jshowing multifocal area of necrosis and apoptotic cells. Hence, 2-aryl- 2,3-dihydro quinazolin-4(1H)-ones opens a new avenue for combating cancer.
Acknowledgement
We thankful to Prof. Suguna Rao, Department of Veterinary Pathology, Veterinary College, Hebbal, Bengaluru for helping in in vivo studies. KSR is grateful to DST, CSIR, for the financial assistance under different projects. No. DST/INT/SA/P-05/2011-12, Dated 10-01-12.INT/Indo-Korea/122/2011-12, Dated 13.09.2011.No. 01 (2434) /10/ EMR-II, Dated 28.12.2010. IFCPAR Sanction No. IFC/4303-1/2010-11 dated 22/12/2010. KHN& KSS is grateful to UGC, RFSMS Govt. of India for Research fellowship. A.H. thanks ICMR for SRF (No 45/54/2013-PHA/BMS, Dated: 08.12.2014) for research fellowship.
References
- Liverton NJ, Armstrong DJ, Claremon DA, Remy DC, Baldvin JJ, et al. (1998) Nonpeptide glycoprotein IIb/IIIa inhibitors: Substituted quinazolinediones and quinazolinones as potent fibrinogen receptor antagonists. Bioorg & Med Chem Lett 8(5): 483-486.
- de Laszlo SE, Quagliato CS, Greenlee WJ, Patchett AA, Chang RSL, et al. (1993) A potent orally active balanced affinity angiotensin II AT1 antagonist and AT2 binding inhibitor. J Med Chem 36(21): 3207-3210.
- Hour MJ, Huang LJ, Kuo SC, Xia Y, Bastow K, et al. (2000) 6-Alkylaminoand 2,3-Dihydro-3‘-methoxy-2-phenyl-4-quinazolinones and Related Compounds: Their Synthesis, Cytotoxicity and Inhibition of Tubulin Polymerization. J Med Chem 43(23): 4479-4487.
- Kamal A, Bharathi EV, Reddy JS, Ramaiah MJ, Dastagiri D, et al. (2011) Synthesis and biological evaluation of 3,5-diaryl isoxazoline/isoxazole linked 2,3-dihydroquinazolinone hybrids as anticancer agents. E J Med Chem 46(2): 691-703.
- Chinigo GM, Paige M, Grindrod S, Hamel E, Dakshanamurthy S, et al. (2008) Asymmetric Synthesis of 2,3-Dihydro-2-arylquinazolin-4-ones: Methodology and Application to a Potent Fluorescent Tubulin Inhibitor with Anticancer Activity. J Med Chem 51(15): 4620-4631.
- Iwashita A, Tojo N, Matsuura S, Yamazaki S, Kamijo K, et.al. (2004) A novel and potent poly(ADP-ribose) polymerase-1 inhibitor, FR247304 (5-chloro-2-[3-(4-phenyl-3,6-dihydro-1(2H)-pyridinyl)propyl]-4(3H)- quinazolinone) attenuates neuronal damage in in vitro and in vivo models of cerebral ischemia. J Pharma Exp Ther 310(2): 425-436.
- Alaimo RJ, Russell HE (1972) Antibacterial 2,3-dihydro-2-(5-nitro-2- thienyl)quinazolin-4-(1H)-ones. J Med Chem 15(3): 335-336.
- Bajaj K, Srivastava VK, Kumar A (2003) Newer substituted benzoxazepinyl-quinazolinones as potent antipsychotic and anticonvulsant agents. Arzneimittel-Forschung 53(7): 480-485.
- Baldazzi C, Barbanti M, Basaglia R, Benelli A, Bertolini A, et al. (1996) A new series of 6-chloro-2,3-dihydro-4(1H)-quinazolinone derivatives as antiemetic and gastrointestinal motility enhancing agents. Arzneimittel-Forschung 46(9): 911-918.
- Birch HL, Buckley GM, Davies N, Dyke HJ, Frost EJ, et al. (2005) Novel 7-methoxy-6-oxazol-5-yl-2,3-dihydro-1H-quinazolin-4-ones as IMPDH inhibitors. Bioorg Med Chem Lett 15(23): 5335-5339.
- Bonola G, Da Re P, Magistretti MJ, Massarani E, Setnikar I (1968) 1-Aminoacyl-2,3-dihydro-4(1H)-quinazolinone derivatives with choleretic and antifibrillatory activity. J Med Chem 11(6): 1136-1139.
- Buyuktimkin S, Buschauer A, Schunack W (1991) [Quinazolinones. 18. Synthesis and H1/H2-antihistaminic action of omega-[2-aryl- 2,3-dihydro-4(1H)-quinazolinone-1-yl]alkyl substituted ureas and cyanoguanidines]. Arch Pharm (Weinheim) 324(5): 291-295.
- Khalil MA, Soliman R, Farghaly AM, Bekhit AA (1994) Non-steroidal anti-inflammatory agents: novel pyrazolyl-1,2-oxazolyl and1,3-diazinyl derivatives of 4(3H)-quinazolinones. Arch Pharm (Weinheim) 327(1): 27-30.
- 14. Manivannan E, Chaturvedi SC (2012) Analogue-based design, synthesis and docking of non-steroidal anti-inflammatory agents. Part 2: Methyl sulfanyl/methyl sulfonyl substituted 2,3-diaryl-2,3-dihydro- 1H-quinazolin-4-ones. Bioorg Med Chem 20(24): 7119-7127.
- Rambabu D, Kiran Kumar S, Yogi Sreenivas B, Sandra S, Kandale A, et al. (2013) Ultrasound-based approach to spiro-2,3-dihydroquinazolin- 4(1H)-ones: their in vitro evaluation against chorismate mutase. Tetrahedron Lett 54(6): 495-501.
- Rambabu D, Raja G, Yogi Sreenivas B, Seerapu GP, Lalith Kumar K, et al. (2013) Spiro heterocycles as potential inhibitors of SIRT1: Pd/Cmediated synthesis of novel N-indolylmethyl spiroindoline-3,2’- quinazolines. Bioorg Med Chem Lett 23(5): 1351-1357.
- Sharma M, Chauhan K, Shivahare R, Vishwakarma P, Suthar MK, et al. (2013) Discovery of a new class of natural product-inspired quinazolinone hybrid as potent antileishmanial agents. J Med Chem 56(11): 4374-4392.
- Roopan SM, Nawaz Khan F, Jin JS, Senthil Kumar R (2011) An efficient one pot–three component cyclocondensation in the synthesis of 2-(2-chloroquinolin-3-yl)-2,3-dihydroquinazolin-4(1H)-ones: potential antitumor agents. Res Chem Intermed 37(8): 919-927.
- Sharath Kumar KS, Swaroop TR, Harsha KB, Narasimhamurthy KH, Rangappa KS, et al. (2012) T3P®-DMSO mediated one pot cascade protocol for the synthesis of 4-thiazolidinones from alcohols. Tetrahedron Letters 53(42): 5619-5623.
- Narasimhamurthy KH, Chandrappa S, Kumar KSS, Swaroop TR, Rangappa KS, et al. (2013) Synthetic Utility of Propylphosphonic Anhydride & ndash; DMSO Media: An Efficient One-pot Threecomponent Synthesis of 2-Arylquinolines. Chemistry Letters 42(9): 1073-1075.
- Girish YR, Kumar KSS, Muddegowda U, Lokanath NK, Rangappa KS, et al. (2014) ZrO 2-supported Cu (ii)–β-cyclodextrin complex: construction of 2, 4, 5-trisubstituted-1, 2, 3-triazoles via azide–chalcone oxidative cycloaddition and post-triazole alkylation. RSC Advances 4(99): 55800-55806.
- Narasimhamurthy KH, Chandrappa S, Sharath Kumar KS, Harsha KB, Rangappa KS, et al. (2014) Easy access for the synthesis of 2-aryl 2,3-dihydroquinazolin-4(1H)-ones using gem-dibromomethylarenes as synthetic aldehyde equivalent. RSC Advances 4(65): 34479-3448
- Srinivas V, Mohan CD, Baburajeev CP, Rangappa S, Jagadish S, et al. (2015) Synthesis and characterization of novel oxazines and demonstration that they specifically target cyclooxygenase 2. Bio org Med Chem Lett 25(15): 2931-2936.
- Rangappa KS, Kumar KSS, Mohan CD, Jagadish S, Rakesh KS, et al. (2015) Synthesis and acetylcholinesterase/butyrylcholinesterase inhibition activity of arecoline, 4-thiazolidinone and piperidine based conjugates. A J Pharma Clin Res 8(1).
- Sharath Kumar KS, Hanumappa A, Hegde M, Narasimhamurthy KH, Raghavan SC, et al. (2014) Synthesis and antiproliferative effect of novel 4-thiazolidinone, pyridine and piperazine-based conjugates on human leukemic cells. E J Med Chem 81(0): 341-349.





