Research Article
An Expedient One-Pot Synthetic Protocol to the Preparation Of Isoxazolo and Pyrazolo Annulated Analogues of Benzoxazepino Condensed Carbazoles and Azacarbazoles of Medicinal Interest
Simpal Kumari, Nirupama Mishra* and Dharma Kishore
Department of Chemistry, Banasthali University, India
Submission: November 24, 2015; Published: December 22, 2015
*Corresponding author: Nirupama Mishra, Department of Chemistry, Banasthali University, P.O. Banasthali, Vidyapith, Rajasthan, 304022, India; Email: nirupmaphd@gmail.com
How to cite this article: Simpal K, Nirupama M, Dharma K. An Expedient One-Pot Synthetic Protocol to the Preparation Of Isoxazolo and Pyrazolo Annulated Analogues of Benzoxazepino Condensed Carbazoles and Azacarbazoles of Medicinal Interest. Organic & Medicinal Chem IJ. 2015; 1(1): 555552. DOI: 10.19080/OMCIJ.2015.01.555552
Abstract
A facile one-pot synthetic protocol for the preparation of isoxazolo and pyrazolo annulated analogues of the benzoxazepine condensed carbazoles and azacarbazoles from the corresponding enolic ethers, α, β-unsaturated ketones, oxoketene dithioacetals and dimethylaminomethylene ketones derived from benzoxazepino condensed oxocarbazoles and oxoazacarbazoles has been described. Compounds were realized from the Japp-Klingemann reaction of the diazonium salt of 2-aminodibenzo [b,f] [1,4]oxazepine with 2-hydroxymethylideno cyclohexanone and N-benzyl-3-hydroxymethylidenopiperidin-4-one followed by Fischer indolization of the obtained hydrazone with kent’s acid. The synthesized heterocyclic compounds were characterized by IR, NMR and MS spectral data and in vitro antimicrobial activity of the synthesized compounds was screened against the standard bacterial and fungal strains. Some of these benzoxazepine derivatives have been found to display excellent antimicrobial activity against bacteria and fungi.
Keywords:
Japp-Klingemann Reaction; 2-Aminodibenzo [b,f] [1,4] Oxazepine; Fischer Indolization; Enolic Ether; Chalcone; oxoketenedithioacetals; dimethylaminomethylene ketone; oxocarbazoles and oxoazacarbazoles.
Introduction
Benzoxazepine is one of the privileged heterocyclic template in medicinal chemistry, dibenz[b,f ][1,4]oxazepine (CR) is an important incapacitating agents of this class which is also more potent and less toxic than widely used other riot control agents [1,2]. Analogues of 11H-dibenz [b,e] azepine and dibenz [b,f] [1,4] oxazepine (tear gas) are used as human transient receptor potential ankyrin 1 (TRPAI) antagonists [3]. Sintamil, a new dibenzoxazepine derivative has been extensively studied during the last decade for anti depressant action with fairly good results in animals and human beings [4-6]. N-substituted dibenz [b,f] [1,4] oxazepin-11(10H)-ones have been reported to exhibit antidepressant [6], calcium antagonist [7], anticancer [8-10] and squalene synthase inhibiting activities [11]. Dibenz [b,f] [1,4] oxazepines are found in many physiochemically active compounds. Numerous derivatives have been prepared and evaluated for PGEs antagonists and analgesic activities [12] (Figure 1).
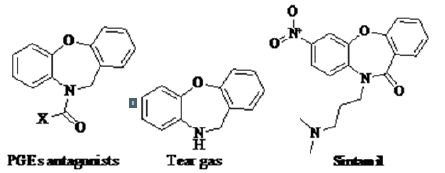
Condensed heterocyclic systems containing carbazole, azacarbazole, azepine, benzoxazepine, isoxazole, pyrazole etc. represent such pharmacophoric scaffolds which have a wide range of biological applications such as analgesic, anti inflammatory, anti-bacterial, antifungal and anticancer activity [1-8, 13-20]. Carbazoles, azacarbazoles (including pyridocarbazoles) show impressive cytotoxic activity and form interesting targets in synthesis since such structures have potential for the development of anticancer drugs [5-8]. Coupled with this, encouraging findings of anticancer and antileukemic activity of triazolo and pyrrolo condensed benzoxazepines which was hailed as a major step forward in the battle against cancer [9-11], stimulated many eyes to examine the versatility of this nucleus in combating this disease. It firmly affirmed the observation that incorporation of additional rings onto the benzoxazepine core tended to exert a profound influence in conferring novel biological activities in this molecule. Another class of heterocycles which exhibit similar versatility in providing pharmacophoric scaffolds in medicinal field are the isoxazoles and pyrazoles whose derivatives have emerged as potent materials useful as potential anti HIV agents [21- 25]. In view of the prodigious range of activities of the above molecules, it was considered of interest in the present work to develop the bioactive materials incorporating benzoxazepine nucleus on one side of carbazole or azacarbazole core and isoxazole and pyrazole nucleus on its other side, on this premise that their presence in tandem in the same molecular framework could contribute significantly to the biological activity in the resulting molecules. Here in, in this communication, we report the applications of facile synthetic protocols which have allowed an easy incorporation of isoxazole and pyrazole motifs on to the benzoxazepine condensed carbazole nucleus.
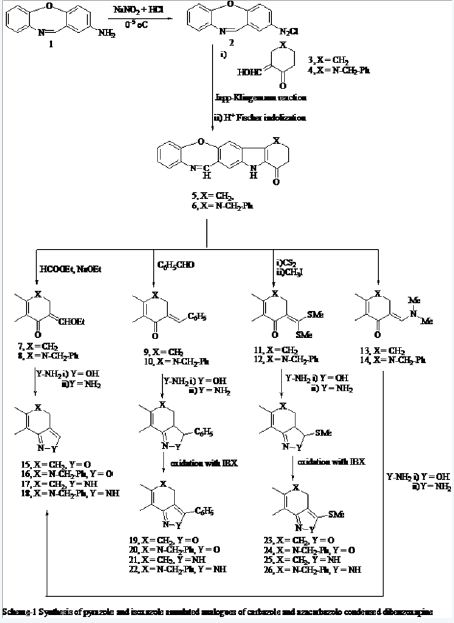
In pursuance of our interest in the biological potential of isoxazolo and pyrazolo annulated analogues of benzoxazepine condensed carbazoles and azacarbazoles 15-26 shown in scheme-1 [26-29], these were required to be obtained. On brainstorming in the direction of finding easy routes to their formation, we envisioned that these could possibly be readily available from the corresponding enolic ethers 7, 8, α,β- unsaturated ketones 9, 10, oxoketenedithioacetals 11, 12 and dimethylaminomethyleneketone 13, 14, which in turn, in principle be readily formed from the carbocyclic ketones derived from the benzoxazepino condensed oxocarbazoles and oxoazacarbazole 5 and 6 respectively on their reaction with-
- HCOOEt, NaOEt
- C6H5CHO
- CS2, CH3I (base)
- dimethylformamide dimethylacetal (DMF-DMA).
These intermediates have been known to undergo reaction with the bidentate nucleophiles to provide a very convenient synthetic entry to the five, six, and seven membered heterocycles [30-33]. In accord to their versatility in synthesis the procedures reported in the literature were applied on 5- and 6 to obtain 7-14, whose treatment with hydroxyl amine hydrochloride, and hydrazine hydrate afforded the corresponding isoxazole and pyrazole annulated analogues of benzoxazepine condensed carbazole and azacarbazoles 15-26 respectively (scheme-1).
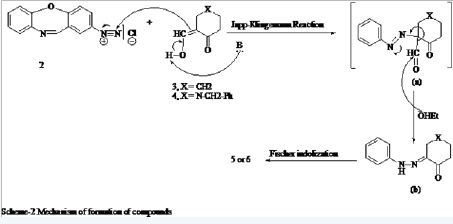
The potential of 5- and 6 in the above synthesis prompted us to explore the feasibility of their preparation through an expedient route from the easily accessible starting materials. A perusal of literature on the synthesis of oxocarbazoles revealed that the most recorded method of their preparation is use of the Japp-Klingemann reaction on the corresponding aryl amines, followed by Fischer indolization of the obtained hydrazone in acid, to give the corresponding tetrahydrocarbazol-4-one derivative [34,35]. This methodology was applied on the diazotized 2-amino-dibenzo [b,f] [1,4] oxazapine (2) which was allowed to condense with 2-(hydroxy-methylideno) cyclohexanone (3) and 3-(hydroxymethylideno)-N-benzyl-piperidin-4-one (4) (which were prepared in situ from the reaction of cyclohexanone or N-benzyl-4-piperidone with ethyl formate in presence of sodium ethoxide) followed by cyclization in Kent acid (AcOH+HCl 20:5 ml v/v) to give 5 and 6 respectively (scheme-1) (Figure 2).
2-Amino-dibenzo [b,f] [1,4] oxazepine (2) was obtained by the reduction of the corresponding 2-nitroderivative. The later was in turn realized through a reported procedure [36] which consisted of the base catalyzed cyclocondensation of o-aminophenol with 2-chloro-5-nitrobenzaldehyde. The formation of 5 and 6 is believed to take place through the cyclocondensation of the corresponding phenyl hydrazone derivative (scheme-2) which results from the reaction of 2 with 2-hydroxymethylideno cyclohexanone (3) or N-benzyl- 3-hydroxymethylidenolpiperidin-4-one (4). The reaction is suggested to proceed through the sequence of reactions shown in scheme-2 (Figure 3).
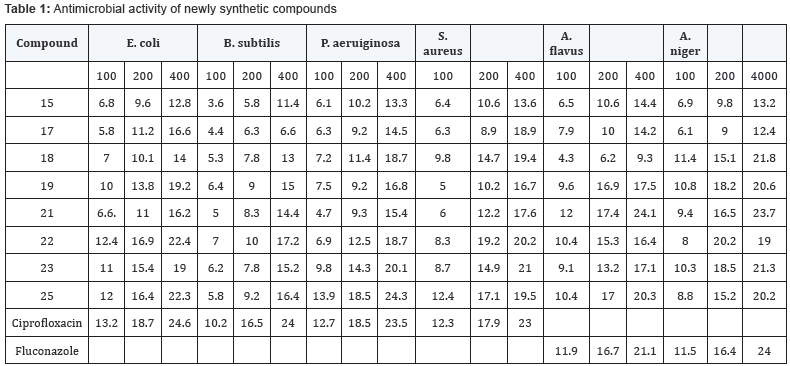
In order to find out the biological potential of synthesized compounds, antimicrobial activity of compound 15, 17, 18, 19, 21, 22, 23, 25 was studied towards four bacterial Escherichia coli (MTCC119), Bacillus subtilis (MTCC7419), Pseudomonas aeruginosa (MTCC1688) and Staphylococcus aureus (MTCC9886) using ciprofloxin as standard and two fungal species A flavus (MTCC871) and A.niger (MTCC282) using fluconazole as standard antifungal agent by disc diffusion method. By following a reported method by Kumar et al. [37] with some modifications. Antimicrobial activity of indicated compounds was recorded as zone of inhibition in mm and results are summarized in (Table 1). Results showed that majority of the compounds display varying degree of inhibition against the tested microorganisms. In general the potency against gram positive is higher than gram negative. Some tested compound showed remarkable antibacterial and antifungal activities. Compound 22 inhibited both gram positive and gram negative bacterial strains but show less efficiency towards fungal strains. Instead, compound 25 showed activity against both bacterial and fungal strains. Compound 21 showed excellent antifungal activity against both fungal strains comparable to standard drug Fluconazole.
In summary, an efficient methodology for easy access to the pyrazolo and isoxazolo annulated analogues of carbazole and azacarbazole fused benzoxazepines has been described. The application of Japp-Klingemann reaction on 2-aminodibenzoxazepine and 2-hydroxymethylideno cyclohexanone/3-hydroxymethylideno-N-benzyl-4-piperidone followed by Fischer indolization of the obtained hydrazone with acid provided a very convenient synthetic entry to the oxocarbazole and oxoazacarbazole fused benzoxazepine. The later formed a very facile substrate in providing the subsequent annulation of this nucleus with pyrazole and isoxazole through the reaction of the corresponding enol ethers, enones, oxoketene dithioacetals and dimethylaminemethylene ketones with hydrazine hydrate and hydroxylamine hydrochloride respectively. Eight selected compounds from this series were evaluated for antimicrobial activity showing moderate to excellent antibacterial and antifungal activity.
Experimental
All reagents were purchased from HIMEDIA, Aldrich, Fluka and used directly without further purification. The melting points of all compounds were recorded in open glass capillaries on a VMP-D melting point apparatus and uncorrected. 1H-NMR spectra were recorded on Bruker-F-300 (300 MHz) spectrometer using CDCl3 as solvent and trimethylsilane (TMS) as internal reference and chemical shifts were expressed in δ (ppm). Mass spectra were recorded on model Clarus-600 Perkin Elmer; IR spectra were recorded on FTIR-9050S. CE (SCHIMADZU). Elemental analyses were carried out using a PERKIN ELMER CHNS/O analyzer series-II Model 2400. Reactions were monitored through TLC using benzene and methanol as eluent 2,3-dihydo-1H-benzo-[2,3] [1,4] oxazipino [6,7-b] carbazol-4 (5H) -one (5).
Preparation of hydrazone
A solution of 2-amino- dibenzo [b,f] [1,4] oxazepine 1 (525 mg. 2.5 mmol) in aqueous HCl (5ml conc. HCl and 2ml water) was treated with a cold saturated solution of sodium nitrite (650 mg in 5ml of water) while the temperature was kept 0-5°C, It was then added drop wise to ice cold mixture containing 3-hydroxymethylidene cyclohexanone 3 (945 mg 7.5 mmol), sodium acetate trihydrate (810 mg, mmol), methanol (40ml), water (20ml) over a period of 5 hr with stirring. This content was allowed to stand for further 5h and the resulting solid mass was filtered, dried and recrystallized from ethanol to give the hydrazone, which was used in next step without further purification.
Cyclization of hydrazone
A solution of hydrazone (792 mg, 2.5 mmol) in Kent acid (a mixture of acetic acid (20ml) and hydrochloric acid (5ml.) was refluxed on an oil bath at 120-130 oC for 30 min. the content was cooled and poured to cold water with stirring. A yellow solid was obtained which was purified by column chromatography to give 2,3-dihydo-1H-benzo-[2,3] [1,4] oxazipino [6,7-b] carbazol-4 (5H) -one 5, Yield 532 mg (67%), cream-white, crystalline powder, mp 310-311oC. IR spectrum, ν, cm-1: 3110 (C-H str. ArH), 1710 (C=O), 1600 (C=N), 1480 (C=C), 1150 (C-N), 1110 (C-O-C). 1H NMR spectrum (400 MHz, CDCl3), δ, ppm (J, Hz): 11.91 (1H, s, NH); 8.39 (1H, s, N=CH); 7.78 (1H, s, H Ar); 7.47 (1H, s, H Ar); 7.36 (4H, m, H Ar); 3.0 (2H, t, J = 6.1, CH2); 2.58 (2H, t, J = 6.1 CH2); 2.11 (2H, m, CH2). Mass spectrum (ES+, 70 eV), m/z (Irel, %): 302.10 [M]+ (65). Found, %: C 75.12; H 4.63; N 9.21. C19H14N2O2. Calculated, %: C 75.48; H 4.67; N 9.27/9.21. Similarly compound oxazipino derivative 6 was prepared. Yield 64%, cream-white, crystalline powder, mp 322-324 oC. IR spectrum, ν, cm-1: 3120 (C-H); 1720 (C=O); 1600 ((C=N); 1440 (C=C); 1150 (C-N); 1110 (C-O-C). 1H NMR spectrum (400 MHz, CDCl3), δ, ppm (J, Hz): 11.91 (1H, s, NH); 8.39 (1H, s, N=CH); 8.19 (1H, s, H Ar); 7.55 (1H, s, H Ar); 7.28 (8H, m, H Ar); 4.51 (2H, s, CH2); 3.39 (2H, t, J = 6.1, CH2); 2.63 (2H, t, J = 6.1, CH2); Mass spectrum (ES+, 70 eV), m/z (Irel, %): 393.15 [M]+ (47). Found, %: C 75.94; H 4.90; N 10.60. C25H19N3O2. Calculated, %: C 76.32; H 4.87; N 10.68.
To a solution of sodium ethoxide (1300 mg) in dry benzene (10ml) at 0 oC, a solution of ethyl formate (520 mg 1.75mmol) in dry benzene (3 ml) was added. To this mixture 2,3-dihydo- 1H-benzo-[2,3][1,4]oxazepino[6,7-b]carbazol-4(5H)-one 5 (302 mg, 1 mmol) in benzene (10ml) was then added and the mixture was stirred for 4h at room temperature and allowed to stand overnight. It was then diluted with cold water, acidified with dil. HCl and extracted with ether. The solid mass obtained was recrystallized from ethanol to give 7, Yield 210 mg (70 %), light-yellow, crystalline powder, mp 318-320 oC. IR spectrum, ν, cm-1: 3180 (C-H str. ArH); 1700 (C=O); 1660 (C=C); 1550 (C=N); 1590 (C=C); 1200(C-O-C). 1H NMR spectrum (400 MHz, CDCl3), δ, ppm (J, Hz): 11.91 (1H, s, NH); 8.39 (1H, s, N=CH); 7.78 (1H, s, HAr); 7.47 (1H, s, HAr); 7.36 (4H, m, HAr);; 6.99 (1H, s, CH); 4.49 (2H, q, J = 6.9, CH2); 3.1 (2H, t, J = 6.1, CH2); 2.82 (2H, t, J = 6.1, CH2); 1.21 (3H, t, J = 7.5, CH3). Mass spectrum (ES+, 70 eV), m/z (Irel, %): 359.14 [M]+ (48). Found, %: C 73.37, H 5.02, N 7.76. C22H18N2O3. Calculated, %: C 53.73/; H 5.06; N 7.82.
Similarly 8 was prepared from 6. Yield 260 mg (66%), paleyellow, crystalline powder mp 314-316 oC. IR spectrum, ν, cm-1: 3150 (C-H); 1730 (C=O), 1630 (C=C); 1610 (C=N); 1480 (C=C str.); 1220 (C-O-C). 1H NMR spectrum (400 MHz, CDCl3), δ, ppm (J, Hz): 11.91 (1H, s, NH); 8.39 (1H, s, N=CH); 8.19 (1H, s, H Ar); 7.84 (1H, s, H Ar); 7.28 (8H, m, H Ar); 7.55 (1H, s, H Ar); 7.11 (1H, s, CH); 4.71 (2H, s, CH2); 4.49 (2H, q, J = 6.9, CH2 ); 3.73 (2H, s, CH2); 1.21 (3H, t, CH3). Mass spectrum (ES+, 70 eV), m/z (Irel, %): 449 [M]+ (36). Found, %: C 74.80; H 5.11; N 9.01. C28H23N3O3. Calculated, %: C 74.82; H 5.16; N 9.35..
3 - b e n z y l i d e n e - 2 , 3 - d i h y d r o - 1 H - b e n z o [ 2 , 3 ] [1,4[oxazepino[6,7-b]carbazol-4(5H)-one (9). A mixture of 2,3-dihydro-1H-benzo-[2,3][1,4]oxazepino[6,7-b]carbazol- 4(5H)-one 5 (453 mg. 1.5 mmol), benzaldehyde (265 mg. 2.5 mmol) and fused sodium acetate (300 mg. 3.7 mmol) in glacial acetic acid was refluxed for 5h. The reaction mixture was cooled in ice water. The resulting solid was filtered and washed with water, dried and recrystallized from ethanol to give pure oxazepino derivative 9, Yield 300 mg (yield 66%), brawn, crystalline powder, mp 326-328 oC. IR spectrum, ν, cm-1: 3200 (C-H str. ArH); 1680 (C=O); 1600 (C=N); 1400 (C=C), 1350 (CN); 1000 (C-O-C). 1H NMR spectrum (400 MHz, CDCl3), δ, ppm (J, Hz): 11.91 (1H, s, NH); 8.39 (1H, s, N=CH); 7.78 (1H, s, H Ar); 7.50 (5H, m, H Ar); 7.47 (1H, s, H Ar); 7.36 (4H, m, H Ar); 7.15 (1H, s, CH); 3.1 (2H, t, J = 5.9, CH2); 2.82 (2H, t, J = 6.1, CH2). Mass spectrum (ES+, 70 eV), m/z (Irel, %): 390.8 [M]+ (63). Found, %: C 79.64; H 4.61; N 7.12. C26H18N2O2. Calculated, %: C 79.98; H 4.65; N 7.18.
Similarly 24 was prepared from 12. Yield 837 mg (68%), light-brawn, crystalline powder, mp 260-263 oC. IR spectrum, ν, cm-1: 1630 (C=C); 1600 (C=N); 1220 (C-O-C); 1100 (C-H), 900 (C-O-N); 700 (C-S). 1H NMR spectrum (400 MHz, CDCl3), δ, ppm (J, Hz): 11.8 (1H, s, NH); 8.3 (1H, s, CH); 8.1 (1H, s, H Ar); 7.6 (1H, s, H Ar); 7.3 (4H, m, H Ar); 7.2 (5H, m, H Ar); 4.7 (2H, s, CH2); 4.3 (2H, s, CH2); 2.5 (3H, s, CH3). Mass spectrum (ES+, 70 eV), m/z (Irel, %): 464 [M]+ (71). Found, %: C 69.38; H 4.31, N 12.82; S 6.56. C27H20N4O2S. Calculated, %: C 69 80; H 4.34; N 12.06; S 6.90.
3-(methylthio)-1,4,5,15-tetrahydrobenzo[2,3][1,4] oxazepino[7,6-b]pyrazole[3,4-a]carbazole (25). Hydrazine hydrate (1-.5 ml) and bis(methylthio)methylene compound 11 (405 mg. 1mmol) was taken in 10 ml ethanol and refluxed for 3h. The resulting residue was poured in water and extracted with ethyl acetate, washed with saturated solution of sodium bicarbonate and dried over anhydrous sodium sulfate. Removal of the solvent gave pyrazole derivative 25, Yield 259 mg (64%), pale-yellow, crystalline powder, mp 288-289 oC. IR spectrum, ν, cm-1: 3300 (NH str. pyrazole); 3000 (C-H str. ArH); 1590 (C=N); 1570 (C-H str. ArH); 1200 (C-O-C); 1150 (C-N); 700 (C-S). 1H NMR spectrum (400 MHz, CDCl3), δ, ppm (J, Hz): 12.3 (1H, s, NH); 11.8 (1H, s, NH); 8.3 (1H, s, CH); 7.9 (1H, s, H Ar); 7.4 (1H, s, H Ar); 7.3 (4H, m, H Ar); 2.8 (4H, m, CH2); 2.5 (3H, s, CH3). Mass spectrum (ES+, 70 eV), m/z (Irel, %): 372.44 [M]+ (62). Found, %: C 67.24; H 4.28; N 14.73; S 8.21. C26H16N4OS. Calculated, %: C 67.7; H 4.33; N 15.05; S 8.61.
Similarly 26 was prepared from bis(methylthio)methylene compound 12. Yield 330 (66 %), pale-yellow, crystalline powder, mp 274-275 oC. IR spectrum, ν, cm-1: 3320 (NH str. pyrazole); 3050 (C-H), 1590 (C=N); 1570 (C=C); 1210 (C-O-C); 1150 (C-N str.); 720 (C-S). 1H NMR spectrum (400 MHz, CDCl3), δ, ppm (J, Hz): 12.3 (1H, s, NH); 11.8 (1H, s, NH); 8.3 (1H, s, CH); 8.1 (1H, s, H Ar); 7.6 (1H, s, H Ar); 7.3 (4H, m, H Ar); 7.2 (5H, m, H Ar); 4.7 (2H, s, CH2); 4.3 (2H, s, CH2). Mass spectrum (ES+, 70 eV), m/z (Irel, %): 463.15 [M]+ (86). Found, %: C 69.58; H 4.52; N 14.82, S 6.54. C32H23N5OS. Calculated, %: C 69.95; H 4.56; N 15.11; S 6.92.
The authors are grateful to the Head, Sophisticated Analytical Instrument Facility (SAIF), CDRI Lucknow India for providing spectral data of the compounds. We are are thankful to the Department of Science and Technology, New Delhi (India), for granting project to “Banasthali Centre of Education for Research in Basic Sciences” under their CURIE (Consolidation of University Research for Innovation and Excellence in Women Universities) program.
References
- Brone B, Peeters PJ, Marrannes R, Mercken M, Nuydens R, et al. (2008) Tear gasses CN, CR, and CS are potent activators of the human TRPA1 receptor. Toxicol Appl Pharmacol 231(2): 150-156.
- Gijsen HJ, Berthelot D, Zaja M, Brone B, Geuens I, et al. (2010) Analogues of morphanthridine and the tear gas dibenz[b,f][1,4] oxazepine (CR) as extremely potent activators of the human transient receptor potential ankyrin 1 (TRPA1) channel. J Med Chem 53(19): 7011-7020.
- Danneberg P, Weber KH (1983) Chemical structure and biological activity of the diazepines. Br J Clin Pharmacol 16 Suppl 2 231S-244S.
- Smith AJ, Tett SE (2010) BMC Health Services Research 30(10): 321. Wieczorek J, Peczynska-Czoch W, Mordarski M, Kaczmarek L, Becalski A, et al. (1986) Antineoplastic activity of azacarbazoles. III. Synthesis and antitumor evaluation of selected 2-, 3- aza- and diazaanalogues of carbazole. Arch Immunol Ther Exp (Warsz) 34(3): 323-326.
- Wieczorek J, Peczynska-Czoch W, Mordarski M, Kaczmarek L, Becalski A, et al. (1986) Antineoplastic activity of azacarbazoles. III. Synthesis and antitumor evaluation of selected 2-, 3- aza- and diazaanalogues of carbazole. Arch Immunol Ther Exp (Warsz) 34(3): 323-326. Ulrich J, Sylvain R, Arnaud T, Jerome K, William L, et al. (2004) Org Biomol Chem 2: 1476.
- Jasztold Howorko R, Tylińska B, Biaduń B, Gebarowski T, Gasiorowski K (2013) New pyridocarbazole derivatives. Synthesis and their in vitro anticancer activity. Acta Poloniae Pharmaceutica 70(5): 823-832.
- Lescot E, Muzard G, Markovits J, Belleney J, Roques BP, Le Pecq JB (1986) Synthesis of 11H-pyridocarbazoles and derivatives. Comparison of their DNA binding and antitumor activity with those of 6H- and 7H-pyridocarbazoles. Med Chem 29(9): 1731-1737.
- Nathwani SM, Butler S, Fayne D, McGovern NN, Sarkadi B, et al. Novel microtubule-targeting agents, pyrrolo-1,5-benzoxazepines, induce apoptosis in multi-drug-resistant cancer cells. Cancer Chemother Pharmacol 66(3): 585-596.
- Greene LM, Fleeton M, Mulligan J, Gowda C, Sheahan BJ, et al. (2005) The pyrrolo-1,5-benzoxazepine, PBOX-6, inhibits the growth of breast cancer cells in vitro independent of estrogen receptor status and inhibits breast tumour growth in vivo. Oncol Rep 14(5): 1357-1363.
- Banerji B, Pramanik SK, Sanphui P, Nikhar S, Biswas SC (2013) Synthesis and cytotoxicity studies of novel triazolo-benzoxazepine as new anticancer agents. Chem Biol Drug Des 82(4): 401-409.
- Li R, Farmer PS, Wang J, Boyd RJ, Cameron TS, et al. (1995) Molecular geometries of dibenzothiazepinone and dibenzoxazepinone calcium antagonists. Drug Des Discov 12(4): 337-358.
- Ndubaku CO, Heffron TP, Staben ST, Baumgardner M, Blaquiere N, et al. (2013) Discovery of 2-{3-[2-(1-isopropyl-3-methyl-1H-1,2-4- triazol-5-yl)-5,6-dihydrobenzo[f]imidazo[1,2-d][1,4]oxazepin-9-yl]- 1H-pyrazol-1-yl}-2-methylpropanamide (GDC-0032): a β-sparing phosphoinositide 3-kinase inhibitor with high unbound exposure and robust in vivo antitumor activity. J Med Chem 56(11): 4597-4610.
- Schmidt AW, Reddy KR, Knölker HJ (2012) Occurrence, biogenesis, and synthesis of biologically active carbazole alkaloids. Chem Rev 112(6): 3193-3328.
- Ghazala y, Abdul H, Erum A, Muhammad U, Almas H, et al. (2013) Synthesis, Antibacterial, and Antifungal Activities of Novel Pyridazino Carbazoles. Journal of Chemistry.
- Mowbray CE, Burt C, Corbau R, Gayton S, Hawes M, et al. (2009) Pyrazole NNRTIs 4: selection of UK-453,061 (lersivirine) as a development candidate. Med Chem Lett 19(20): 5857-5860.
- Cichero E, Fossa P (2012) Docking-based 3D-QSAR analyses of pyrazole derivatives as HIV-1 non-nucleoside reverse transcriptase inhibitors. J Mol Model 18(4): 1573-1582.
- Han Q, Chang CH, Li R, Ru Y, Jadhav PK, Lam PY (1998) Cyclic HIV protease inhibitors: design and synthesis of orally bioavailable, pyrazole P2/P2’ cyclic ureas with improved potency. J Med Chem 41(12): 2019-2028.
- Loh B, Vozzolo L, Mok BJ, Lee CC, Fitzmaurice RJ, Caddick S, Fassati A (2010) Inhibition of HIV-1 replication by isoxazolidine and isoxazole sulfonamides. Chem Biol Drug Des 75(5): 461-474.
- Ali MA, Ismail R, Choon TS, Yoon YK, Wei AC, et al. (2011) Synthesis and anti-HIV activity of novel 3-substituted phenyl-6,7-dimethoxy- 3a,4-dihydro-3H-indeno[1,2-c]isoxazole analogues. Acta Pol Pharm 68(3): 343-348.
- Singh B, Vashishtha B, Kishore D (2010) Book Proceedings of International Conference on Pure and Applied Chemistry-2010 (ICPAC-2010). Springer.
- Jhanwar A, Tyagi R, Vashistha B, Srivastava V, Singh B, et al. (2009) Application Of Japp-Klingemann Reaction In The Synthesis Of Azacarbazole Derivatives. Intl J Chem Sci 7(4): 2222-2226.
- Tyagi R, Singh B, Kishore D (2012) Synthesis of novel precursors of Pfitzinger reaction: A facile one-pot strategy to the synthesis of quinoline carboxylic acid derivatives of pyrazolo-carbazoles and azacarbazoles. J Chem Sci 124(2): 431-435.
- Anand A, Kaur N, Kishore D (2014) An Efficient One Pot Protocol to the Annulation of Face “d” of Benzazepinone Ring with Pyrazole, Isoxazole, and Pyrimidine Nucleus through the Corresponding Oxoketene Dithioacetal Derivative. Advances in Chemistry.
- Venkatapuram P, Boggu JMR, Akula B, Katta VR, Dandu BR (2000) Molecules, 5: 1281.
- Bhawani S, Deepika M, Lalit BK, Talesara GL (2004) Indian J Chem Sect B 43B(6): 1306.
- Chauhan SMS, Junjappa H (1976) Progress in Heterocyclic Chemistry. Tetrahedron 13(32): 1911.
- Fukuii H, Inoguchi K, Nakano J (2002) Heterocycles 100(16): 257.
- Archana R, Yamuna E, Rajendra Prasad KJ, Thiruvalluvar A, Butcher RJ (2010) Acta Crystallogr Sect E Struct Rep Online 66(9): 2299-2300.
- Anand A, Kaur N, Kishore D (2014) An Efficient One Pot Protocol to the Annulation of Face “d” of Benzazepinone Ring with Pyrazole, Isoxazole, and Pyrimidine Nucleus through the Corresponding Oxoketene Dithioacetal Derivative. Advances in Chemistry.
- Tyagi R, Kaur N, Singh B, Kishore D, Heterocyclic J (2014) Novel Synthetic Protocol for the Heteroannulation of Oxocarbazole andOxoazacarbazole Derivatives through Corresponding Oxoketene Dithioacetals. Chem 51(18).
- Clarke HT, Read RR (1941) A Publication of Reliable Methods for the Preparation of Organic Compounds. Org Synth.1: 514.
- P. Kumar, N. Chandak, P. Kaushik, C. Sharma, D. Kaushik, K. R. Aneja, P. K. Sharma (2012) Synthesis and biological evaluation of some pyrazole derivatives as anti-inflammatory–antibacterial agents. Med Chem Res 21(11): 3396-3405.





