Research Article
Is US Patent Policy Strong Enough to Withstand the Winds of Change: A Study of the Need to Change United States Patent Policy
Kent R Acheson
Department of Business, University of Phoenix, USA
Submission: November 06, 2015; Published: December 03, 2015
*Corresponding author: Kent R Acheson, Department of Business, University of Phoenix, USA; Email: drkentraymon@gmail.com
How to cite this article: Kent R Acheson. Is US Patent Policy Strong Enough to Withstand the Winds of Change: A Study of the Need to Change United States Patent Policy. Organic & Medicinal Chem IJ. 2015; 1(1): 555551. DOI: 10.19080/OMCIJ.2015.01.555551
Abstract
The purpose of this case study was to learn how US patent policy requirements differ for the Software and Pharmaceutical Industries, specifically if United States Patent Policy adequately protects intellectual property rights [1] for two divergent industries while still encouraging research and development (R & D) investment sufficient to increase profits and innovation. Data for this study consisted of 38 witness testimonies delivered to US Congress between the years 2005 and 2010 by experts representing the two industries of interest: pharmaceutical and software. Key findings from the data analysis of these 38 testimonies revealed both within industry differences and between industry differences in patent law protection. Within industry differences showed variance based on size of the company and the degree to which they relied on their own R & D. Between industry differences reflected divergent ‘products’ with Pharmaceutical Industries needing long-term protection to recover R & D expenditures that include expenses for human trials research to proceed from patent application to market. Software industry, on the other hand, uses follow-on innovation of others to continue technological advancement by constantly improving upon existing software. The data show that these two industries use patent policy protection in different ways for different reasons. This information will enable Policymakers to develop another form of product protection in lieu of the current patent law to better meet the needs of these two industries rather than try to modify the existing law.
Introduction
Patent law was developed in parts, building on one another with a single purpose in mind of protecting all innovations in a society and this created the basis for patent laws imposed on the current and future generations. Bessen [2,3] stressed that patents may not be valuable in protecting innovation [4-6] but are used solely to diffuse new ideas in the public. Bessen and Maskin [7] had previously highlighted that little research and development (R&D) in the Software Industry is used to gain patent protections and the enforcement issue with patents is difficult, as many patents are issued for products that are not new. Levin [8] and others found much earlier that patents were rated weak at protecting the returns on innovation, far behind the protection gained through lead time and learning curve advantages.
Patent’s role in different industries
The purpose of this qualitative case study was to explore the different requirements for patent policy for the Software and Pharmaceutical Industries. All transcripts from testimonies from the spokespersons representing the two industries introduced to either House between the years 2005 to 2010 concerning the U.S. Patent Reform Bills were collected and analyzed to answer the research question in this case study. The findings could be useful in further adjusting patent policy to encourage innovation for diverse industries, or suggest the creation of another form of idea protection.
A similar problem may be in the type of intellectual property protection that a company chooses to obtain to avoid the constraints of getting a patent and extend the time frame for protection, such as copyright protection that extends protection from the 20 years for a patent to 120 years. Apple Inc. obtained a copyright protection for their popular iPhone [9], which recently lost in a suit against the Federal Government. The landmark decision helps to control copyright creep. Initially when buying an iPhone, Apple Inc. limited the service provider to AT&T and applications had to be bought from the Official Apple Store. Now, however, through a hack on the iPhone, users can choose a different service provider and load other, unofficial, applications not supported by Apple Inc., and hackers are not in violation of Copyright Law.
Policy Makers can use the findings of this study to explore new directions for the United States Patent Policy to optimize advancement of technology in the Software and Pharmaceutical Industries. Historically in the United States, there has been one patent policy. Scholars, academicians, and the United States Government still do not know the ideal amount of IPRs mainly because the objective has been to uphold one uniform policy. This study clarified if further changes were needed for patent policy through a Patent Reform Act, which enables Policy Makers to understand the needs of the Software Industry, or design another form of protection designed specifically for the Software Industry.
Crowe [10] and others stated that a case study design is most appropriate when little is known of a phenomenon in its natural context. A case study is “used to generate an in-depth, multifaceted understanding of a complex issue in its real-life context” (p. 1). The Pharmaceutical Industry has a profitable track record using the existing Patent Law to protect their R&D investments. The Software Industry is comparatively new and therefore their issues are only just now becoming obvious. Case law is outside the boundaries of this study.
The multiple dimensions of the phenomenon of the nature of protecting intellectual property rights in the Software Industry property and the Pharmaceutical Industry are worthy of study to allow all voices to be heard, including large corporations from both software and pharmaceutical companies, generic drug companies, and smaller software startups. After carefully examining all relevant transcripts, these diverse opinions can be given venue to state their needs.
Methodology and main results
The research question addressed in this study was: How do the patent policy requirements differ for the Software and Pharmaceutical Industries? From the Software and Pharmaceutical Industries’ objectives and needs for the United States Patent Policy to address, the questions spotlighted the sufficiency and effectiveness of the United States Patent Policy.
The focus of this study has two parts, they are:
1. What is the evidence United States Patent Policy adequately protects Intellectual Property Rights (IPRs) for both the Software and Pharmaceutical Industries?
2. How does the United States Patent System encourage companies to make R&D investment in the Software and the Pharmaceutical Industry?
The first research question dealt with the effectiveness of the United States Patent Policy in protecting IPRs in two industries: software and pharmaceutical. The second research question related to how companies invest in R&D with support of the United States Patent Policy. The study explored the ability of the United States Patent Policy to foster innovation with satisfactory IPR protection to encourage R&D spending focusing on two specific industries. The Software Industry experiences a sequential and complementary nature of innovations, building on previous discoveries; and may not use the patent policy in effect in the United States. If patent policy does not consider the different requirements within the Pharmaceutical Industry and is too lax then enough R&D spending will not be invested and technological advancements, including new medications, may come to the market slower or not at all.
The scope of the study is to understand how the Software and Pharmaceutical Industries use the patent system and how better to adjust the patent system to optimize technological advancement. As discussed in assumptions, because of the nature of the source of data and the possible bias that was not fully known, the assumptions may or may not have had a credible or dependable basis and may therefore have biased the findings. Qualitative designs such as the case study have inherent limitations that may threaten validity, they may lack rigor and they may not be generalizable. These limitations may be mitigated with transparency in data analysis and reporting. Crowe 5 and others explains on page 8 “seeking potential, alternative explanations, and being explicit about how interpretations and conclusions were reached, helped readers to judge the trustworthiness of the case study report.
Evidence from various sources highlight the United States Patent system does not work as intended and needs a solution to continue or increase innovative activity. The principal problem deals with innovative activity that is sequential in nature and innovative activity that involves much R&D investment to bring a product to market. Sequential inventions build on previous breakthroughs and do not require much R&D investment. Secrecy would hinder follow-on discoveries of sequential innovative products.
This study used a content analysis of witness [11] testimonies to Congress on the Software and Pharmaceutical Industries from the years 2005 to 2010, and the possibility to develop more than one patent policy to accommodate different sectors of the economy. The study concentrated on software and pharmaceutical companies, as these two industries are most at odds with each other, and have prevented the passage of the Patent Reform Act of 2005 through 2010. The Patent Reform Act of 2010 [12,13] is the result of non-passage of the 2009 Act, as was each successive year from 2005. The stance of the Software and Pharmaceutical Industries remained relatively unchanged in their requirements, but the patent reform acts changed to incorporate the majority opinion of industry. The most important recommendations of the Federal Trade Commission (FTC 11) and National Academies of Sciences (NAS) studies that were first introduced in 2005 by Senator [14] Lamar Smith were considered.
The purpose of this descriptive analysis was to examine the current United States Patent Policy and the proposed changes to United States Patent Policy, and answer the research question – How do the patent policy requirements differ for the Software and Pharmaceutical Industries? This study will help decide if the Software and Pharmaceutical Industries effectively use the U.S. Patent Policy through protecting Intellectual Property Rights (IPRs) and encouraged investment research and development (R&D). The qualitative case study was the most suitable approach to study the issues and answer the research questions because it explored real-life experiences of industries looking to patent Intellectual Property (IP).
Data and Sample Statistics
Data were collected and analysis began using the Content Analysis Guide developed for this study. The testimonies of the BSA representatives, other computer software witnesses, Computing Technology Industry Association, PhRMA representatives, other generic pharmaceutical representatives, and the Generic Pharmaceutical Association, Biotechnology Industry Association (BIO), Intellectual Property Owners Association (IPO) [15-18], and venture capital organizations were included in this study. The IPO was included because IPO members represent 30% of patent applications at the USPTO and include members from the Software and Pharmaceutical Industries, among others. The study included Venture capitalists because some members of BSA [19] and other smaller software companies began with venture capital dollars. Each data point was examined for inclusion of any reference to R&D, including duration and support for R&D, the need for patent protections [20,21], and future needs for patent policy.
The 38 documents submitted to the congressional hearings were analyzed. Documents relating to software and pharmaceutical companies reviewed were not ambiguous but very clear and straight forward following a consistent format, so that anyone conducting another study would reach the same conclusions. They all stated who authored the document, who the document represented, who presented opinion to Congress, their position on the patent reform act, and agreements and disagreements with specific points of the patent reform act. No ambiguity existed and no information required subjective judgments to interpret the information reported. The nature of the data supported the reliability of the findings.
Figure 1 shows the percentage of witness testimonies in favor of and those against passing the patent reform bill. The characteristics of the total sample data points follow: Twentytwo United States Government documents studied and 38 witness testimonies with 12 (31.6%) Software Industry and 26 (68.4%) Pharmaceutical Industry. The data were separated into two general categories: software companies and pharmaceutical companies. Other companies of similar characteristics aligned with one of these two categories and were included in the patent policy needs and the resulting analysis. The two categories were identified by the principle industry of each category, which also followed the characteristics of companies similar to and aligned with either the Software or Pharmaceutical Industries. For example, biotech companies were included if they also had a pharmaceutical component.
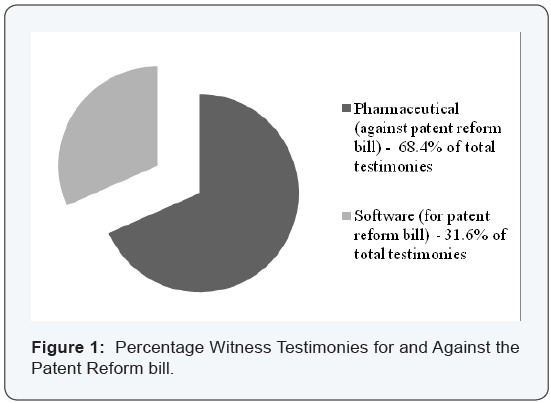
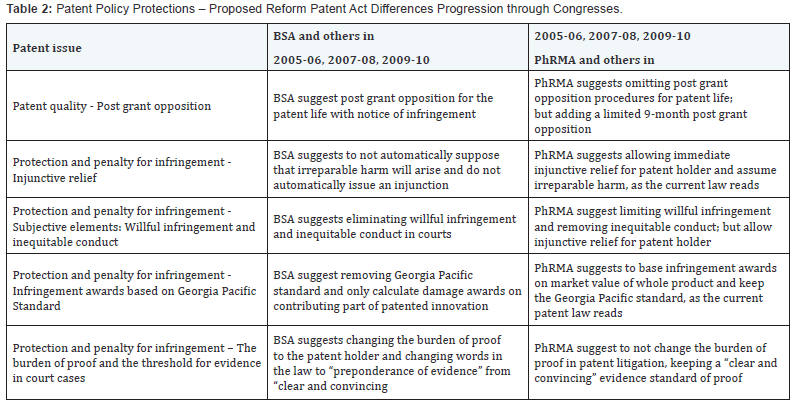
The total percentages of those in favor and against passage of the Patent Reform Act gradually shifted from the majority opposing passage of the Patent Reform Act in 2005 to the majority in favor of passage of the Patent Reform Act in 2010. The major opponents agreed to some form of compromise in the Patent Code. Even though changes moderated some, the Act still did not pass Congress [22-35] in 2010. Congressional modifications of the patent reform act resulted in the shift in the users’ support of the patent policy rather than a change in industries’ requirements. Table 1 illustrates the total number of documents analyzed for each year for each industry.
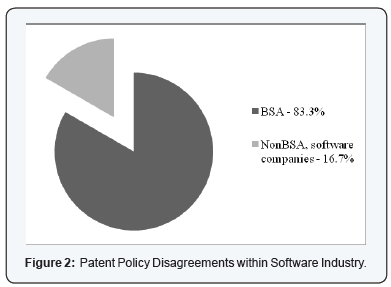
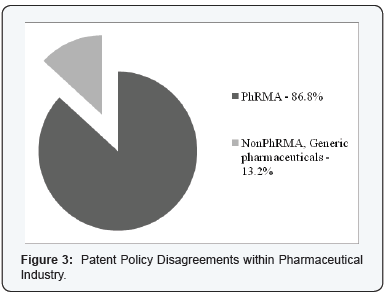
BSA and PhRMA did not represent the entire Software and Pharmaceutical Industries, as differences within each industry were obvious in the expert’s witness testimonies. Figure 2 & 3 is a visual reference to the opposition in each association, that is, the brand-name pharmaceutical companies versus the generic pharmaceutical companies and BSA member companies versus non-BSA member companies. The BSA represented only about 83% of computer software companies’ expert witness testimonies for this study. The PhRMA [36-38] represented only about 86% of pharmaceutical companies’ expert testimony in this study.
Cisco, Hewlett-Packard, and other big high-tech companies began pushing for reform legislation to limit the number of patent infringement lawsuits and therefore the amounts paid in damages. The United States Patent and Trademark Office’s (USPTO) proceedings’ transcript from the public hearings showed the patent policy needs for BSA’s principle member and founder Microsoft. The public hearing titled Use of the Patent System to Protect Software Related Inventions took place in 1994 at the San Jose Convention Center, California, and at the Crystal Forum in Arlington, Virginia. A brief summary of Microsoft’s speech follows. Microsoft (BSA) recommended that patent protection allow an accused infringer to identify readily the activity forbidden under the claim. The success of a particular claim in meeting these objectives may depend less on the form and more on claim substance and the supporting details.
BSA represents a large base of computer software and hardware companies in the United States. Phelps (2005) from Microsoft Corporation stated that BSA does not want the patent holder to have automatically injunctive relief. Injunctive relief occurs when the courts rule an infringement occurred and automatically issue a ruling to stop the infringer from continuing operations. From the congressional hearing in 2005 on harmonization and other matters, Phelps for BSA supports publication in 18 months. Phelps [39] expressed support for establishing a post grant opposition procedure and supported third-party opportunity to alert USPTO to questionable patents during review. Phelps also supported allowing third parties the opportunity to suggest relevant prior art to examiner during review, supported a limit on damages for willful infringement to include only egregious behavior, and supported limiting damages to only the contributing, patented piece of the invention and not the market value of the whole product, as it is now.
In a congressional hearing in 2005, Simon [40] from Intel , a BSA representative, stated the patent system is difficult to maneuver because of many pieces that comprise computers and software contain “potentially hundreds of patents [that] may be relevant to a particular computer or software technology” [40]. The primary way to challenge a patent under current law is through costly litigation. Intel suggests Congress create a balanced post grant opposition enabling third parties to challenge issued patents that includes a post grant opposition of 2 years from patent grant or 1 year from receiving notice of patent infringement. Simon also encouraged Congress to create a second window to make the post grant review meaningful. Simon suggested a limit on patent application continuations and for the court not to issue a continuation on any claim broader than the broadest claim previously published or issued. BSA suggested a stay on the lower court’s decision in interlocutory appeals before final determination by the Federal Circuit Court of Appeals. Micron Technology, Inc., a non-BSA member, suggested the same patent law reforms as BSA.
In a congressional hearing in 2006, Chandler [41] of Cisco (BSA) suggested a second window triggered by receipt of an infringement complaint. During the first window, the patent issues with thousands or millions of parts making the effectiveness of the patent examination questionable. Chandler (2006) encouraged Congress to make changes to remove venue shopping, and prevent suits from worldwide damages in United States Courts like the Microsoft and AT&T case. The only patent policy need described on the BSA website dated 1994 had no updates, which is understandable because United States Patent Policy has not changed significantly for more than 50 years and the proposed changes have not made it into law. The agreement with the Patent Reform Act was from the most influential voice for the Software Industry; nevertheless, there were disagreements within the Software Industry mainly arising from smaller companies and individual inventors. Software companies wanted patent reform by Congress but differences remained among large software companies and smaller organizations. An overhaul of the patent system and other measures to promote tech development efforts are top priorities of the Business Software Alliance, Cisco, Hewlett-Packard, and other big high-tech companies . BSA members began pushing for reform legislation to limit the number of patent infringement lawsuits, and therefore, the amounts paid in damages.
In an article in PC World dated March 9, 2008, patent reform leads a list of five legislative priorities expressed by BSA in 2008. The opinion article stated that BSA members want Congress to approve the Patent Reform Act but the legislation stalled in the United States Senate because of objections from inventors, pharmaceutical companies and some small tech (computer software) firms. In addition the article proclaimed, more than 170 California businesses and organizations oppose the Patent Reform Act in its current form. They mention that research to stay competitive is both expensive and risky, but strong protections from patent policy attract the necessary investments to commercialize a new product. This is especially the case for the hundreds of smaller, venture capital-backed firms in the state, of which many spun from California’s world-class research universities and private research institutes. According to GlaxoSmithKline, California Wireless and Mi5 Networks in paragraph eight on page one of Gross [42] (2008), the Patent Reform Act “would increase costs to obtain and maintain patents, undermine patent certainty, incentivize infringement, and weaken the enforceability of patent rights and intellectual property protections.”
Dr. Myhrovold [43-45] started Dynamical Systems, a software company, in 1984 that Microsoft bought in 1986. He worked with Microsoft from 1986 to 2000 (14 years). Myhrovold retired from Microsoft in 2000 to start another company, Intellectual Ventures, which files more than 300 patents a year making it the 25th largest inventing organization in America. Dr. Myhrovold stated “[Software is] a complex topic…and it’s all about company culture and how companies use patents” (Perspectives on Patents [46,47]. Continuing Dr. Myhrovold stated “…for most tech companies patents have never been important; they have never been a way to make money” (p. 76, para. 2), and “…patents are, at best, a distraction and most tech companies have made a deliberate decision to ignore the patent system” (p. 76, para. 5). Many other non-BSA members agreed with Myhrovold.
Defensive patenting by software companies explains if a company holds enough patents then this company can steal another product company’s ideas with impunity, but the problem enters when the other entity does not create a product to attack (Myhrovold, 2006, p. 77, para 3). These are the battle lines in the patent reform debate with universities, small inventors and pharmaceutical companies whose lifeblood is the patent system on one side, and companies who decide to infringe or at least do not care about infringing on the other side. Dr. Myhrovold is a witness from the vantage point of a Microsoft senior executive in the 1990s who discussed this role with other firms in the earlier rounds of patent reform debate.
Technology companies exaggerate the problem when over the last 20 years patents have remained in last place of lawsuits for the three forms of idea protection: trademark, copyright, and patents. A study of four high-tech companies that are active in the patent reform debate paid out $3.7 billion in patent lawsuit settlement from 1993 to 2005, but those same four companies earned $1.4 trillion in revenue over the same period making the sums for infringement only 0.26% of revenues on average. The company with the highest number of lawsuits experienced sums for infringement at only 0.51% of revenues. “Patent trolls” are companies that do not market a product but only the idea for a product. Companies that do not produce a product comprise only 2% of the patent infringement lawsuits. Software companies like to blame an innocuous group of patent troll companies when they themselves perform the same litigious practices blamed on trolls. Myhrovold stated the need to embrace the trend to make the alternate resolutions more like a court trial by creating a separate Patent Court, much like the Tax Court, Bankruptcy Court, or Divorce Court to try only specific cases.
Inter Digital is a technology and software company that disagrees with BSA’s proposed changes to patent law. Inter Digital’s Bernstein summarized the differences in the Software industry on page 220 last paragraph at the 2007 congressional hearings: “…the IT industry is absolutely not united in its support for mandatory apportionment, post grant opposition, expansive USPTO rulemaking authority, and interlocutory appeals fall outside the realm of patent ‘reform’.” Bernstein continues by expressing how such an action would degrade patent rights and increase litigation for smaller innovators. The weakening of legitimate patents would protect a few corporate giants and increase the number of lawsuits Bernstein (2007), [48,49].
An article by Mc Dougall [50] and Chabrow (2006), [51,52] in InformationWeek explains the problems as they perceive them with the Patent Reform Act from other software and computer companies. Hans Hxu, founder of online gift registry Felicite.com, says the industry’s large players want the appearance of IP openness but do not practice it. “IBM patents almost everything they do, and then they sit on it, which does not encourage innovation” (Microsoft Agenda, para. 3) says Hxu, a McKinsey consultant although other critics suggest the sellers’ moves cement their advantages when they face rising [53] competition from startups. In an August 2005 essay, Harvard Law School professor and tech entrepreneur James Moore argued the collaborative patent review proposed by IBM, Microsoft, and others would result in fewer patents issued because it would give examiners more ammunition to shoot down patent applications. “If fewer patents are issued, but existing patents are not revoked, IBM and Microsoft win because they already possess vast existing portfolios,” Moore writes (Microsoft Agenda, para. 4). Some Web 2.0 companies dismiss IBM’s argument that business-method patents protect obvious ideas. “Everything is obvious after someone has done it,” says a spokesperson for online movie renter Netflix (Microsoft Agenda, para. 5), which has patents on its queue-ordering system--and is suing Blockbuster for allegedly copying the system.
Small tech companies are taking matters into their own hands, forming patent cooperatives through which they share IPRs. Search company Wink shares in Creative Commons, a group that encourages sharing of copyrights and open source licenses, but there is a line between sharing and protecting intellectual property that creates competitive advantage, says Wink’s Chief Executive Officer (CEO) Michael [54,55] Tanne. “When companies have invested in the development of technologies, they really ought to be able to protect it,” Tanne says (Microsoft Agenda, para. 6). Resolving these issues will influence developing and commercializing tech innovations. Too many lengthy and expensive legal battles will persuade IT departments to stick with familiar technology, and this is something tech vendors should consider as they take one another to court.
The largest and best known pharmaceutical companies in the Pharmaceutical Industry represented by Pharmaceuticals Researchers and Manufacturers of America (PhRMA), Biotechnology Industry Organization (BIO), and the Professional Inventors Alliance disagree with the weakening of patent protection and the long, time frame proposed for patent reexamination. High R&D characterizes these industries and the Pharmaceutical Industry realizes a shortened patent protection because patent protection begins before FDA approval. This shortens patent protection to commercialize the product to the remaining years.
On September 17, 2007, The Professional Inventors Alliance expressed through a letter to President Bush the flaws in the Patent Reform Act of 2007. The Patent Reform Act of 2007 did not pass the United States Senate because of the opposition from PhRMA, small inventors, and small tech firms . The letter from the Professional Inventors Alliance expressed that if the Patent Reform Act of 2007 passed into law it would harm the United States’ innovative character because of the inability to enforce patents and would reduce the royalties associated with a patented technology. In 1980, PHRMA’s members invested $2 billion in R&D for new medicines; although, nearly 30 years later (in 2009), PHRMA’s members invested $50.3 billion in R&D out of the $65.2 billion industry-wide total. Pharmaceutical companies rely on government-granted patents to protect their substantial investments in researching and developing new drugs. It takes 10-15 years and costs $800 million on average to bring a new medicine to market. The Pharmaceutical Research and Manufacturers of America (PhRMA) represents the country’s leading pharmaceutical research and biotechnology companies.
Without patents to protect all the inventions necessary to develop a drug for a limited time, others could simply copy the drugs immediately, offering their versions at a reduced price because they did not incur the high costs to develop the drug. This would seriously affect the pharmaceutical companies’ ability to recoup their costs and reinvest in other research projects. PhRMA stated in 2010 that “a strong patent system is crucial to our economic [56,57] competitiveness, especially in these economically trying times” (PhRMA’s website, 2001, p. 1). The companies in favor and against the Patent Reform Act of 2010 divided into the companies that have favored and opposed the previous patent reform acts, that is, computer software favoring patent reform and pharmaceutical companies and biotechnology companies opposing patent reform. Those opposing and in favor of the patent reform acts through the six years in this study have not changed their needs but, instead, Congress changed trying to create a patent policy agreeable to most patent users.
The large pharmaceutical companies also known as the name brand pharmaceutical companies and the smaller, generic pharmaceutical companies were in general agreement on most issues. Both wanted strong patent protection and both sides were against the Patent Reform Bill [58] of 2005 and 2006 as stated in the congressional hearings on patent reform. The firstinventor- to-file patent system while harmonizing with the large United States trading partners also poses some difficulties and disagreements with United States patentees. The problems lay in the grace period of 1-year and the best mode requirement in the patent application. Harmonizing with other countries’ patent systems as currently written, such as Japan and Europe, would remove the United States grace period of 1 year to file a patent application and would remove the best mode requirement when filing a patent application. The best mode requirement is the descriptive part of the patent application the inventor has to include the inventor’s idea of how best to use or combine the chemicals for complete effectiveness.
The differences between the brand name and generic pharmaceutical companies lay in eliminating the best mode factor of the patent application and the inequitable conduct defense. Brand name pharmaceutical companies say the best mode provision of the patent law is subjective, and therefore should be removed. The generic pharmaceutical companies believe the best mode provision should remain because they cannot copy the patented medication without the recipe or the “best mode” of making the drug. By removing the inequitable conduct defense, brand name pharmaceutical companies will misuse the patent system to the harm of the public and generic pharmaceutical companies. Differences exist between the brand name pharmaceuticals and the generic pharmaceuticals. One example is the issue of patent quality: Best mode. Generic pharmaceuticals want to keep the “best mode” in the patent law language because it lowers cost of medications by allowing generic companies to copy name brand drugs more easily. Ely Lilly [59,60] and PhRMA want to remove the best mode language . The Generic Pharmaceutical Association also has qualms with weakening the inequitable conduct saying that weakening this provision gives brand-name pharmaceutical companies incentive to misrepresent their inventions.
The differences between the brand name and generic pharmaceutical companies lay in eliminating the best mode factor of the patent application and the inequitable conduct defense. Brand name pharmaceutical companies say the best mode provision of the patent law is subjective, and therefore should be removed. The generic pharmaceutical companies believe the best mode provision should remain because they cannot copy the patented medication without the recipe or the “best mode” of making the drug. By removing the inequitable conduct defense, brand name pharmaceutical companies will misuse the patent system to the harm of the public and generic pharmaceutical companies. Differences exist between the brand name pharmaceuticals and the generic pharmaceuticals. One example is the issue of patent quality: Best mode. Generic pharmaceuticals want to keep the “best mode” in the patent law language because it lowers cost of medications by allowing generic companies to copy name brand drugs more easily. Ely Lilly [59,60] and PhRMA want to remove the best mode language . The Generic Pharmaceutical Association also has qualms with weakening the inequitable conduct saying that weakening this provision gives brand-name pharmaceutical companies incentive to misrepresent their inventions.
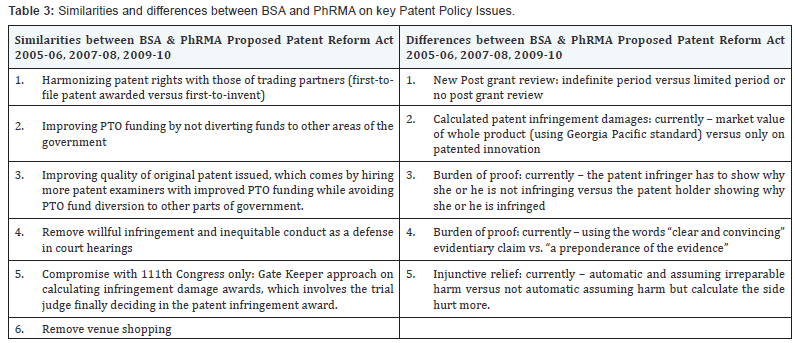
Table 2 compares the important differences between the Software and Pharmaceutical Industries, and shows the large industry organizations’, BSA and PhRMA, position on individual aspects of patent reform and what they want in a patent policy. Table 3 shows the commonalities and disagreements on specific items within the individual industries. This table shows on which issues agreements and disagreements exist categorized by specific points of the patent reform legislation. The principle differences lie in patent infringement culpability, determining the responsible party, and setting time limits to challenge the validity of patents issued. Major developments occurred after Congress began deliberation on patent reform in 2005 that changed the patent reform debate. Examples of the change include injunctions, defining the obviousness standard, treble damages and willful infringement test, and patent venue rules. The Supreme Court and Federal Circuit have reshaped the patent landscape for software patentability that has both strengthened the bargaining position of patent users and created more doubt for patent holders. Cassidy (2009) [61,62] stated the marketplace must have the opportunity to adjust and apply these decisions, and mused legislation that would decrease the value of patents would harm the United States economy. Pharmaceutical and Biotechnology need a patent policy that preserves the foundational strength of patent rights and improves the fairness and efficiency of pre-grant and post grant examination of patents.
Together the Case Lawre presented the most comprehensive line of court-led patent reforms, which makes patent reform substantially different in 2010 than 2005. Patent lawyers and the law association, AIPLA [63,64], believe that legislation is not necessary and the court system will eventually find a solution for compromise for the different users of the patent system and will define patent law through successive Case Law. Larger, more market capitalized firms make more noise and are heard more clearly than smaller, less capitalized companies or individual inventors, including companies that specialize in innovation but do not concurrently produce a product, also known as patent trolls. More innovation comes from smaller firms and individual inventors than large entities. The larger software enterprises that often infringe on patents held by companies that do not produce a product (patent trolls) behave similarly to the patent trolls. IBM and Microsoft sit on patents without an accompanying product, when another company discovers something similar the patent surprises the unsuspecting company, and a licensing or royalty agreement can avoid costly litigation. IBM earned over a billion dollars in 2005 solely from license agreements and royalties. Licensing and royalty agreements are another possible direction that companies take to avoid patent infringement suits; however, their use threatens other companies to ransom licensing or royalty agreements but is cheaper and the outcome more certain than litigation.
The Pharmaceutical Industry appreciates the current patent policy and is leery of any changes that would disrupt the current manner in which they use the patent system to optimize patent protection; also the Pharmaceutical Industry like the Software Industry makes the best of the current patent policy . Although pharmaceutical firms have to wait until after drug trials and resulting FDA approval to market the medication, which includes the 20-year patent term and drug approval sometimes lasts as much as 10 years, they too have found ways to evade current patent law to extend the patent length. The Pharmaceutical Industry commonly increases the shortened patent length by adding a known chemical to the patent protected drug therapy, and adds another patent protection term of 20 years by increasing the number of patents on a drug. One specific drug therapy created by a name-brand pharmaceutical firm that a generic company was exploring to copy had patent protection by more than 200 patents spanning 40 years.
Discussion and Conclusions
The specific research questions that framed this qualitative case study were 1. What is the evidence United States Patent Policy adequately protects Intellectual Property Rights [65] (IPRs) for both the Software and Pharmaceutical Industries? 2. How does the United States Patent Policy encourage companies to make research and development (R&D) investment in both the Software Industry and in the Pharmaceutical Industry? Based on the differences on how patent policy should read, issues of effectiveness of the United States Patent Policy to both protect and encourage IPRs and R&D investment should be considered. Patent policy in the United States has remained unchanged for the last 55 years, and has been effective in protecting IPRs and encouraging R&D investment. Pharmaceutical firms have been around many years and have flourished in the current patent policy environment. Only with the creation of the personal computer have software companies entered the scene and have expressed concern for the patent policy changes to reduce the software company’s purposeful infringement. In a few words, the large software companies want to weaken patent protections and reduce their costs to defend against patent infringement lawsuits because big software companies do not care about patents or patent infringement.
Three important findings from this study are
1. The Pharmaceutical and Software Industries use patent policy differently
2. BSA explicitly states they want a strong patent policy, but, in effect, want to weaken the current patent policy, and
3. Differences exist within each industry. Congress has attempted to improve patent law 6 years without success because there is not agreement pleasing all industries, but the principle differences embodied the Software and Pharmaceutical Industries.
Firstly, pharmacy and software use patent policy differently: Pharmacy to protect R&D and Software for defensive purposes. Software Industry (BSA) does not use the patent policy as designed to protect R&D, but to defend against the threat of patent infringement lawsuits. The testimonies to Congress provided evidence to answer my research question of how the patent policy requirements differ between the Software and Pharmaceutical Industries. The testimonies to Congress were clear and straightforward. I did not have to infer the meaning or needs of the witnesses. They clearly stated their position and what they wanted in patent policy. Many people in the Pharmaceutical Industry and smaller software companies specifically stated that larger software and computer companies began calling for patent reform to limit the many patent infringement suits against them. Myhrovold shared his experience working for Microsoft in the late 90s stating that large software companies are not concerned with infringing on another’s patents and the only reason they care at all about patents is to defend against patent infringement lawsuits.
Secondly, the data from congressional testimonies clearly showed that the Software Industry (BSA) verbalized they want a strong patent policy but, instead, they want to weaken the rights of patent holders. This weakening is from: An unlimited post patent review period, placing the burden of proof for infringement on the patent holder (instead of the offender), and limiting the damage awards for infringement to only the infringing part of an innovation. The testimonies clearly stated their position and what they wanted. The previous list clearly communicated to Congress what the Software Industry (BSA) wanted in a patent policy, and refuted by other expert testimonies in the Software Industry.
All BSA representatives stated they wanted strong patent protection, and continued with the above reasons, which amount to weakening a patent holders’ legal rights to their Intellectual Property Rights (IPRs). Many testimonies contrary to BSA stated specifically the reasons BSA wants to limit a patent holders’ IPRs is to stave off patent infringement lawsuits. Myhrovold (2006) shared that patent policy did not enter into Microsoft’s and other BSA members’ culture. Patents are not how software companies protect innovation, but, rather, secrecy, and lead time or economies of scale are more effective to protect innovation in a short product lifecycle industry. Thirdly, the entire Software Industry is not united with BSA, and the entire Pharmaceutical Industry is not united with PhRMA. Differences exist between the two industries and differences exist within each industry, such as difference between larger companies and smaller companies in Software Industry and brand name pharmaceutical versus generic pharmaceutical. Each expert clearly stated what they wanted, why they wanted it, and differences within their respective industries. The witnesses to the congressional hearings succinctly stated that the BSA or PhRMA did not represent the entire industry, and the industry was not united in its desires for patent policy. Siwik [66] said in the exact words that the Pharmaceutical Industry is not united, and based on the non-BSA members’ testimonies with them vehemently disagreeing with BSA’s stance, anyone would reach the same conclusions that BSA is far from united too.
The evidence suggests the two industries use patent policy in different ways. For instance, The Software Industry does not use the patent system to protect intellectual property but rather use the patent system for defensive purposes not so much to protect innovation but to defend against infringement lawsuits. Pharmaceutical industry relies heavily on a patent protection to recover large R&D spending. The evidence was found in examples of how each industry effectively uses the patent system. Based on research of the patent system and the evidence of how each industry uses the patent system, the data would suggest agreement with many of the pharmaceutical, biotechnology, and other industries that use the patent system effectively to protect research and development dollars that the system does not need major change. Research shows the answer to the question of how the United States Patent System encourages R&D and promotes innovation; the patent system performs well according to its design. It protects ideas. The current patent policy is effective in protecting innovation and encouraging research and development spending.
References
- Chu AC (2009) Macroeconomic Effects of Intellectual Property Rights: A Survey. Institute of Economics, Academia Sinica. Academia Economic Papers 37(3): 283-303.
- Bessen J (2005) Patents and the Diffusion of Technical Information. Economics letters 86(1): 121-128.
- Bruce AL (1994) Patent and Trademark Office. Use of the Patent System to Protect Software Related Inventions: Transcript of Proceedings from Public Hearings by the Patent and Trademark Office. Transcript of Proceedings. 1-99.
- Biberstein, Kathryn L (2007) Witness Testimony from Alkermes, Inc. (Bio). In U.S. Senate on Patent Reform: The future of American innovation. 217-245. Washington, DC.
- Berkowitz, Leonard (2005) Innovation and Its Discontents: How Our Broken Patent System Is Endangering Innovation and Progress and What to Do About It.(Book Review). Research-Technology Management. 48(2).
- Blaxill, Mark, Ralph Eckardt (2009) “Witness Testimony for the Innovation Imperative.” In US Senate on Patent Reform in the 111th Congress: Legislation and recent court decisions, 113-146. Washington, DC.
- Bessen J, Maskin E (2000) Working Paper No. 00-01, Sequential Innovation, Patents, and Imitation. Department of Economics: Massachusetts Institute of Technology. 0-33.
- Levin RC, Klevorick AK, Nelson RR, Winter SG (1987) Appropriating the Returns from Industrial R&D. Brookings Papers on Economic Activity 3. 783-831.
- Cohen, Adam (2010) The Iphone Jailbreak: A Win against Copyright Creep.
- Crowe, Sarah, Kathrin Cresswell, Ann Robertson, Guro Huby, et al. (2011) The Case Study Approach. BMC Medical Research Methodology 11. 100-00.
- Doyle, Mary E (2009) Witness Testimony from Palm, Inc. In US Senate on Patent Reform: The future of American innovation. 1-107. Washington, DC.
- Ambrogi, Robert (2010) Patent Reform Bill Implications. In BullsEye Bulletin: IMS ExpertServices™.
- Bale, Harvey E (1996) Patent Protection for Pharmaceutcials: A Plateform for Investmet, Markets and Improved Health in the Americas. In Paper presented to Workshop ID. Cartagena.
- Philip Nelson, Stuart Gurrea, Gloria Hurdle, Kevin Marshal, David Smith, et al. (2002) The Economics of Innovation: A Survey. Section of Antitrust Law American Bar Association. 0-63.
- Hatch, Orrin (2005) Testimony of Utah Senator. In U.S. Senate - Patent law reform: Injunctions and damages, 110. Washington, DC: U.S. Government Printing Office. 1-67.
- Hawley J (2005) Witness Testimony from Intellectual Property Association (Ipo). In U.S. Senate - Patent reform: Injunctions and damages. Washington, DC: U.S. Government Printing Office. 115-136.
- Subcommittee Intellectual Property of the Committee on the Judiciary. <Perspectives on Patents: Harmonization and Other Matters, First, July 26 2005.
- Wamsley, H. C. “Witness Testimony for Intellectual Property Owners Association (Ipo).” In U.S. Senate - Patent reform in the 111th Congress: Legislation and recent court decisions, 322-38. Washington, DC: US Government Printing Office, 2009.
- BSA website. “Bsa Website.” BSA, bsa.org.
- Jaffe AB, Lerner (2004). Innovation and Its Discontents: How Our Broken Patent System Is Endangering Innovation and Progress, and What to Do About It. Princeton and Oxford: Princeton University Press.
- Leahy Leahy Press Release.
- Noyes, Andrew. “Boehner Leads New Republican Push for Patent Reform.” NationalJournal.com, http://swtuopproxy.museglobal.com/ MuseSessionID= a927316723f8c7bdd11f4b5af88814c5 /MuseHost=www.nationaljournal.com/ MusePath/ congressdaily/cda_20090429_6657.php.
- Burk, Dan L, Mark A Lemley (2009) The Patent Crisis and HowCourts Can Solve It. The University of Chicago Press, Chicago, USA.
- Appleton, Steven R (2009) Witness Testimony for Micron Technology, Inc. In US Senate on Patent Reform in the 111th Congress: Legislation and recent court decisions, Washington DC, USA, p. 89-97.
- Biotechnology Industry Organization (2009) Witness Testimony for Bio.” In US Senate on Patent Reform in the 111th Congress: Legislation and recent court decisions, Washington DC, USA, p. 98- 112.
- Agricultural members of Biotechnology Industry Organization (2007) “Witness Testimony for Bio.” In US Senate on Patent Reform: The future of American innovation, Washington DC, USA, 195-197.
- Johnson, Philip S (2009) Witness Testimony for Johnson & Johnson. In US Senate on Patent Reform Act, Washington DC, USA, p. 47-69.
- Cannon W Stephen (2009) Witness Testimony for America’s Specialty Medicines Company.” In US Senate on Patent Reform in the 111th Congress: Legislation and recent court decisions. Washington DC, USA, p. 87-88.
- Witness Testimony for Johnson & Johnson (2009) In US Senate on Patent Reform in the 111th Congress: Legislation and recent court decisions, Washington DC, USA, 154-155.
- Witness Testimony for Johnson & Johnson (Phrma). In US Senate on post-grant review, Washington DC, USA, p. 57-67.
- Witness Testimony from Johnson & Johnson (2005) In US House of Representatives on An Amendment in the Nature of a Substitute to H.R. 2795. Washington DC, USA.
- Witness Testimony for Intel Inc. (2009) In US Senate on Patent Reform Act, 32-44. Washington, DC.
- Copyright Basics (2010) edited by US Copyright Office - Library of Congress. US Government Printing Office, Washington, USA.
- Jaeger Kathleen D (2009) Witness Testimony for Generic Pharmaceutical Association. In US Senate on Patent Reform in the 111th Congress: Legislation and recent court decisions. Washington, DC, USA, pp. 151-153.
- Kelly David, Rob Schulman (2008) Federal Circuit Hits Pharmaceutical Patentees Hard. The National Law Journal.
- PhRMA’s website. “Phrma Website.” PhRMA.
- Beier David (2005) Witness Testimony for Amgen (Phrma). In US Senate Harmonization and other matters, edited by Amgen, Washington, DC: Amgen, USA, pp. 24-33.
- Kappos David J (2009) Witness Testimony for International Business Machines Corporation. In US Senate on Patent Reform in the 111th Congress: Legislation and recent court decisions. Washington, DC, USA, pp. 156-203.
- Phelps Marshall C (2005) Witness Testimony Microsoft Corporation (Bsa). In US Senate on harmonization and other matters, edited by Microsoft Corporation, Washington, DC, USA, pp. 80-85.
- Simon David (2005) Witness Testimony for Intel Corporation. In US Senate on persepectives of patents, Washington, DC, USA, pp. 167- 179.
- Chandler Mark (2006) Witness Testimony for Cisco Systems. In US Senate on post-grant review. Washington, DC, USA, pp. 40-52.
- Gross, Grant. “Software Companies Want Patent Reform by Congress.” PC World Communications.
- Myhrvold NP (2006) “Witness Testimony for Intellectual Ventures.” In US Senate on post-grant review. Washington, USA, pp. 68-97.
- Kushan JP (2005) “Witness Testimony of Attorney Specializing in Biotechnology, Pharmaceuticals and Software “ In US senate on injuctions and damages. Washington, USA, pp. 132-153.
- Lasersohn, Jack W (2010) “Witness Testimony to the House of Representatives for Vertical Group.” In Patent Reform Act of Washington, USA Government Printing Office.
- Parker, William (2005) “Witness Testimony from Diffraction.” In US Senate on perspectives on patents. Washington, USA, pp. 146-155.
- Lee, Steven J, Creel LT (2005 “Witness Testimony Teva North America.” In US Senate: Perspectives on Patents: Harmonization and Other Matters,. Washington, USA, Government Printing Office. pp. 103-28.
- Bernstein, Bruce (2007) “Witness Testimony for Interdigital Communications Corporation.” In US Senate on Patent Reform: The future of American innovation, Washington, USA, pp. 198-216..
- Maghame, Taraneh (2009) “Witness Testimony for Tessera.” In US Senate on Patent Reform in the 111th Congress: Legislation and recent court decisions, Washington, DC, USA, pp. 240-262.
- McDougall, Paul, and Eric Chabrow. “How to Avoid the Patent Trap.” InformationWeek, http://www.informationweek.com/news/ internet/webdev/showArticle.jhtml?articleID=193402991
- Chabrow, Eric. “The Us Patent System in Crisis.” United Business Media, LLC, http://www.informationweek.com/news/global-cio/ showArticle.jhtml?articleID=180204145.
- Lerner, Josh. “The Patent Crisis.” 2007.
- Friedman, Thomas L. “Rising above the Gathering Storm: Two Years Later: Accelerating Progress toward a Brighter Economic Future.”. National Accademies Presses (2009): 24.
- Kirk, Michael. “Witness Testimony to House of Representatives from Aipla.” In US House of Representatives on perspectives on patents. Washington DC, 2005.
- Michel, Paul R. “Witness Testimony as the Chief Judge on the Us Court of Appeals for the Federal Circuit.” In US Senate on Patent Reform: The future of American innovation, 277-81. Washington, DC, 2007.
- Chu, Angus C (2007) Economic Growth and Patent Policy: Quantifyingthe Effects of Patent Length on R&D and Consumption. In MPRA Paper: University of Michigan.
- Gallini, Nancy, and Suzanne Scotchmer. “Intellectual Property: When Is It the Best Incentive System?” In Economics Working Papers E01-303. University of California at Berkeley, 2001.
- Silverman, E. “Patent Bill Stalls in Us Senate.” Cannon Communications, http://www.pharmalot.com/2008/04/patentreform- bill-stalls-in-us-senate/.
- Armitage, Robert A. “Witness Testimony for Eli Lilly (Phrma).” In US Senate on Perspectives on Patents, 44-52. Washington, DC, 2005.
- Peterman, Anthony (2007) “Witness Testimony for Dell Inc.” In US House of Respresentatives on Patent Reform Act. Washington, DC.
- Cassidy, Bernard J (2009) “Witness Testimony for Tessera, Inc.” In US Senate on Patent Reform Act, 101-46. Washington, DC.
- Poppen, Joel L (2005) “Witness Testimony for Micron Technology, Inc.” In US Senate on perspectives on patents, 156-66. Washington, DC.
- Kasper AJ (2010) “Support for Manager’s Amendment to S. 515 “the Patent Reform Act of 2010”.” AIPLA., http://www.aipla.org/Content/ContentGroups/ Legislative_Action/111th_Congress1/Testimony7/ LetterSupportingS515-040210.pdf.
- AIPLA. American Intellectual Property Law Association (Aipla). Report of Economic Survey 1999. Arlington, VA: AIPLA.
- Greenhalgh Christine, Mark Rogers (2007) The Value of Intellectual Property Rights to Firms and Society. Oxford Review of Economic Policy 23(4): 541-567.
- Siwik, Christine. “Witness Testimony for Barr Pharmaceuticals, Inc.” In US Senate on harmonization and other matters, 86-102. Washington, DC: Barr Pharmaceuticals, Incl, 2005.





