Cigarettes Contains Arsenic: A Study of Tobacco in New Delhi Region using AAS-VGA
Rohit Kanojia1, Niyati Khurana2, AK Jaiswal3 and Sunita Bhagat4*
1Ph.D. Research Scholar, Department of Chemistry, University of Delhi, currently serving as Assistant Professor (Forensic Chemistry and Toxicology), Department of Forensic Science, National Forensic Science University Delhi Campus, Delhi, India
2Student, Integrated Forensic Science, Dept. of Forensic Science, National Forensic Science University Delhi Campus, Delhi, India
3Chemist, Department of Forensic Medicine & Toxicology, All India Institute of Medical Sciences (AIIMS), New Delhi, India
4Professor, Department of Chemistry, Atma Ram Sanatan Dharma (ARSD) College, Dhaula Kuan, University of Delhi, New Delhi, India
Submission:March 10, 2025;Published:March 19, 2025
*Corresponding author:Sunita Bhagat, Department of Chemistry, Atma Ram Sanatan Dharma (ARSD) College, Dhaula Kuan, University of Delhi, New Delhi – 110021, India Email: sunitabhagat28@gmail.com
How to cite this article: Rohit Kanojia, Niyati Khurana, AK Jaiswal, Sunita B. Cigarettes Contains Arsenic: A Study of Tobacco in New Delhi Region using AAS-VGA. Open Acc J of Toxicol. 2025; 6(3):555688.DOI: 10.19080/OAJT.2025.06.555688.
Abstract
Arsenic, a very toxic metalloid, presents significant health hazards from multiple environmental exposures, including the inhalation of arsenic-laden tobacco smoke. This pertains to the accumulation of arsenic in combustible tobacco product and the related health hazards for smokers and anyone exposed to second hand smoke. Inorganic arsenic, the predominant substance in tobacco, is converted into less harmful metabolites during smoking. Nonetheless, the methylation process in smokers is suboptimal, resulting in increased concentrations of harmful arsenic compounds in blood. Atomic Absorption Spectroscopy with a Vapor Generator Assembly (AAS-VGA) was used to determine arsenic content in tobacco product, owing to its superior sensitivity and cost-effectiveness. Prior to the analysis of concentration of As, the process entailed closed vessel digestion of tobacco samples using Microwave Digestion System (MDS-10). This research revealed elevated arsenic concentrations, yet no threshold for tobacco as such by WHO or any organization. This increases the risk of developing arsenic-related health issues, such as lung cancer, cardiovascular disease, and other chronic ailments, to both active and passive smokers. Smoking therefore also has the potential to make a significant contribution to total indoor air pollution. This study underscores the need for more stringent public health measures to diminish arsenic exposure from smokable tobacco, while promoting the implementation of advanced detection techniques such as AAS-VGA for efficient monitoring and reduction of contamination.
Keywords:Arsenic; Cigarettes; Tobacco; AAS-VGA; WHO; Indoor Pollution
Abbreviations:As: Arsenic, AAS-VGA: Atomic Absorption Spectroscopy with a Vapor Generator Assembly, PPM: Parts Per Million, PPB: Parts Per Billion, WHO: World Health Organization, MA: Methyl arsenic Acid, DMA: Di Methyl arsenic Acid, ICP-MS: Inductively Coupled Plasma Mass Spectroscopy, MG: Milligram, L: liter, HCl: Hydrochloric Acid, ML: Milliliter, CM: centimeters, G: Gram, MA: Milliampere, MM: Millimeter, μg: Microgram, Min: Minute, MPA: Mega Pascal, NHV: Negative High Voltage, HCL: Hollow Cathode Lamp, R2: Correlation coff, Au: Absorbance, MDS: Microwave Digestion System, TS: Tobacco Sample, HNo3: Nitric Acid, NaBH4: Sodium Borohydride, KOH: Potassium Hydroxide, W: Watts
Introduction
The element arsenic (As) being the 53rd most abundant element, is an extremely toxic metalloid. Primarily, arsenic exists in oxidation states of +3 (arsenite), +5 (arsenate), 0 (elemental), and -3 (arsine). The most deleterious forms are arsenite (As³⁺), which is acutely poisonous because of its interference with cellular processes, and arsenate (As5⁺), which hampers cellular energy generation. Among these, arsenite is universally regarded as the most hazardous to human health [1]. Arsenic poisoning is a serious global health issue that affects millions of people due to intentional poisoning as well as exposure in the workplace and environment [2,3]. Arsine gas (AsH3), which is both colorless and odorless, is highly hazardous and is commonly linked to industrial activities such as mining and semiconductor manufacturing. Administering even little amounts of arsine can be fatal, as symptoms including headache, nausea, vomiting, diarrhea, dark urine, and jaundice and can occur several hours after exposure. Arsenic predominantly enters the human body from internal consumption, inhalation, or dermal absorption, where it accumulates in the lungs, liver, kidneys, and skin. Following absorption, arsenic undergoes intricate metabolic transformations, whereby certain intermediates exhibit higher toxicity compared to the initial state. Acute arsenic poisoning includes gastrointestinal symptoms such as nausea, vomiting, abdominal discomfort, and severe diarrhea. Chronic exposure, particularly by the consumption of polluted drinking water and food, can result in the development of skin lesions, skin cancer, and increased susceptibility to lung and bladder cancer. In addition, exposure to arsenic is associated with developmental adverse effects, diabetes, lung disease, and cardiovascular disease [4-6].
The absorption of arsenic from polluted soil and water by tobacco plants, namely Nicotiana Benthamian a Domin, results in the buildup of both inorganic and organic arsenic species. Within tobacco, inorganic arsenic is the predominant type, frequently making up a significant amount of the entire arsenic content. The process of arsenic metabolism in tobacco entails its conversion into less harmful byproducts such as methyl arsenic acid (MA) and dimethyl arsenic acid (DMA). Nevertheless, the consumption of tobacco is linked to a less effective process of methylating arsenic in blood, leading to elevated concentrations of harmful inorganic arsenic in those who smoke. This increases the potential health hazards, particularly when coupled with other forms of exposure such as contaminated drinking water. Tobacco smoking results in transfer of arsenic into both the mainstream and side stream smoke, therefore exposing both active and passive smokers. Inhalation of arsenic directly by active smokers poses a significant risk for the development of lung cancer and skin lesions. Smoking hinders the process of arsenic methylation, leading to elevated concentrations of harmful byproducts such as mono methyl arsenic acid (MA) in urine, so posing heightened health hazards. People who passively smoke, or are exposed to second hand smoke, also encounter health hazards associated with arsenic exposure, but to a lesser extent as compared to active smokers. The cumulative impacts of arsenic exposure from tobacco and other sources greatly increase the likelihood of health issues associated with arsenic, underscoring the necessity for public health measures aimed at minimizing exposure.
Traditional approaches for identifying arsenic in matrices like tobacco, such as colorimetric methods and Inductively Coupled Plasma Mass Spectrometry (ICP-MS), have significant limitations. Colorimetric techniques, which entail the formation of a colored complex with arsenic and subsequent visual or spectrophotometric measurement, are inherently subjective and have restricted sensitivity, especially when dealing with intricate matrices such as tobacco. The ICP-MS method, although very sensitive, is intricate, necessitating thorough sample preparation and calibration, and expensive, therefore limiting its availability for regular use [7-9]. AAS-VGA is the favored method because to its exceptional sensitivity in identifying extremely low levels of arsenic, which is crucial for precise analysis in many complicated matrices. AAS-VGA technology necessitates a substantial sample volume to guarantee representativeness and enables fast analysis, usually within a 30-minute timeframe. The device is designed to be easily used by experts, which minimizes mistakes and mitigates the risk of interference between different elements, therefore improving the accuracy of measurements. Moreover, AAS-VGA is more economically efficient than ICP-MS, thereby enhancing its availability for regular chemical analysis in laboratory settings. The aforementioned benefits render AAS-VGA a dependable and effective approach for the detection of arsenic in comparison to previous methodologies [7,10,11].
Materials and Methods
Materials
Experimental glassware: High-quality PFA glassware, manufactured by Borosil Pvt. Ltd. in India. Following an overnight immersion in a chromic acid solution, all the glassware was rinsed with water, ultrapure water, and dried.
Chemicals and reagents: 1000 mg/L (1000 ppm) Arsenic produced by Loba Chemicals Pvt. Ltd., L-ascorbic acid and 37% Hydrochloric Acid (HCl) of Emparta grade, Sodium borohydride, Merck India provided Potassium hydroxide of Emsure grade, thiourea, Sodium Boro hydrate and Potassium Hydroxide were used for the analysis along with Ultrapure water from Rion’s India Pvt. Ltd. was used throughout the experiment. Argon gas of purity 99.99% from Laser Gases Pvt. Ltd., New Delhi, was also used throughout the experiment.
Miscellaneous Items: The pipette of a capacity of 1 ml of Corning Company was used. Tarson’s Company Pipette Tips of 1 ml were used and the weighing Balance of Igene Lab serve was used throughout the experiment.
Instrumentation: Atomic Absorption Spectrophotometer (AAS), Model No. AAS9000 and Hydride Generator (HG), Model No. HG600s from Jiangsu Sky ray Instrument Co. Ltd., China was used for the analysis.
Methods Preparation of Standard Solutions
Dilution method was used to prepare 1ppm of arsenic stock solution was prepared from 1000 ppm arsenic solution. 5 ppb standard solution was prepared by adding 0.5 ml from 1 ppm arsenic stock solution, 1 g of L-ascorbic acid (to make the solution equivalent to 1% L-ascorbic acid), 1 g of thiourea (to make the solution equivalent to 1% thiourea) and 13.5 ml of HCl (to make the solution equivalent to 5% HCl) in a100 ml volumetric flask, shaken well and made up to the mark with ultrapure water. Similarly, to prepare 10 ppb, 20 ppb, and 40 ppb an amount of 1 ml, 2ml, and 4 ml is added from 1 ppm arsenic stock solution in respective 100 ml volumetric flasks by using the above procedure.
Preparation of Standard Blank Solution
1 g of thiourea (to make the solution equivalent to 1% thiourea), 1 g of L-ascorbic acid (to make the solution equivalent to 1% L-ascorbic acid), and 13.5 ml of HCl (to make the solution equivalent to 5% HCl) were taken in 100 ml volumetric flask, shaken well and made up to the mark with ultrapure water.
Preparation of Tobacco Samples
The tobacco from a cigarette was taken in a glass vial and labelled. The tobacco was then pulverized into a fine powder using a mortar and pestle. A 0.5g quantity of tobacco powder was measured and placed in an MDS digestion vial. Subsequently, 4 mL of concentrated HNO3 was added to it and kept for digestion. The parameters of MDS were configured in accordance with the instructions outlined in the MDS-10 manual accompanying the device (Table 1). The sample was renamed as TS1 (Tobacco stock 1, Similarly TS2, TS3 ....... TS14). The digested sample was collected and subsequently transferred in a separate 100 mL volumetric flask. 2 mL of TS1, 0.5 g of thiourea (to obtain a concentration of 1% thiourea), 0.5 g of L-ascorbic acid (to produce a concentration of 1% L-ascorbic acid), and 6.7 mL of HCl (to achieve a concentration of 5% HCl) were added to the 50 ml volumetric flask. The solution was vigorously agitated and subsequently diluted with ultrapure water until the flask reached the 50 mL mark. This procedure was replicated for each of the tobacco samples.
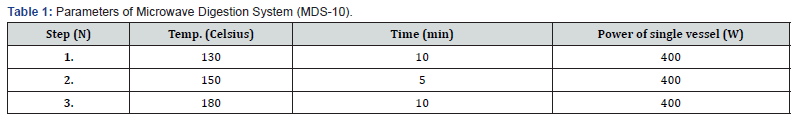
Preparation of Tobacco Sample Blank
In a 100 mL volumetric flask, 4 mL of conc. HNO3 and 0.5 mL of ultrapure water were taken. 1g of thiourea, 1 g of L-ascorbic acid, and 13.5 mL of HCl were added to the 100 mL volumetric flask. The flask was then shaken well and filled up to the mark with ultrapure water.
Preparation of reagents for VGA
Carrier Reagent: A carrier reagent, consisting of 5% HCl,
was prepared by dissolving 135.5 mL of 37% HCl in a 1-liter
volumetric flask. The solution was then brought to the desired
volume by adding ultrapure water.
Reducing Agent: The reducing agent was prepared in a 1-liter
volumetric flask by dissolving 20 g of NaBH4 to create a solution
equivalent to 2% NaBH4, and 5 g of KOH to create a solution
equivalent to 0.5% KOH. The flask was thoroughly shaken, and
then the volume was raised to 1- liter by adding ultrapure water.
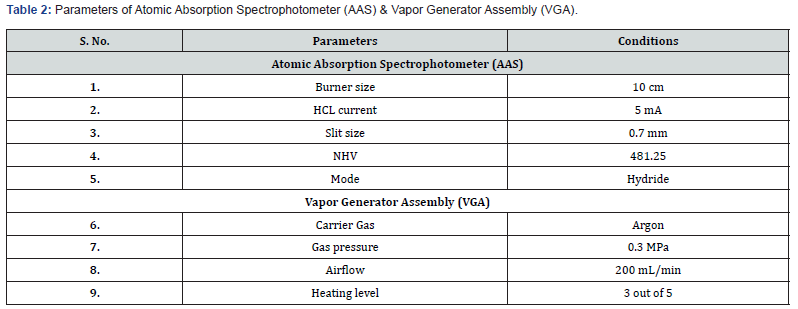
Procedure for calibration of AAS-VGA for Arsenic
A clean and dry volumetric flask was taken. From a 1000 ppm standard sample solution, a stock solution of 1 ppm was prepared. From this 1 ppm stock solution, standard sample solutions of 5 ppb, 10 ppb, 20 ppb, and 40 ppb were prepared. A standard blank solution was also prepared by adding 100 mL of distilled water to a volumetric flask, with no metal sample added.
To prepare the 5ppb solution
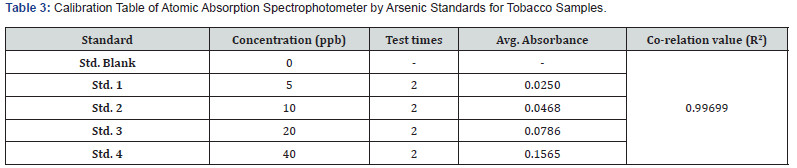
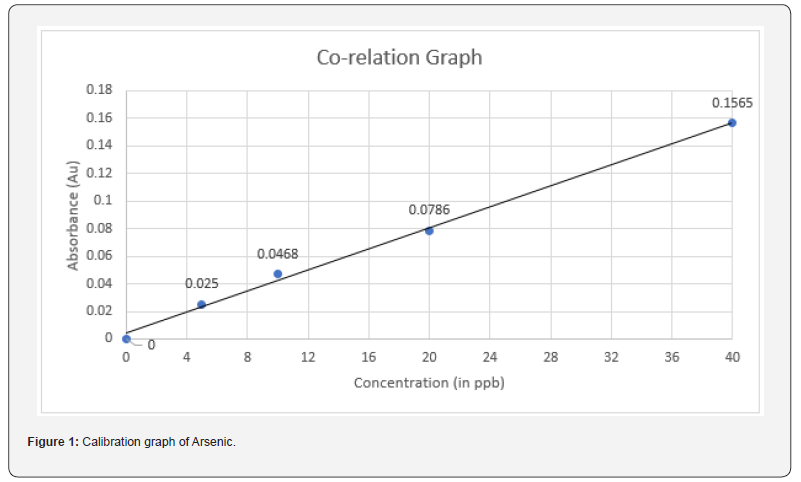
From the 1000 ppm standard solution, 100 μL of solution was taken using a micropipette to make a stock solution of 1 ppm. From this 1 ppm stock solution, 0.5 mL was taken using a micropipette into a beaker to make a standard solution of 5 ppb. Similarly, 1 mL, 2 mL, and 4 mL were taken from the stock solution to prepare standard solutions of 10 ppb, 20 ppb, and 40 ppb, respectively. The system was then turned on, and the application was run. Instrumental parameters were set, the Argon gas knob was turned on, and its pressure was adjusted (Table 2). The As Lamp was selected and executed. A search was started, and the absorbance peak of as was checked. The instrument was prepared for calibration. A new project blank was selected, and an operation table was created for 5 standards: blank, 5 ppb, 10 ppb, 20 ppb, and 40 ppb. Standard 1 (blank sample) was selected, and the capillary of the nebulizer was inserted in the sample. The instrument was set to work, and after 20 seconds, the record button (or space bar) was hit to record the absorbance value. This process was repeated for all calibration table standards (5 ppb, 10 ppb, 20 ppb, and 40 ppb). The calibration table was obtained, and the calibration (correlation) graph was prepared by the instrument, with the correlation value as 0.99699. Calibration was thereby completed. The AAS-VGA calibration was conducted using arsenic standards with concentrations of 5 ppb, 10 ppb, 20 ppb, and 40 ppb (Table 3). The absorbance was determined following the completion of two trials for each standard. The correlation coefficient (R2) obtained was 0.99699. The samples underwent double testing in a single analysis to provide an average measurement, hence minimizing potential errors. The concentration of arsenic in the tobacco sample ranged from 164.4 ppb to 965.2 ppb after considering the dilution factor.
Result and Discussion
Result
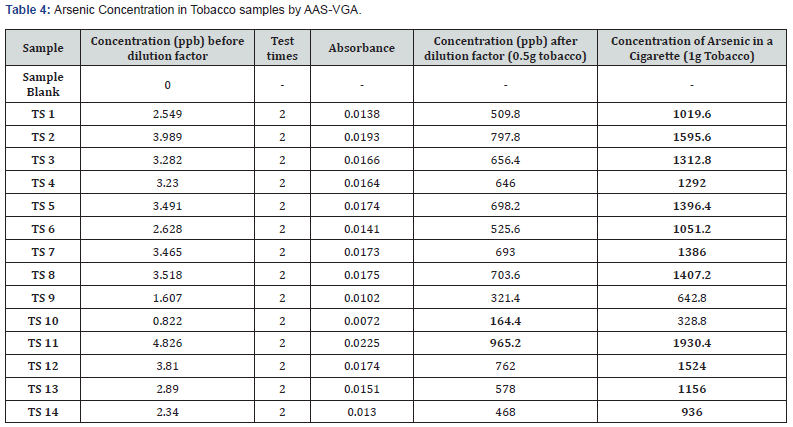
Atomic absorption spectrophotometer, in conjunction with a Vapor Generator Assembly, was employed to quantify the concentration of arsenic in tobacco samples from cigarettes. The calibration technique covered a spectrum from 5 ppb to 40 ppb. The samples underwent dual analysis in a singular run, and the mean concentration of arsenic was determined by taking into account the dilution effect. The arsenic content varied between 164.4 ppb and 965.2 ppb (Table 4), in 0.5g of tobacco taken.
Discussion
Tobacco plants, recognized for their ability to absorb and retain heavy metals from the soil, can contribute to human exposure to arsenic. Tobacco plants predominantly assimilate arsenic from contaminated soils, fertilizers, or pesticides utilized in their cultivation. The levels of arsenic in tobacco may vary based on geographic location and agricultural methods. Nonetheless, the consumption of tobacco products presents a possible avenue for exposure to arsenic. Tobacco is consumed by humans through several methods, including the regular smoking of cigarettes and cigars, chewing or dipping tobacco, excessive use of hookah or shisha, and the continuous intake of significant quantities of tobacco. Tobacco via cigarettes can also be consumed through its exposure to water. Arsenic included in tobacco can be inhaled and then absorbed into the bloodstream when the plant material is combusted. Prolonged tobacco consumption might result in significant health hazards owing to the presence of nicotine and other compounds. Smoking tobacco releases arsenic into the body, leading to elevated levels of arsenic in the urine of smokers. Consequently, there is a necessity to do inspections on cigarettes available in the Indian market to ascertain the arsenic levels, given the absence of particular regulations regarding the arsenic content in tobacco utilized for cigarette production [12,13]. The amount of arsenic found in 0.5 g of tobacco is rather insignificant, despite the fact that a cigarette usually contains over 1-2 g. Hence, increasing the concentration of arsenic twofold will unquestionably result in detrimental effects on both active and passive smokers, as well as the surrounding ecosystem.
The WHO or any other health organization does not establish a precise threshold for the permissible amount of arsenic in tobacco. It is crucial to acknowledge that arsenic has the ability to transform into vapor and build up in the respiratory tract of both active and passive smokers [14]. The present investigation entailed the collection of 14 randomly chosen cigarettes of national and international brands from local pan shops in the New Delhi area. Four standards were taken to calibrate AAS-VGA, resulting in a R2 of 0.99699 (Figure 1). The samples were tested subsequent to the device’s calibration. The arsenic concentration in a single cigarette was found to range from 328.8 ppb to 1930.4 ppb (Table 4). The term ‘Sample’ (TS1, TS2, …...TS14) has been utilized to obscure the brand’s identity. Amongst the total samples, only 3 brands demonstrated the arsenic content below 1 ppm, whilst the other 11 brands exhibit an arsenic concentration around 2 ppm. Sample 10 exhibits the lowest arsenic concentration at 328.8 ppb, whilst sample 11 has the highest value at 1930.4 ppb. Indian customers are subjected to an average of 1212.77 ppb or 1.212 ppm of arsenic per cigarette. The issue of arsenic pollution continues to be a significant global health problem, as ingestion of contaminated crops, water, and seafood are among the many routes through which individuals are exposed. The World Health Organization advises a maximum threshold of 10 μg/L for arsenic in drinking water, although several areas surpass this prescribed limit. Thorough surveillance and mitigation measures, including the implementation of safe irrigation techniques, provision of uncontaminated drinking water, and advocating food safety, are crucial in order to minimize human exposure and associated health hazards. This leads to the overall accumulation of pollutants in the atmosphere, adversely affecting air quality and posing health risks to persons exposed to the contaminated air [15].
Conclusion
Arsenic is a very toxic substance that presents a significant threat to human health when ingested, breathed, or absorbed through the skin. The toxicity of this drug is insidious and may manifest as either acute or chronic poisoning. Upon analysis of tobacco of cigarettes sold in New Delhi, arsenic concentration was found to vary from a low of 328.8 ppb to a maximum of 1930.4 ppb. No specific organization has established a fixed limit for the permissible level of arsenic in tobacco yet. Hence, there is a need for the officials to look upon this matter.
Acknowledgement
The authors would like to acknowledge Toxicology Laboratory, All India Institute of Medical Sciences (AIIMS), New Delhi, India for providing all possible help to carry out the research. They would also like to thank UGC to providing all financial help (NFSC fellowship).
Competing Interests
All the authors find no conflict of interest.
References
- Liu C, Wright C, McAdam K, Taebunpakul C, Heroult J, et al. (2012) Arsenic Speciation in Tobacco and Cigarette Smoke. Beiträge Zur Tabakforschung International/Contributions to Tobacco Research 25(2): 375-380.
- Dhaware D, Deshpande A, Khandekar RN, Chowgule R (2009) Determination of Toxic Metals in Indian Smokeless Tobacco Products. The Scientific World Journal 9: 1140-1147.
- Prabhakar V, Jayakrishnan G, Nair SV, Ranganathan B (2013) Determination of Trace Metals, Moisture, pH and Assessment of Potential Toxicity of Selected Smokeless Tobacco Products. Indian J of Pharmaceutical Scie 75(3): 262-269.
- Ferreccio C, Yuan Y, Calle J, Benítez H, Parra RL, et al. (2013) Arsenic, Tobacco Smoke, and Occupation. Epidemiology (Cambridge, Mass.) 24(6): 898-905.
- Jiang C, Chen Q, Xie M (2020) Smoking increases the risk of infectious diseases: A narrative review.
- Omare MO, Kibet JK, Cherutoi JK, Kengara FO (2022) A review of tobacco abuse and its epidemiological consequences. Zeitschrift Fur Gesundheitswissenschaften 30(6): 1485-1500.
- Bhat A, O Hara T, Tian F, Singh B (2023) Review of analytical techniques for arsenic detection and determination in drinking water. Environmental Science: Advances 2(2): 171-195.
- Hughes MF, Beck BD, Chen Y, Lewis AS, Thomas DJ (2011) Arsenic Exposure and Toxicology: A Historical Perspective. Toxicol Sci 123(2): 305-332.
- Mishra S, Verma SK (2022) Methods to Detect Arsenic Compounds. In Arsenic in Plants pp. 345-366.
- Alidadi H, Ramezani A, Davodi M, Peiravi R, Paydar M, et al. (2015) Determination of Total Arsenic in Water Resources: A Case Study of Rivash in Kashmar City. Health Scope 4(3) Article 3.
- Valskys V, Hassan HR, Wołkowicz S, Satkūnas J, Kibirkštis G, et al. (2022) A Review on Detection Techniques, Health Hazards and Human Health Risk Assessment of Arsenic Pollution in Soil and Groundwater. Minerals. 12(10) Article 10.
- Campbell RC, Stephens WE, Meharg AA (2014) Consistency of arsenic speciation in global tobacco products with implications for health and regulation. Tobacco Induced Diseases 12(1): 24.
- Regassa G, Chandravanshi BS (2016) Levels of heavy metals in the raw and processed Ethiopian tobacco leaves. Springer Plus 5: 232.
- Satterlee HS (1956) The problem of arsenic in American cigarette tobacco. The New England Journal of Medicine 254(25): 1149-1154.
- Dhane AS, Sarode SC, Sarode GS, Sharma NK (2024) Rise in arsenic pollution and oral cancer: A call for action. Oral Oncology Reports 9: 100-238.






























