Chemical Composition and In vivo Oral Toxicity of Essential Oil from Ocimum gratissimum (Scent Leaf) found in Nigeria
Ayoola Florence O1, Nike Jesutofunmi I2, Micheal Abimbola O1, Moses Adondua A3*, Anthony Olalekan A4, Akinniyi O1, Rapheal Akinwunmi A5, Adutwum Ebenezer A6, Priscilla Titilope A1, Mosunmola Vewande F7, Babalola Marvellous T8, Oluwatosin Timothy I7, Daniel Chukwuebuka E8, Ekele Jiata U9 and Luqman Olaitan S10 Rabiyya Halliru Abdullahi11, Osazuwa Ephraim Eghosa12 , and Olowoyo Oluwatomisin Sunday1
1Department of Biochemistry, Faculty of Basic Medical Sciences, University of Lagos, Lagos State, Nigeria
2Department of Chemistry, University of Nebraska-Lincoln, Lincoln, Nebraska, USA
3Department of Biochemistry, Faculty of Biosciences, Federal University Wukari, Taraba State, Nigeria
4College of Public Health, Division of Environmental Health Sciences, The Ohio State University,Ohio, USA
5Department of Biology, College of Science and Technology, North Carolina Agriculture & Technical State University, USA
6Family and Consumer Sciences, College of Agriculture and Environmental Sciences, North Carolina Agriculture & Technical State University, USA
7Department of Pharmaceutical Chemistry, Faculty of Pharmacy, Olabisi Onabanjo University, Ogun State, Nigeria
8Department of Biochemistry, Faculty of Biological Sciences, Abia State University, Uturu, Nigeria
9Department of Biological and Environmental Sciences, Liverpool John Moores University, Byrom Street, Liverpool, L33AF United Kingdom
10Department of Chemistry, Faculty of Science, University of Abuja, Nigeria
11Department of Community Medicine/Public health, Nile University of Nigeria
12National Defense College Medical Centre, Abuja, Nigeria
Submission:February 25, 2025;Published:March 5, 2025
*Corresponding author:Moses Adondua Abah, Department of Biochemistry, Faculty of Biosciences, Federal University Wukari, Taraba State, Nigeria
How to cite this article: Ayoola Florence O, Nike Jesutofunmi I, Micheal Abimbola O, Moses Adondua A, Anthony Olalekan A, et al. Chemical Composition and In vivo Oral Toxicity of Essential Oil from Ocimum gratissimum (Scent Leaf) found in Nigeria. Open Acc J of Toxicol. 2025; 6(3):555687.DOI: 10.19080/OAJT.2025.06.555687.
Abstract
In recent decades, there has been a significant increase in the interest of plant essential oil which constitute an important source of biological active compounds. Essential oils of Ocimum gratissimum species possess medicinal potentials that are used in food industry and medicinal plant as a result of its anti-inflammatory analgesic, hepatoprotective, anti-mutagenic, antihypertensive activities and insect repellant. However, little research has been done on its in vivo oral toxicity effect. The present study aimed at evaluating the chemical composition and in vivo oral toxicity of essential oil from Ocimum gratissimum (scent leaf) found in Nigeria. Fresh leaves of Ocimum gratissimum was chopped into small pieces. 300g of the chopped leaves was mixed with 1000ml of water and the mixture was then boiled in a round-bottom flask subjected to hydro distillation using a Clevenger-type apparatus for 6 hours. GC-MS was carried out in order to identify the bioactive compounds in the extract. Thirty-two (32) male Wistar strain albino rats weighing 120-150g were obtained from the Animal House, College of Medicine, University of Lagos, Nigeria and kept in well-ventilated standard plastic cages with eight animals per cage. The rats were acclimatized for 1 week and maintained on oral standard rats’ pellets and water ad libitum. The experimental animals were randomly divided into four groups of eight animals per group, for a total experimental period of 8 weeks as presented below. After acclimatization, administrations were done orally with 24 hours interval. Three animals from each group were sacrificed by cervical dislocation and the blood was collected for biochemistry and hematology analysis. They were dissected in order to excise the liver, lungs, spleen, brain and kidney for histopathological studies. Statistical analysis was carried out using Analysis of Variance (ANOVA) and Dunnet’s Multiple comparison test at P < 0.05 level of significance. The results obtained from this study revealed that most of the compounds detected are fatty acid esters. Bis (2-ethylhexyl) phthalate was observed to have the highest retention time recorded as 24.029 with a percentage proportion of 0.33%. Carvacrol recorded the lowest retention time (7.625) with a percentage proportion of 3.16. There was no significant difference observed in the body weight of rats between control group and any of treated groups (2-4) at any period of time. After 60 days of treatment, there was significant increase in the concentration of AST (UI/L) and Tot. Protein (g/L) across all treated groups compared to the control group. A decrease was also observed in the concentration of K+ (mmol/L), HCO3 (mmol/L), Ca+ (mmol/L), Creatinine (μmol/L), Total Cholesterol (mmol/L), Triglyceride (mmol/L), LDL (mg/dL), Tot. Bilirubin (μmol/L), Dir. Bilirubin (μmol/L), and ALT (UI/L) across all treated groups compared to the control. There were no signs of apoptosis or neurodegeneration in the brains of experimental rats. The results of this study revealed that O. gratissimum essential oil maybe toxic when given at high doses for a longer period of time which could be responsible for the clinical manifestations and the pathological changes observed in the organs and tissues of the treated rats.
Keywords:Ocimum gratissimum; Essential oil; Bioactive compounds; Toxicity; Wistar rats
Abbreviations:ANOVA: Analysis of Variance, NIST: National Institute of Standards and Technology, NIH: National Institute of Health, EO: Essential Oil, WBC: White Blood Cell, HGB: Hemoglobin, HCT: Hematocrit, MCH: Mean Corpuscular Hemoglobin, MCHC: Mean Corpuscular Hemoglobin Concentration, MCV: Mean Corpuscular Volume, PC: Platelets Count, MPV: Mean Platelet Volume, HDL: High Density Lipoprotein, LDL: Low Density Lipoprotein, AST: Aspartate Aminotransferase, CHOL: Cholesterol, ALT: Alanine Aminotransferase, TG: Triglycerides, ALP: Alkaline Phosphatase, BC: Bowman’s capsule, CT: convoluted tubule
Introduction
The use of synthetic and chemically based substances in the treatment of various pests and diseases in food crops has leads to a long-term complication to the human health, since most of the chemically synthetic substances possess serious side effects that make their disadvantages to outweigh their advantages, because some chemical constituents are carcinogenic, cytolytic or cytotoxic when administered in large doses [1,2]. Majority of synthetic chemicals have serious adverse effects to humans which may lead to temporary or permanent disability and incapacitations [3,4]. Also, gastrointestinal disorders, dysentery, diarrhea and candidiasis are very serious infections that can lead to frequent morbidity and mortality in tropical countries like Nigeria. These disorders are serious diseases that can affect many people at various stages of their lives causing distress and discomfort [5,6]. Therefore, the use of natural plants as substitutes of chemically synthetic substances is imperative in order to prevent negative side effects and toxicity of the synthetic chemicals with the natural means of treatment [7]. Plant essential oils serve this purpose. They are volatile substances, mainly composite mixtures of terpenoids which are used for their aromatic qualities. Essential oils and their constituents cause lethal and sublethal effects on insects, such as biocide activity, infertility, irritability, phagoinhibition and repellency [8].
Ocimum gratissimumis an herbaceous plant which belongs to the Labiatae family. The plant is indigenous to tropical areas especially India and it is also in West Africa. In Nigeria, it is found in the Savannah and coastal areas. It is cultivated in Ceylon, South Sea Islands, and also within Nepal, Bengal, Chittagong and Deccan [9]. It is known by various names in different parts of the world. In India it is known by its several vernacular names, the most commonly used ones being “Vriddhutulsi (Sanskrit), “Ram Tulsi” (Hindi), “Nimma Tulasi” (Kannada). In the southern part of Nigeria, the plant is called “Effinrin-nla” by the Yoruba speaking tribe. It is called “Ahuji” by the Igbos, while in the Northern part of Nigeria, the Hausas call it “Daidoya” [8]. O. gratissimum has been used extensively in the traditional system of medicine in many countries. In the Northeast of Brazil, it is used for medicinal, condiment and culinary purpose. The flowers and the leaves of this plant are rich in essential oils so it is used in preparation of teas and infusion [10]. In the coastal areas of Nigeria, the plant is used in the treatment of epilepsy, high fever and diarrhea. In the Savannah areas, decoctions of the leaves are used to treat mental illness [11]. O. gratissimum is used by the Ibos of Southeastern Nigeria in the management of the baby’s cord, to keep the wound surfaces sterile. It is also used in the treatment of fungal infections, fever, cold and catarrh [12].
O. gratissimum essential oils have also proven to be an alternative for pest control with low pollution and quick degradation in the environment, making them suitable for managing insects even in organic farming. The plant kingdom is a treasure house of potential drugs and there has been an increasing awareness about the importance of O. gratissimum. Scientific research has focused more in discovering and identifying local plant species with bioactive compounds as an essential oil, their characterization, extraction and purification processes and application in drug industries [13]. Presently 25 to 50% of drugs are available from plants [14] and numerous other aromatic plants are under evaluation for their valuable essential oil. Previous research has been done on the importance of Ocimum gratissimum essential oil in Health and Agricultural sectors but there is also need to understand its trend of toxicity from the acute through the subacute to the sub chronic level in in vivo studies. Therefore, this research aimed at determining the chemical composition and in vivo oral toxicity of essential oil from Ocimum gratissimum (scent leaf) found in Nigeria..
Materials and Method
Sample Collection
The aerial part of Ocimum gratissimum was harvested from a farm in Ota (6ᵒ 40, 29.5716’’N, 3ᵒ 11’ 52.9908” E) Ogun state, Southwest, Nigeria. Botanical identification and authentication of the plant were done in the Department of Botany, University of Lagos. The plant was given a voucher specimen number (LUH 7414) and then deposited at the herbarium of the Department of Botany.
Plant Extraction and Identification of Bioactive Compounds
Fresh leaves of Ocimum gratissimum was chopped into small pieces. 300g of the chopped leaves was mixed with 1000ml of water and the mixture was then boiled in a round-bottom flask subjected to hydro distillation using a Clevenger-type apparatus for 6 hours. GC-MS was performed using an Agilent 7890 gas chromatograph (Agilent Technologies, U.S.A) system equipped with Agilent 5975C mass spectrometer detector operating in electron impact mode (ionization voltage, 70 eV). 1.0 μL of the essential oil was injected, using split less mode. The compounds were identified using standard reference compounds and also by matching the mass spectra fragmentation pattern with the National Institute of Standards and Technology (NIST) Mass Spectra Library stored in the GC-MS database.
Animal Studies Ethical approval
The protocol of this study was approved by the Health Research Ethics Committee of the College of Medicine, University of Lagos, Lagos State, Nigeria.
Experimental animals
Thirty-two (32) male Wistar strain albino rats weighing 120- 150g were obtained from the Animal House, College of Medicine, University of Lagos, Nigeria and kept in well-ventilated standard plastic cages with eight animals per cage. The animals were maintained at a constant temperature of 25 ± 2oC and 84 ± 4% relative humidity with a 12h (7:00 – 19:00) light/dark cycle. The rats were acclimatized for 1 week and maintained on oral standard rats’ pellets (Pfizer Livestock Feeds, Lagos, Nigeria) and water ad libitum. All experiments were conducted without anesthesia, and the protocol was in conformity to the guidelines of the National Institute of Health (NIH publication 85-23, 1985) for laboratory animal care and use.
Experimental design and treatment of animals
The experimental animals were randomly divided into
four groups of eight animals per group, for a total experimental
period of 8 weeks as presented below. After acclimatization,
administration began as described below. Administrations were
done orally with 24 hours interval.
Group 1: Group 1 served as the Control Group (CO). Animals
in this group received only water and feed for eight weeks;
Group 2: Group 2 were considered low dose group. Animals
in this group received 125mg/kg bodyweight of Ocimum
gratissimum Essential Oil (EO);
Group 3: Group 3 were considered medium dose group.
Animals in this group received 250mg/kg bodyweight of Ocimum
gratissimum Essential Oil (EO);
Group 4: Group 4 were considered high dose group. Animals
in this group received 500mg/kg bodyweight of Ocimum
gratissimum Essential Oil (EO).
Blood collection and organ harvest
24 hours after the last dose, all animals were anesthetized with diethyl ether and blood samples were obtained by retro-orbital puncture. The bodyweight of the animals was measured weekly. Also, the weight of animals was recorded before sacrifice. Three animals from each group were sacrificed by cervical dislocation and the blood was collected for biochemistry and hematology analysis. They were dissected in order to excise the liver, lungs, spleen, brain and kidney for histopathological studies.
Effect of Ocimum gratissimum essential oil on the weight of experimental rats and their organs
The bodyweight of the animals was measured weekly. Also, the weight of animals was recorded before sacrifice. After the sacrifice of all animals, the kidneys, liver, lungs, spleen, brain, were carefully removed and weighed individually using a weighing balance (the absolute organ weight was determined).
Effect Ocimum gratissimum essential oil on hematological parameters
Hematological analysis was performed using an automatic hematological analyzer Mindray 3000 at the Central Research Laboratory of Lagos University Teaching Hospital, Lagos State, Nigeria. The parameters assayed for were: White Blood Cell (WBC) count, Hemoglobin (HGB), Hematocrit (HCT), Mean Corpuscular Hemoglobin (MCH), Mean Corpuscular Hemoglobin Concentration (MCHC), Mean Corpuscular Volume (MCV), Platelets Count and Mean Platelet Volume (MPV).
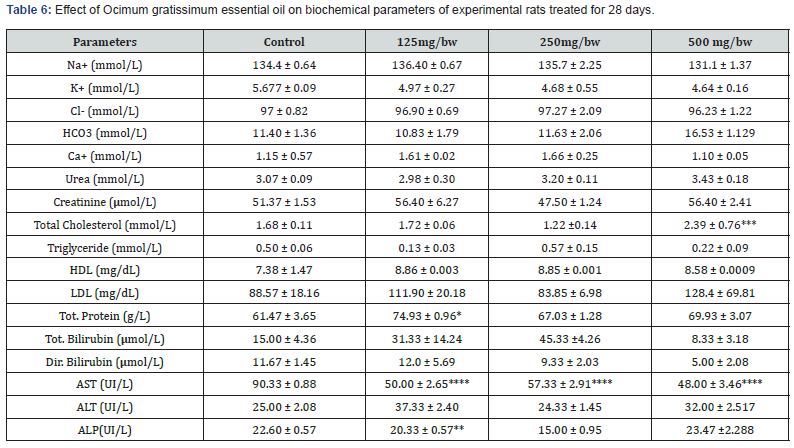
Effect Ocimum gratissimum essential oil on biochemical parameters
For the biochemical analysis, the following parameters were assayed for: Total Cholesterol (CHOL), Urea, Creatinine, Total Protein, Total Bilirubin, Direct Bilirubin, High Density Lipoprotein (HDL), Low Density Lipoprotein (LDL), Aspartate Aminotransferase (AST), Alanine Aminotransferase (ALT), Triglycerides (TG), and Alkaline Phosphatase (ALP). Micro lab 300 automatic analyzer was used in the analysis of the aforementioned parameters. The electrolytes assayed for were: Na+, K+, Ca+, Cland HCO3- using the Ion Selective Electrode automatic analyzer.
Statistical analysis
All experiments were carried out in triplicate. Data obtained were expressed as mean values and standard error of mean. Statistical analysis was carried out using Analysis of Variance (ANOVA) and Dunnet’s Multiple comparison test in Graph Pad Prism, version 6.0 at P < 0.05 level of significance.
Results
Identification of the Chemical Components in Ocimum gratissimum Essential oil
GC-MS analysis of Ocimum gratissimum essential oil revealed a total of 42 compounds with different retention times and proportions. The compounds and with their retention times are captured in Table 1 presented below. Most of the compounds detected are fatty acid esters. Bis (2-ethylhexyl) phthalate was observed to have the highest retention time recorded as 24.029 with a percentage proportion of 0.33%. Carvacrol recorded the lowest retention time (7.625) with a percentage proportion of 3.16.
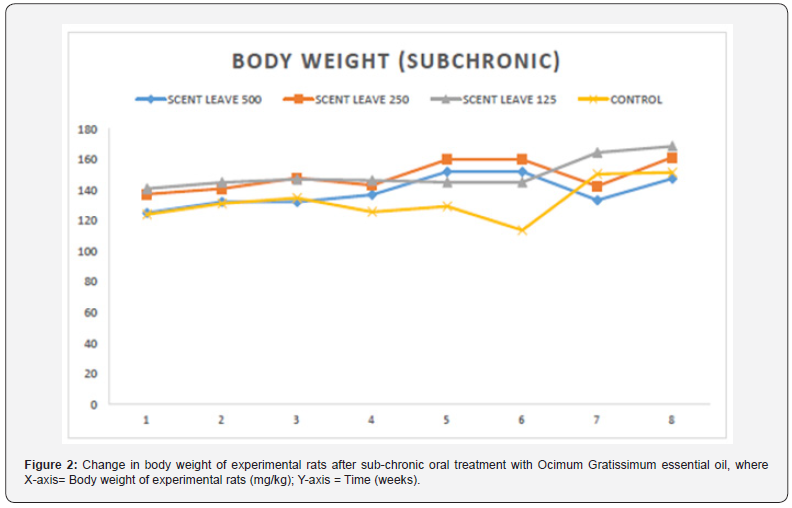
Effect of Sub-acute and Sub-chronic Administration of Ocimum gratissimum Essential Oil on the Body Weight of Experimental Rats
The result of the effect of sub-acute administration of Ocimum gratissimum essential oil on the body weight of experimental rats is presented in Figure 1 below. There was no significant difference observed in the body weight of rats between control group and any of treated groups (2-4) at any period of time. Also, no toxicity signs or death were recorded during the 60 consecutive days of treatment via oral route with Ocimum gratissimum essential oil dissolved in Tween 20 following subacute administration. Figure 2 reveals the result of the effect of sub-chronic administration of Ocimum gratissimum essential oil on the body weight of experimental rats within a given period of time. Again, no toxicity signs or death were recorded during the 60 consecutive days of treatment via oral route with Ocimum gratissimum essential oil dissolved in Tween 20 at doses of 125, 250 or 500 mg/kg of bodyweight.


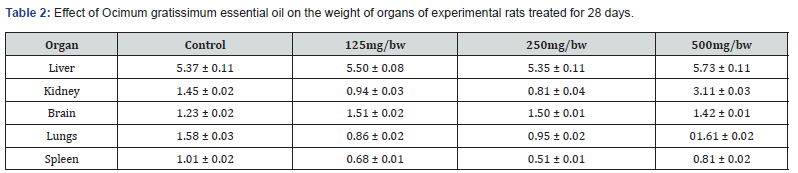
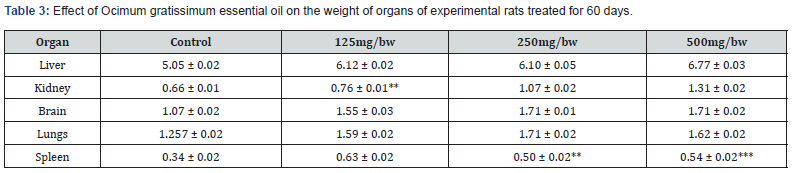
Effect of Ocimum gratissimum Essential Oil on the Weight of Organs of Experimental Rats Treated for 28 days (Subacute) and 60 days (Sub chronic)
The result of the effect of Ocimum gratissimum essential oil on the weight of organs of experimental rats treated for 28 days is presented in Table 2 below. There was no significant increase in the weight of the liver of the experimental rats compared to the control. Also, experimental rats who received 125mg/ bw and 250mg/bw body weight of essential oil showed no significant weight gain in their kidneys. However, there was significant gain in the weight of the kidneys of rats administered 500mg/ kg bodyweight of Ocimum gratissimum essential oil. Rats administered 125mg/bw, 250mg/bw, and 500mg/kg body weight of essential oil showed no significant weight gain in their brains. There was also no significant weight gain in the lungs and spleens of Group 2, Group 3, and Group 4 rats compared to the control. The result of the effect of Ocimum gratissimum essential oil on the weight of organs of experimental rats treated for 60 days is presented in Table 3 below. There was significant increase in the weight of the liver of the experimental rats compared to the control. Group 4 rats had the highest gain in weight of their liver (6.77 ± 0.03). Compared to the control, rats administered 125mg/bw, 250mg/bw, and 500mg/kg body weight of essential oil showed no significant weight gain in their kidney, brain, lungs and spleen.
*Significant difference between control group and experimental group: (*) = P < 0.05, (**) = P =<0.01, and (***) =P<0.001. Mean ± SEM (n=3).
*Significant difference between control group and experimental group: (*) = P < 0.05, (**) = P =<0.01, and (***) =P<0.001. Mean ± SEM (n=3).
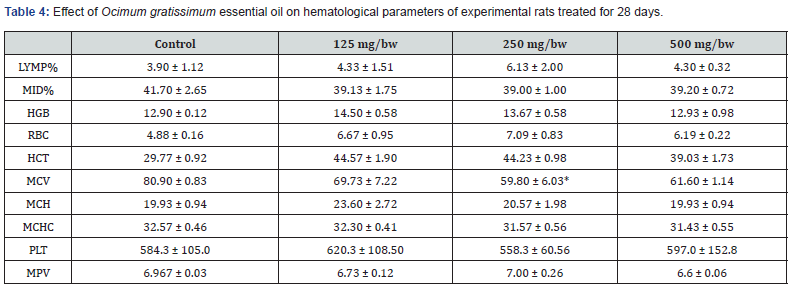
Effect of Ocimum gratissimum Essential Oil on Biochemical Parameters of Experimental Rats Treated for 28 days (Subacute) and 60 days (Sub chronic)
The result for the effect of Ocimum gratissimum essential oil on hematological parameters of experimental rats treated for 28 days is presented in Table 4 below. After 28 days of treatment, there was significant increase in LYMP% for rats treated with 250 mg/bw of Ocimum gratissimum essential oil compared to the control and the other groups. However, there was no significant difference in the value of MID% across all the groups of experimental rats. This was same for MCH, MPV, and MCHC values. A significant increase in the value of RBC was observed across all treated groups compared to the control group with 250 mg/bw Ocimum gratissimum essential oil treated rats having the highest value of RBC. A significant decrease in the value of MCV was also observed across all treated groups compared to the control group with 250 mg/bw Ocimum gratissimum essential oil treated rats having the lowest value of MCV. The result for the effect of Ocimum gratissimum essential oil on hematological parameters of experimental rats treated for 60 days is presented in Table 5 below. After 60 days of treatment, there was significant increase in the value of MCV for all treated groups compared to the control. However, there was no significant difference in the value of MPV and MPV across all the groups of experimental rats. A significant decrease in the value of MCHC, MCH, and MCH was also observed across all treated groups compared to the control group.
*Significant difference between Control group and Experimental group: ns implies non- significant difference; (*) = P < 0.05, (**) = P =<0.01, and (***) =P<0.001. Mean ± SEM (n=3).
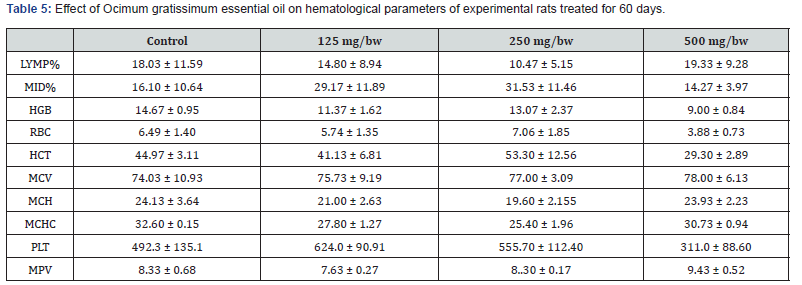
*Significant difference between control group and experimental group: ns implies non- significant difference; (*) = P < 0.05, (**) = P =<0.01, and (***) =P<0.001. Mean ± SEM (n=3).
*Significant difference between control group and experimental group: ns implies non-significant difference; (*) = P < 0.05, (**) = P =<0.01, and (***) =P<0.001. Mean ± SEM (n=3).
The result for the effect of Ocimum gratissimum essential oil on biochemical parameters of experimental rats treated for 28 days is presented in Table 6 below. After 28 days of treatment, there was significant increase in the concentration of Tot. Protein (g/L) and HDL (mg/dL) across all treated groups compared to the control. A significant decrease was also observed in the concentration of K+ (mmol/L) and AST (UI/L) across all treated groups compared to the control. There was no significant change observed in the concentration of Urea (mmol/L), Ca+ (mmol/L), and Cl- (mmol/L) across all treated groups compared to the control group. It was observed that rats treated with 500 mg/bw essential oil had the highest concentration of HCO3 (mmol/L), Urea (mmol/L), Total Cholesterol (mmol/L), LDL (mg/dL), and ALP(UI/L) compared to other treated groups and control group. The result for the effect of Ocimum gratissimum essential oil on biochemical parameters of experimental rats treated for 60 days is presented in Table 7 below. After 60 days of treatment, there was significant increase in the concentration of AST (UI/L) and Tot. Protein (g/L) across all treated groups compared to the control group. A decrease was also observed in the concentration of K+ (mmol/L), HCO3 (mmol/L), Ca+ (mmol/L), Creatinine (μmol/L), Total Cholesterol (mmol/L), Triglyceride (mmol/L), LDL (mg/dL), Tot. Bilirubin (μmol/L), Dir. Bilirubin (μmol/L), and ALT (UI/L) across all treated groups compared to the control. There was no significant change observed in the concentration of Triglyceride (mmol/L) and Na+ (mmol/L) across all treated groups compared to the control. It was observed that rats treated with 500 mg/bw essential oil had the highest concentration of Tot. Protein (g/L) compared to other treated groups and control group.
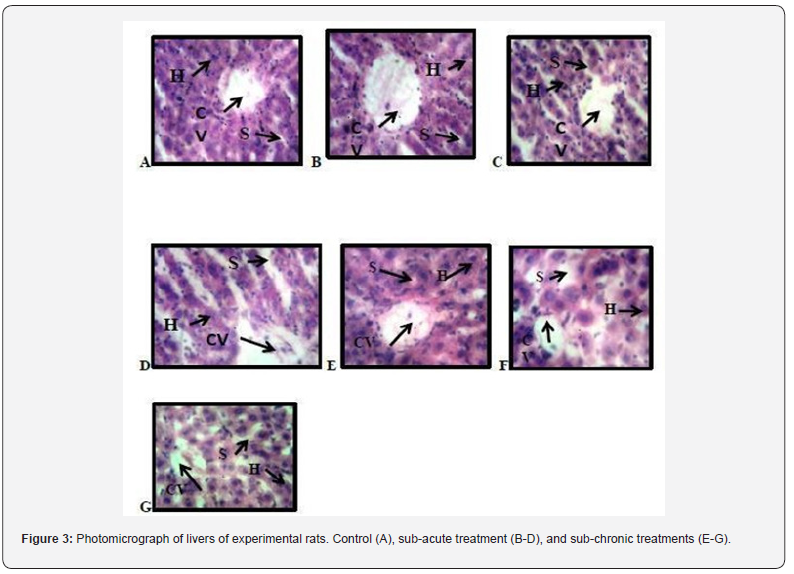
Histopathological Examination of the Effect of Ocimum gratissimum Essential Oil on Selected Organs of Experimental Rats Histopathological examination of livers of experimental rats
Figure 3 presents the photomicrograph of the livers of treated and untreated experimental rats. A, B and E represents photomicrograph of a high-power magnification (x400) of liver showing typically sized halo spaced central vein surrounded by densely distributed hepatocytes. (CV: Central vein, S: f Sinisoidal space, H: Hepatocyte). C and G reveals typically sized halo spaced central vein surrounded by densely distributed hepatocytes. Mild sinusoidal space dilation is apparent (CV: Central vein, S: Sinisoidal space, H: Hepatocyte) D indicate typically sized halo spaced central vein surrounded by less densely distributed hepatocytes. sinusoidal space dilation is apparent (CV: Central vein, S: Sinisoidal space, H: Hepatocyte. F reveals typically sized halo spaced central vein surrounded sparsely densely distributed hepatocytes. sinusoidal space dilation is apparent (CV: Central vein, S: Sinisoidal space, H: Hepatocyte).
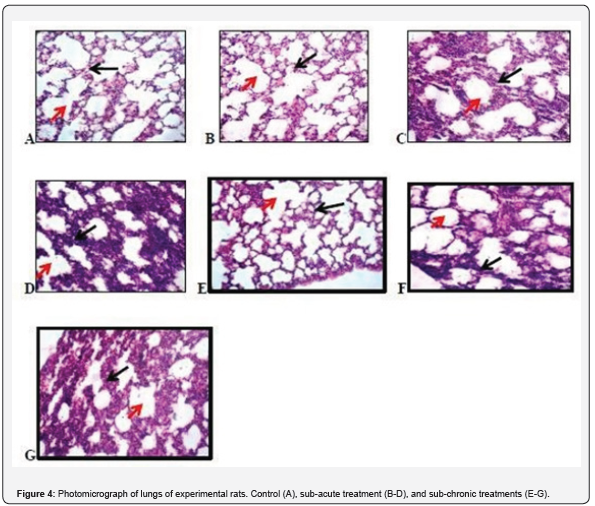
Histopathological examination of lungs of experimental rats
Figure 4 presents the photomicrograph of the lungs of treated and untreated experimental rats. A, B and E captures photomicrograph of lung of experimental rats showing a high-power magnification (X100) of the lung with normal histomorphologies presentation showing the alveolus (red arrow) and the alveoli wall (black arrow) H and E stain X 100. C and D: (red arrow: visceral pleura, black arrow: alveolus) H and E stain X100. F and G shows some degree of collapsed alveoli and thickening in the alveoli wall and an infiltration of different inflammatory cells in the alveoli wall. (red arrow: visceral pleura, black arrow: alveolus) H and E stain X100.
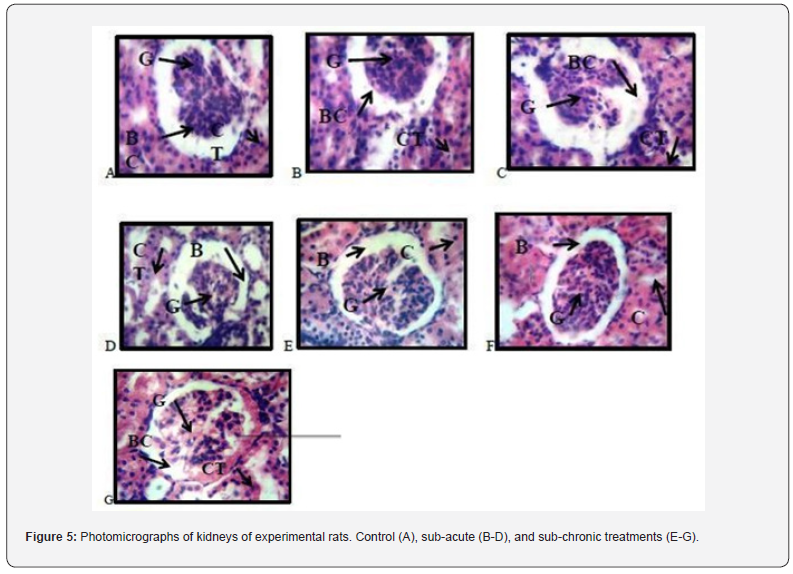
Histopathological examination of kidneys of experimental rats
Figure 5 presents the photomicrograph of the kidneys of treated and untreated experimental rats. A, B, C and E reveals photomicrograph of kidney of experimental rats showing normal renal corpuscle with typical cellular delineation, distribution, density and staining intensity. (BC- Bowman’s capsule, GGlomerulus, CT- convoluted tubule). D, F and G indicate atypical presentation of collapsed alveoli and thickening in the alveoli wall and an infiltration of different inflammatory cells in the alveoli wall. (BC- Bowman’s capsule, G- Glomerulus, CT- convoluted tubule)
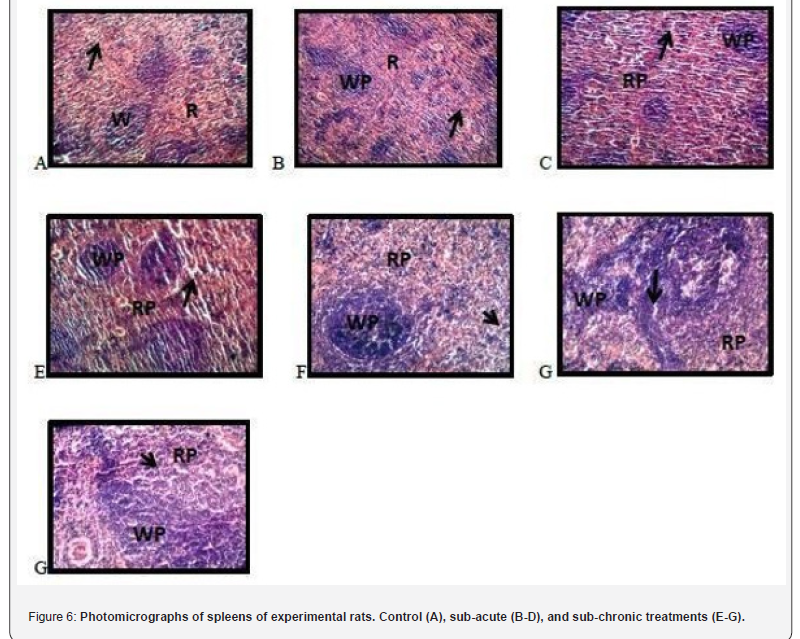
Histopathological examination of spleens of experimental rats
Figure 6 presents the photomicrograph of the spleens of treated and untreated experimental rats. A, B, and C represents photomicrographs of spleen of experimental rats showing a high-power magnification (X100) of the spleen with normal histomorphologies presentation of the red pup and white pulp. (RP- Red pulp, WP- White pulp, black arrow: splenic sinusoid). D reveals a high-power magnification (X100) of the spleen with areas of cellularity admixed with mild congestion. Splenic sinusoidal dilation is apparent. (RP- Red pulp, WP- White pulp, black arrow: splenic sinusoid). E shows a high-power magnification (X100) of the spleen with normal histomorphologies presentation of the red pup and white pulp. (RP- Red pulp, WP- White pulp, black arrow: splenic sinusoid). F reveals a high-power magnification (X100) of the spleen with areas of cellularity admixed with mild congestion.
(RP- Red pulp, WP- White pulp, black arrow: splenic sinusoid). G shows a high-power magnification (X100) of the spleen with normal histomorphologies presentation of the red pup and white pulp. Splenic sinisoidal dilation is apparent. (RP- Red pulp, WPWhite pulp, black arrow: splenic sinusoid).
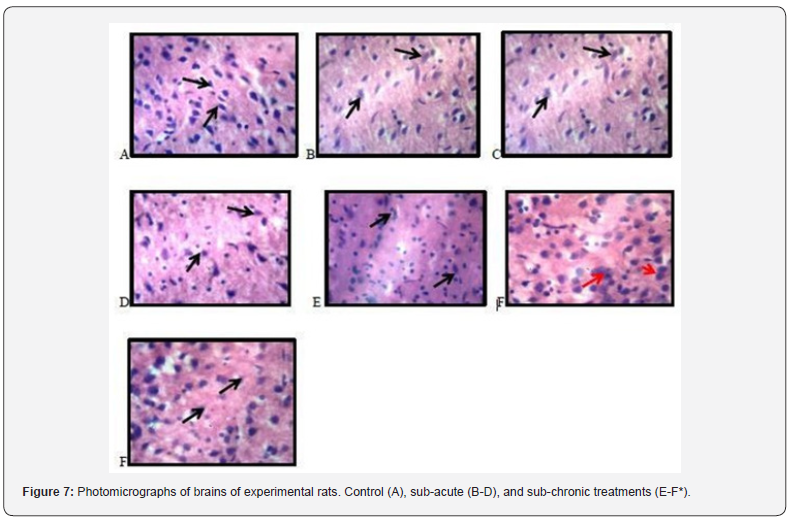
Histopathological examination of brains of experimental rats
Figure 7 presents the photomicrograph of the livers of treated and untreated experimental rats. A, B and C reveals typical cortical histomorphology with characteristic cellular delineation, distribution, density, staining intensity and characteristic cellular distribution and staining of the neuronal and nonneuronal cells in their respective neurophils. There are no signs of apoptosis or neurodegeneration D and E reveals typical cortical histomorphology with characteristic cellular delineation, distribution, density, staining intensity and characteristic cellular distribution and staining of the neuronal and non-neuronal cells in their respective neurophils. There are no signs of apoptosis or neurodegeneration. In the presentation of F, there appears to be cortical neuron presenting with pyknosis (red arrow) depicted from the dark stained cytoplasm and enlarged neurophil. F* shows typical cortical histomorphology with characteristic cellular delineation, distribution, density, staining intensity and characteristic cellular distribution and staining of the neuronal and non-neuronal cells in their respective neurophils.
Discussion
The use of natural product for treatment of different human ailment is increasing day by day. Toxicity evaluation of such natural products is very important for their safe and correct use. Sometimes the natural products may not be toxic but its higher dose may induce the toxicity [15]. This study was designed to evaluate the in vivo oral toxicity of Ocimum gratissimum essential oil, an essential oil with various medicinal values in male rat at different doses. The chemical components of Ocimum gratissimum essential oil were found to be carvacrol, caryophyllene, aromadendrene, D-Germacrene, alloaromadendrene, gamma-murolene, 1-methyl decyl benzene, 1-phenyloctylbenzene. They are responsible for the various biological activities of O gratissimum and its unique odor. Most of the compounds identified are fatty acid esters. The subacute and sub chronic in vivo oral toxicity of O. gratissimum was studied in rat model. The body weight of the male rats revealed a non-significant change compared with the control. In the biochemical studies carried out in this work, Total Cholesterol increased significantly in rats treated with 500mg/bw essential oil (Sub-acute). In sub chronic administration of O gratissimum, 500mg/bw essential oil, Total Cholesterol was reduced which may be due to the inhibition of 3-Hydroxy-3- methylglutaryl-CoA reductase which is a key enzyme that regulate hepatic cholesterol synthesis or by reduction in the expression of these enzyme [16]. AL P was reduced at 125mg/bw in subacute administration of O. gratissimum essential oil while creatinine and Dir bilirubin was reduced in sub chronic administration of O gratissimum essential oil but AST significantly increased which depict a liver damage thereby leading to the release of cytosolic enzymes into the blood stream [17].
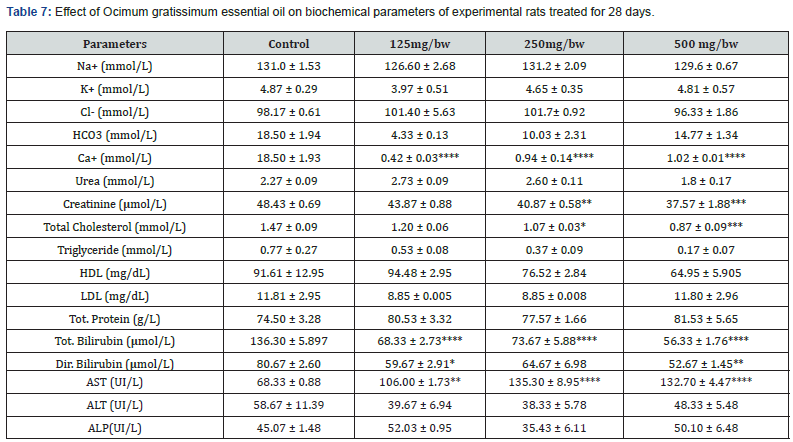
*Significant difference between control group and experimental group: ns implies non- significant difference; (*) = P < 0.05, (**) = P =<0.01, and (***) =P<0.001. Mean ± SEM (n=3).
Therefore, the fact that the oral administration of O. gratissimum essential oil produce changes in these biomarkers suggest presence of renal and hepatic toxicity. In hematological studies carried out in this work, there were no significant changes in the profile groups administered essential oil of O. gratissimum at the different doses given in male rats except for the significant decrease of MCV at 250mg/bw in subacute administration of O gratissimum essential oil. With the exception of MCV, no other hematological parameter was changed. This finding is consistent with the results obtained by Orafidiya et al. [8] who carried out studies on the acute and sub-chronic toxicity of the essential oil of Ocimum gratissimum L. leaf. This data indicated that the essential oil of O. gratissimum had no effect on the circulating blood cells or on their production. The analysis of blood parameters is important for risk evaluation as any changes in the hematological system have a higher predictive value for human toxicity when data are translated from human studies. In histopathological studies, the significant increase in the weight of the spleen observed may be due to the splenic sinisoidal dilation that is apparent. kidney gain more weight as a result of the significant increase of AST found in it and may also be due to inflammatory edema [18]. The renal veins were enlarged and congested with blood the renal tubules show wide lumen and separation of the epithelial cells from its membrane [19]. In lungs, some indicate degree of collapsed alveoli and thickening in the alveoli wall and also infiltration of different inflammatory cells in the alveoli wall was reported by [20]. Typically-sized halo spaced central vein surrounded sparsely, densely distributed hepatocytes were observed in the liver. Sinusoidal space dilation was also apparent. The brain weight in subacute was significantly reduce which may be due to the appearance of cortical neuron presenting with pyknosis depicted from the dark stained cytoplasm and enlarged neurophilie [21].
Conclusion
In conclusion, the results of this study reveal that O.gratissimum essential oil maybe toxic when given at high doses for a longer period of time which could be responsible for the clinical manifestations and the pathological changes observed in the organs and tissues of the treated rats
Acknowledgment
We acknowledge all authors for their contributions towards the success of this research.
Conflicts of interest
All authors declare that they have no conflict of interest associated with this research article.
References
- Liu C, Wright C, McAdam K, Taebunpakul C, Heroult J, et al. (2012) Arsenic Speciation in Tobacco and Cigarette Smoke. Beiträge Zur Tabakforschung International/Contributions to Tobacco Research 25(2): 375-380.
- Dhaware D, Deshpande A, Khandekar RN, Chowgule R (2009) Determination of Toxic Metals in Indian Smokeless Tobacco Products. The Scientific World Journal 9: 1140-1147.
- Prabhakar V, Jayakrishnan G, Nair SV, Ranganathan B (2013) Determination of Trace Metals, Moisture, pH and Assessment of Potential Toxicity of Selected Smokeless Tobacco Products. Indian J of Pharmaceutical Scie 75(3): 262-269.
- Ferreccio C, Yuan Y, Calle J, Benítez H, Parra RL, et al. (2013) Arsenic, Tobacco Smoke, and Occupation. Epidemiology (Cambridge, Mass.) 24(6): 898-905.
- Jiang C, Chen Q, Xie M (2020) Smoking increases the risk of infectious diseases: A narrative review.
- Omare MO, Kibet JK, Cherutoi JK, Kengara FO (2022) A review of tobacco abuse and its epidemiological consequences. Zeitschrift Fur Gesundheitswissenschaften 30(6): 1485-1500.
- Bhat A, O Hara T, Tian F, Singh B (2023) Review of analytical techniques for arsenic detection and determination in drinking water. Environmental Science: Advances 2(2): 171-195.
- Hughes MF, Beck BD, Chen Y, Lewis AS, Thomas DJ (2011) Arsenic Exposure and Toxicology: A Historical Perspective. Toxicol Sci 123(2): 305-332.
- Mishra S, Verma SK (2022) Methods to Detect Arsenic Compounds. In Arsenic in Plants pp. 345-366.
- Alidadi H, Ramezani A, Davodi M, Peiravi R, Paydar M, et al. (2015) Determination of Total Arsenic in Water Resources: A Case Study of Rivash in Kashmar City. Health Scope 4(3) Article 3.
- Valskys V, Hassan HR, Wołkowicz S, Satkūnas J, Kibirkštis G, et al. (2022) A Review on Detection Techniques, Health Hazards and Human Health Risk Assessment of Arsenic Pollution in Soil and Groundwater. Minerals. 12(10) Article 10.
- Campbell RC, Stephens WE, Meharg AA (2014) Consistency of arsenic speciation in global tobacco products with implications for health and regulation. Tobacco Induced Diseases 12(1): 24.
- Regassa G, Chandravanshi BS (2016) Levels of heavy metals in the raw and processed Ethiopian tobacco leaves. Springer Plus 5: 232.
- Satterlee HS (1956) The problem of arsenic in American cigarette tobacco. The New England Journal of Medicine 254(25): 1149-1154.
- Dhane AS, Sarode SC, Sarode GS, Sharma NK (2024) Rise in arsenic pollution and oral cancer: A call for action. Oral Oncology Reports 9: 100-238.






























