Heparin-induced Thrombocytopenia: A Narrative Review of Clinical Implications
Pushan Aggarwal1, Aishwarya Yannamani1, Jhon Nicol Navarro Gonzalez2, Javier Isaac Solorzano Leon3, Ghena Khasawneh4, Bayan Khasawneh4, Andrea Galecio Chao5, Antonia Lisseth Valle Villatoro3, Elsa Carolina Bonilla la Rosa6, Laura Sofía Trivino Cuellar7, Vivaswan Dutt Mishra8, Xavier F Vela Parada9 and Maria Isabel Gomez10*
1Kasturba Medical College, Manipal, Karnataka, India
2Universidad del Zulia. Venezuela
3Universidad de El Salvador. El Salvador
4Jordan University of Science and Technology, Jordan
5Universidad de Guayaquil, Ecuador
6Universidad Peruana Cayetano Heredia, Peru
7Fundacion Universitaria Juan N. Corpas, Colombia
8Moti Lal Nehru Medical College, Allahabad, India
9Berkshire Medical Center, Pittsfield, USA
10Universidad del Valle, México
Submission:April 24, 2023; Published: May 03, 2023
*Corresponding author: Maria Isabel Gomez, Department of Medicine, Universidad del Valle de Mexico, Mexico, USA
How to cite this article: Pushan A, Aishwarya Y, Jhon Nicol Navarro G, Javier Isaac Solorzano L, Ghena K, et al. Heparin-induced Thrombocytopenia: 002 A Narrative Review of Clinical Implications. Open Acc J of Toxicol. 2023; 5(4):555668. DOI: 10.19080/OAJT.2023.05.555668.
Summary
Heparin-induced thrombocytopenia is a rare immune-mediated complication of heparin therapy, characterized by a decrease in platelet count by more than 50% of the baseline. Typically occurs between 5 to 10 days after the initiation of heparin. Incidence in the United States ranges from 0.2% to 3.0%, depending on the population studied and the diagnostic criteria used. HIT has been categorized into two types: HIT type I and II. While HIT type I is a benign, non-immune mediated response presenting with mild self-limited thrombocytopenia, HIT type II is a severe, immune-mediated, potentially fatal complication that requires urgent diagnosis and management. Moreover, Type II is the most common form of HIT. Signs and symptoms may include a sudden onset of pain, redness, and swelling or ecchymosis in extremities. For diagnosis, the 4Ts score is a widely used clinical scoring system that can help assess the probability of HIT based on the timing of thrombocytopenia onset, degree of thrombocytopenia, presence of thrombosis, and other clinical factors. Diagnostic confirmation can be pursued with the help of immunoassays or functional assays such as ELISA and/or SRA. The first step in managing HIT is to discontinue all heparin exposure. Secondly, non-heparin anticoagulation medication must be initiated quickly. As a final step, it may be necessary to switch from parenteral to oral anticoagulation following the resolution of HIT.
2. Abbreviations: HIT: Heparin-Induced Thrombocytopenia; PF4: Platelet Factor 4; DIC: Disseminated Intravascular Coagulation; ELISA: Enzyme-Linked Immunosorbent Assay; SRA: Serotonin Release Assay; LMWH: Low Molecular Weight Heparins; UFH: Unfractionated Heparin; GAGs: Glycosaminoglycans; DVT: Deep Vein Thrombosis; PE: Pulmonary Embolism; CVC: Central Venous Catheter; HEP: Heparin-Induced Thrombocytopenia Expert Probability; HIPA: Heparin-Induced Platelet Activation Assay; DOAC: Direct Oral Anticoagulant; IVIG: Intravenous Immunoglobulin
Keywords: Heparin-induced thrombocytopenia; Heparin; Prevention; Diagnosis; Treatment
Introduction
Heparin-induced thrombocytopenia (HIT) is a rare immune-mediated complication of heparin therapy, characterized by a decrease in platelet count by more than 50% of the baseline, typically occurring between 5 to 10 days after the initiation of heparin therapy. HIT is a rare but potentially fatal condition in approximately 0.2-5% of patients exposed to heparin. There are two types of HIT: type 1 and type 2. Type 1 HIT is a mild, non-immune-mediated thrombocytopenia that occurs within the first 2 days of heparin therapy and typically resolves spontaneously without any sequelae. Type 2 HIT is an immune-mediated reaction that occurs 5 to 10 days after heparin exposure and is associated with the formation of platelet-activating antibodies, which can lead to thrombotic complications. Several risk factors have been identified for HIT, including prolonged exposure to unfractionated heparin, recent surgery, female gender, and history of HIT. While the exact etiology of HIT is not well understood, it is thought to be caused by the formation of antibodies against heparin-PF4 complexes, leading to platelet activation, aggregation, and thrombus formation.
Patients with HIT may present with various symptoms, including thrombocytopenia, skin necrosis, and thrombotic complications such as deep vein thrombosis, pulmonary embolism, arterial thrombosis, disseminated intravascular coagulation (DIC), death. The diagnosis is based on clinical suspicion and laboratory tests, including platelet factor 4 (PF4)/heparin enzyme-linked immunosorbent assay (ELISA) and serotonin release assay (SRA). Prevention of HIT involves careful monitoring of patients receiving heparin therapy, particularly those with known risk factors. In addition, alternative anticoagulants, such as direct oral or fondaparinux, may be considered in patients at high risk of developing HIT. The mainstay of treatment for HIT is the prompt cessation of heparin therapy and the initiation of alternative anticoagulation. In addition, patients with thrombotic complications may require treatment with thrombolytics or mechanical thrombectomy. In severe cases, treatment with immunoglobulins or plasmapheresis may be considered. The purpose of this narrative review is to enhance the overall comprehension of this uncommon but potentially fatal medical condition utilizing current literature evidence.
Epidemiology
Heparin-Induced Thrombocytopenia is a rare but potentially life-threatening complication of heparin therapy. According to epidemiological studies, the incidence of HIT in the United States ranges from 0.2% to 3.0%, depending on the population studied and the diagnostic criteria used [1]. HIT is more common in patients receiving unfractionated heparin than those receiving low molecular weight heparin. HIT risk factors include prolonged heparin exposure, female gender, and underlying conditions such as malignancy and autoimmune disease. Early recognition and prompt management of HIT are critical to prevent complications such as thrombosis and bleeding [2,3].
Etiology & Pathogenesis
Heparin is a negatively charged sulfated glycosaminoglycan with a high binding affinity for platelet factor-4 (PF4) [4]. Low molecular weight heparins (LMWH), molecular weight 2000–10 000 Daltons (Da), are produced by chemical or enzymatic processes from unfractionated heparins (UFH). UFH is a heterogeneous mixture of negatively charged sulfated glycosaminoglycan (3000-30 000 Da) derived from animal sources [5]. PF4 is a positively charged, heparin-neutralizing protein synthesized by megakaryocytes and stored in platelet alpha-granules. When platelets are activated at sites of vascular injury, PF4 is released locally and binds to negatively charged heparin-like GAGs, such as heparan sulfate, on the endothelial cell surface [6]. When heparin binds with PF4, it undergoes a conformational change and becomes immunogenic, generating heparin–PF4 antibodies (HIT antibodies), most frequently IgG. The heparin–PF4–IgG multimolecular immune complex then activates platelets via their FcγIIa receptors, causing the release of prothrombotic platelet‐derived microparticles, platelet consumption, and thrombocytopenia. These microparticles, in turn, promote excessive thrombin generation, frequently resulting in thrombosis. In addition, the antigen–antibody complexes also interact with monocytes, leading to tissue factor production, and antibody‐mediated endothelial injury may occur. Both of these latter processes may contribute further to the coagulation cascade activation and thrombin generation [7].
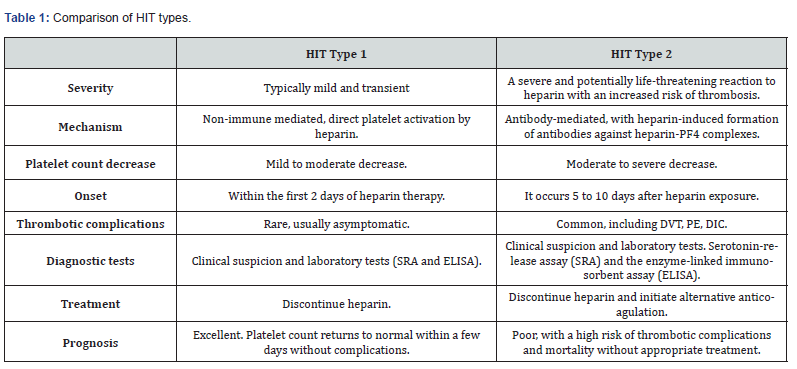
HIT has been categorized into two types: type I and II. HIT type I is a non-immune mediated response to heparin therapy. Its typical presentation includes mild thrombocytopenia (rarely below 100.000/mm3) within the first two days of treatment. It is a self-limited direct effect of heparin and normalization of platelet count occurring spontaneously without discontinuation of therapy [8]. On the other hand, HIT type II is an immune-mediated adverse effect. It represents a potentially catastrophic complication in which heparin administration must be discontinued as soon as possible at the time of clinical suspicion [9]. It commonly develops after five to ten days of treatment and manifests with more severe thrombocytopenia (<100.000 /mm3) or a decrease in platelet count to less than 50% of baseline values [10]. HIT type II occurs with a frequency of 0.5% to 5% of patients treated with unfractionated heparin. Risk factors for HIT type II can be categorized into drug- or host-related factors. Host-related risk factors include sex and age. According to Warkentin et al., there is a higher predisposition - twice the risk - for HIT development in females than in males [11]. An overview of the differences between HIT types 1 and 2 can be found in (Table 1).
Context of OH in EAC
Thrombocytopenia is the hallmark of HIT and is typically associated with exposure to heparin temporally. The most frequent indication of thrombocytopenia in HIT is a decline in platelet count, which occurs at least four days after heparin exposure and generally presents 5 to 14 days after exposure. In addition to thrombocytopenia, HIT causes a hypercoagulable state, and venous, arterial, or micro thrombosis may occur. Lower extremity deep vein thrombosis (DVT) with or without pulmonary embolism (PE), upper extremity central venous catheter (CVC)-associated DVT, splanchnic vein thrombosis, and cerebral vein/dural sinus thrombosis are all associated with HIT [12]. The signs and symptoms of HIT may include a sudden onset of pain, redness, and swelling in an arm or leg, as well as the development of ecchymotic lesions. Patients may also experience weakness, numbness, or pain with the movement of the affected limb. A rash or sore may also develop at the injection site following a heparin shot. Chills, fever, hypertension, tachycardia, shortness of breath, and chest pain may also occur [12].
Despite thrombocytopenia, bleeding is not classically associated with HIT. However, a recent study of 310 patients with suspected HIT found that significant bleeding occurred in 40.6% of patients and was no less common in HIT+ patients (40.9%) [13]. The most common type of bleeding is gastrointestinal, although intracranial hemorrhage, retroperitoneal bleeding, and other types of bleeding may occur. Less frequently, patients with HIT may develop heparin-induced skin necrosis, limb gangrene, or an anaphylaxis-type reaction following a heparin bolus. Rarer and perhaps under-recognized manifestations of HIT include adrenal failure/adrenal hemorrhage secondary to adrenal thrombosis and flap failure [12,14].
Diagnosis
Diagnosing HIT remains challenging due to the rising use of UFH/LMWH and the frequency of thrombocytopenia, especially among critically ill patients. Added to this are the difficulties caused by a poor understanding of the pathogenesis of this condition. Heparin-Induced thrombocytopenia cannot be diagnosed by clinical features or laboratory parameters alone but by combining both. A presumptive/suspected diagnosis can be established based on clinical findings (thrombosis, skin necrosis, etc.) and low platelet counts. Clinical manifestations of HIT can include a myriad of features- Thrombosis (DVT, PE, Cerebral veins, Dural thrombosis, Bleeding (less frequent than thrombosis), limb gangrene, Adrenal hemorrhage or insufficiency (often secondary to thrombosis), and occasionally even anaphylaxis type reactions [15]. The 4Ts score is a widely used clinical scoring system that can help assess the probability of HIT based on the timing of thrombocytopenia onset, degree of thrombocytopenia, presence of thrombosis, and other clinical factors [16-18]. Based on the clinical scoring of the 4Ts score/criteria, patients can be classified as low, intermediate, or high-risk categories [19]. Recently, a newer scoring system called the HIT expert probability (HEP) score has been developed by HIT experts and shown to have similar efficacy to 4Ts scoring [20,21].
In individuals with a presumptive diagnosis of HIT (positive pre-test score), confirmation can be sought with the help of Immunoassays or Functional assays [22]. These specialized biochemical tests can detect the presence of PF4 antibodies or antigen-antibody complexes that underlie the pathogenesis of HIT. Currently, the highest diagnostic sensitivity and specificity is awarded to Functional Washed Platelet Assays and Serotonin Release Assays (SRA)- tests that assess platelet activation in the presence of controlled fractions of patient serum, donor platelets, and heparin [23-25]. The SRA and ELISA are the most commonly used laboratory tests for HIT diagnosis. SRA is considered the gold standard, with a sensitivity and specificity of approximately 95% and 98%, respectively. However, it is a technically challenging and time-consuming assay and is not widely available. The ELISA is a more straightforward and widely available test, with a sensitivity and specificity of approximately 80-90% [16,17]. Other laboratory tests that can support the diagnosis of HIT include the heparin-induced platelet activation assay (HIPA), the platelet factor 4 (PF4)/heparin enzyme immunoassay, and the functional assay for heparin-induced platelet antibodies (HIPA-F). However, these tests have lower sensitivity and specificity than the SRA and ELISA [18].
Treatment & Prognosis
The suspicion index for diagnosing HIT is crucial in management since misdiagnosis raises the risk of bleeding and thrombosis. Using the 4Ts score helps us to classify individuals in different risk groups according to the likelihood of HIT [26,27]. For example, detecting HIT in patients with a low likelihood increases the risk of bleeding; failing to diagnose it when it is highly likely puts patients at risk of thromboembolic events [28]. The risk of thrombotic events following heparin withdrawal over the first 24 hours might range from 40% to 60%, posing a life-threatening situation [29,30]. A combination strategy using the 4Ts score and PF4/H-PaGIA is used to lessen the possibility of missing instances [27].
The first step in preventing HIT is to discontinue all heparin exposure; platelet transfusion may worsen the hypercoagulable state, resulting in additional thrombosis, and is thus not recommended until surgical intervention or severe thrombocytopenia is required [29,27]. Nevertheless, discontinuing heparin will not prevent antibody-mediated platelet activation leading to thrombosis [31]. Additionally, the time to discontinue heparin has shown no difference in the outcome since early heparin discontinuation is insufficient to prevent thrombotic events [30]. Non-heparin anticoagulation medication must be initiated quickly, which raises the question of which non-heparin anticoagulant is optimal for each patient. HIT has various treatment options, including direct thrombin inhibitors like argatroban, bivalirudin, fondaparinux, or direct oral anticoagulants (DOACs) like apixaban, rivaroxaban, or dabigatran [28,32]. Before prescribing an anticoagulant, there are some conditions to consider, as might affect the pharmacokinetics of the drugs, conditions such as chronic kidney disease, individuals that might need an invasive procedure, liver disease, or multiple organ dysfunction. Therefore, anticoagulant therapy will need a dose adjustment. (Table 2) depicts a summary of the different types of non-heparin anticoagulants. Argatroban, a thrombin inhibitor drug, is associated with a decrease in the occurrence of a thrombotic event and death from thrombosis [33]. It also has a short half-life, which means it can be discontinued quickly if required, either because of bleeding or the need to initiate invasive procedures [34]. However, this drug is metabolized in the liver, so it requires an adjustment in the initial dosage and multiple controls in patients with hepatic dysfunction whose serum levels of total bilirubin are higher than 1.5 mg/dL [35]. In patients with liver disease, another option, a hirudin analog called bivalirudin, has been shown to decrease the risk of subsequent thrombotic events. However, argatroban is associated with a lower risk of bleeding [36].
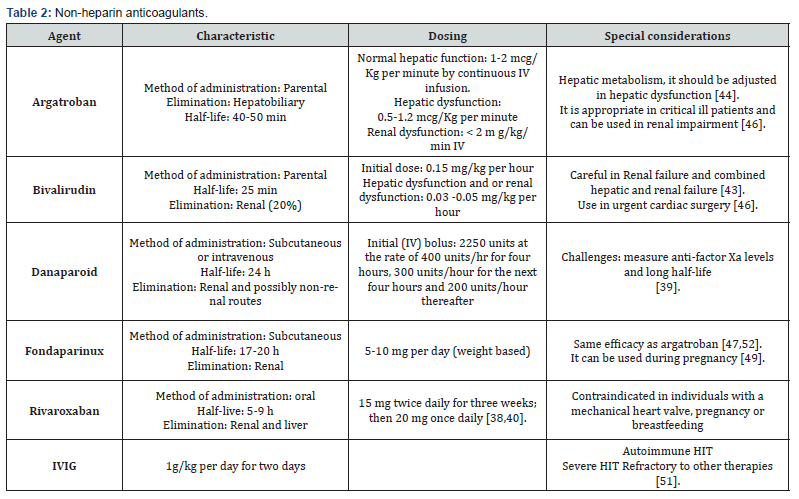
Following the resolution of HIT, a transition from parenteral anticoagulant (thrombin or factor Xa inhibitor) to warfarin or DOAC must occur. Warfarin can be begun only after a non-heparin anticoagulant treatment has been initiated, and the platelet count is at least 150,000/microL [37]. The risk of warfarin generates a thrombotic condition upon its quick onset, resulting in significant adverse effects such as skin necrosis and gangrene. Furthermore, warfarin should be used with a non-heparin anticoagulant for at least 5 days or until the INR is within the desired range [28]. The risk of thrombosis in a patient with HIT can be extended until approximately 4 weeks after starting HIT treatment. Therefore, anticoagulant therapy with non-heparin anticoagulant or warfarin should be continued for up to 3 months in patients with thrombosis secondary to HIT and up to 4 weeks in patients with isolated HIT [38].
Some alternative treatments for patients with refractory thrombocytopenia are intravenous immunoglobulin administration and therapeutic plasmapheresis [39]. On the one hand, therapeutic plasmapheresis consists of removing antibodies against heparin/PF4 large molecular weight complexes and has been described in patients requiring immediate cardiovascular surgery. Moreover, it is a choice / indicated when anticoagulants are contraindicated due to a bleeding event or in the case of refractory HIT [40,41]. On the other hand, the administration of high doses of intravenous immunoglobulin is still under study. For the time being, it is associated with the inhibition of HIT antibody-induced platelet-mediated activation and shows some benefit in patients with refractory thrombocytopenia [42] [43-61].
Conclusion
Heparin-induced thrombocytopenia (HIT) is a severe immune-mediated adverse reaction in patients receiving heparin therapy. It is characterized by a rapid decrease in platelet count and an increased risk of thrombosis, including deep vein thrombosis, pulmonary embolism, and arterial thrombosis. This drug-induced reaction is typically caused by forming antibodies against heparin-platelet factor 4 (PF4) complexes, leading to platelet activation and thrombosis. While HIT type 1 is a non-immune-mediated response to heparin and does not typically lead to thrombosis, HIT type 2 is an immune-mediated response that can result in significant morbidity and mortality. In addition, type 2 HIT is the more severe form, accounting for up to 90% of cases of HIT. Clinical presentation may include a drop in platelet count, new thrombosis or worsening of existing thrombosis, and skin lesions. Therefore, the diagnosis of HIT is based on clinical suspicion and laboratory testing, which may include platelet count, functional assays for HIT antibodies, and imaging studies to evaluate thrombosis. Prevention of HIT involves avoiding heparin therapy in patients with a history of HIT or at high risk for developing HIT. Alternative anticoagulant therapies such as direct oral anticoagulants, fondaparinux, or argatroban may be used in these patients. The treatment involves discontinuation of heparin therapy and initiation of alternative anticoagulation. In addition, in severe cases, immune globulin or plasmapheresis may be considered. Therefore, early recognition and management of HIT are crucial to prevent morbidity and mortality associated with this condition. Future research studies are still needed to understand HIT's pathophysiology further and develop more effective diagnostic and treatment strategies. Improvements in our understanding of the immune-mediated mechanisms involved in HIT could lead to more targeted therapies with improved patient outcomes. Additionally, studies evaluating the long-term outcomes of patients with HIT are needed to guide management strategies and improve patient outcomes.
References
- Warkentin TE (2003) Heparin-induced thrombocytopenia: pathogenesis and management. Br J Haematol 121(4): 535-555.
- Arepally GM (2017) Heparin-induced thrombocytopenia. Blood 129(21): 2864-2872.
- Greinacher A (2015) Heparin-induced thrombocytopenia. N Engl J Med 373(19): 1883-1884.
- Zinkovsky DA, Antonopoulos MS (2008) Heparin-Induced Thrombocytopenia: Overview and Treatment. Pharmacy and Therapeutics 33(11): 642-651.
- Katzung BG Basic and clinical pharmacology, 9th ed McGraw Hill 2004545-549.
- Arepally GM, Ortel TL (2010) Heparin-Induced Thrombocytopenia. Annual rev med 61: 77-90.
- Kelton JG, Smith JW, Warkentin TE, Hayward CP, Denomme GA, et al. (1994) Immunoglobulin G from patients with heparin‐induced thrombocytopenia binds to a complex of heparin and platelet factor 4. Blood 83 (11): 3232-3239.
- Fathi M (2018) Heparin-induced thrombocytopenia (HIT): Identification and treatment pathways. Glob Cardiol Sci Pract 2018(2): 15.
- Zinkovsky DA, Antonopoulos MS (2008) Heparin-induced thrombocytopenia: overview and treatment. P T 33(1): 642-651.
- Cuker A, Cines DB (2012) How I treat heparin-induced thrombocytopenia. Blood 119(10): 2209-2218.
- Warkentin TE, Sheppard JA, Sigouin CS, Kohlmann T, Eicher P, et al. (2006) Gender imbalance and risk factor interactions in heparin-induced thrombocytopenia. Blood 108(9): 2937-2941.
- Warkentin TE, Greinacher A (2004) Heparin-induced thrombocytopenia: recognition, treatment, and prevention: the Seventh ACCP Conference on Antithrombotic and Thrombolytic Therapy. Chest 126(3 Suppl): 311S-337S.
- Linkins LA, Dans AL, Moores LK, Bona R, Davidson BL, et al. (2012) Treatment and prevention of heparin-induced thrombocytopenia: Antithrombotic Therapy and Prevention of Thrombosis, 9th ed: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines. Chest 141(2 Suppl): e495S-e530S.
- Martel N, Lee J, Wells PS (2005) Risk for heparin-induced thrombocytopenia with unfractionated and low-molecular-weight heparin thromboprophylaxis: a meta-analysis. Blood 106(8): 2710-2715.
- Hogan M, Berger JS (2020) Heparin-induced thrombocytopenia (HIT): Review of incidence, diagnosis, and management. Vasc Med 25(2): 160-173.
- Warkentin TE, Greinacher A (2004) Heparin-induced thrombocytopenia: recognition, treatment, and prevention: the Seventh ACCP Conference on Antithrombotic and Thrombolytic Therapy. Chest 126(3 Suppl): 311S-337S.
- Linkins LA, Dans AL, Moores LK, Bona R, Davidson BL, et al. (2008) Treatment and prevention of heparin-induced thrombocytopenia: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines (8th Edition). Chest 133(6 Suppl): 340S-380S.
- Cuker A, Gimotty PA, Crowther MA, Warkentin TE (2012) Predictive value of the 4Ts scoring system for heparin-induced thrombocytopenia: a systematic review and meta-analysis. Blood 120(20): 4160-4167.
- Lee GM, Arepally GM (2013) Heparin-induced thrombocytopenia. Hematology Am Soc Hematol Educ Program 2013: 668-674.
- Cuker A, Arepally G, Crowther MA, Rice L, Datko F, et al. (2010) The HIT Expert Probability (HEP) Score: A novel pre-test probability model for heparin-induced thrombocytopenia based on broad expert opinion. J Thromb Haemost 8(12): 2642-2650.
- Pishko AM, Fardin S, Lefler DS, Padary K, Vega R, et al. (2018) Prospective comparison of the HEP score and 4Ts score for the diagnosis of heparin-induced thrombocytopenia. Blood Adv 2(22): 3155-3162.
- Andreas K, Michael N, Gabor E, Jerrold HL (2022) Heparin-induced Thrombocytopenia: Perioperative Diagnosis and Management. Anesthesiology 136(2): 336-344.
- Pouplard C, Amiral J, Borg JY, Laporte SS, Delahousse B, et al. (1999) Decision analysis for use of platelet aggregation test, carbon 14-serotonin release assay, and heparin-platelet factor 4 enzyme-linked immunosorbent assay for diagnosis of heparin-induced thrombocytopenia. Am J Clin Pathol 111(5): 700-706.
- Padmanabhan A, Jones CG, Curtis BR, Bougie DW, Sullivan MJ, et al. (2016) A novel PF4- dependent platelet activation assay identifies patients likely to have heparin-induced thrombocytopenia/thrombosis (HIT). Chest 150(3): 506-516.
- Warkentin TE, Arnold DM, Kelton JG, Sheppard JI, Smith JW, et al. (2018) Platelet-Activating Antibodies Are Detectable at the Earliest Onset of Heparin-Induced Thrombocytopenia, With Implications for the Operating Characteristics of the Serotonin-Release Assay. Chest 153(6): 1396-1404.
- Cuker A, Arepally GM, Chong BH, Cines DB, Greinacher A, et al. (2018) American Society of Hematology 2018 guidelines for management of venous thromboembolism: heparin-induced thrombocytopenia. Blood Advances 2(22): 3360-3392.
- Linkins LA, Bates SM, Lee AYY, Heddle NM, Wang G, et al. (2015) Combination of 4Ts score and PF4/H-PaGIA for diagnosis and management of heparin-induced thrombocytopenia: prospective cohort study. Blood 126(5): 597-603.
- Linkins LA, Dans AL, Moores LK, Bona R, Davidson BL, et al. (2012) Treatment and prevention of heparin-induced thrombocytopenia: Antithrombotic Therapy and Prevention of Thrombosis, 9th ed: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines. Chest 141(2 Suppl): e495S-e530S.
- Ahmed I, Majeed A, Powell R (2007) Heparin induced thrombocytopenia: Diagnosis and management update. In Postgraduate Medical Journal 83 (983): 575-582.
- Workman DL, Lewis BE, Steen L, Pifarre R, Moran JF (1999) Failure of early heparin cessation as treatment for heparin-induced thrombocytopenia. Am J Med 106(6): 629-635.
- Marini I, Uzun G, Jamal K, Bakchoul T (2022) Treatment of drug-induced immune thrombocytopenias. Haematologica 107(6): 1264-1277.
- Nilius H, Kaufmann J, Cuker A, Nagler M (2021) Comparative effectiveness and safety of anticoagulants for the treatment of heparin-induced thrombocytopenia. Ame J Hematol 96(7): 805-815.
- Lewis BE, Wallis DE, Hursting MJ, Levine RL, Leya F (2006) Effects of argatroban therapy, demographic variables, and platelet count on thrombotic risks in heparin-induced thrombocytopenia. Chest 129(6): 1407-1416.
- Ahmed I, Majeed A, Powell R (2007) Heparin induced thrombocytopenia: diagnosis and management update. Postgraduate medical journal 83(983): 575-582.
- Levine RL, Hursting MJ, McCollum D (2006) Argatroban therapy in heparin-induced thrombocytopenia with hepatic dysfunction. Chest 129(5): 1167-1175.
- Duewell BE, Briski MJ, Feih JT, Rinka JR, Tawil JN (2021) Argatroban versus bivalirudin in the treatment of suspected or confirmed heparin-induced thrombocytopenia. J Pharm Pract 34(4): 529-534.
- Hogan M, Berger JS (2020) Heparin-induced thrombocytopenia (HIT): Review of incidence, diagnosis, and management. Vasc Medicine 25(2): 160-173.
- Kearon C, Akl EA, Comerota AJ, Prandoni P, Bounameaux H, et al. (2012) Antithrombotic therapy for VTE disease: Antithrombotic Therapy and Prevention of Thrombosis, 9th ed: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines. Chest 141(2 Suppl): e419S-e496S.
- Arepally GM, Padmanabhan A (2021) Heparin-Induced Thrombocytopenia: A Focus on Thrombosis. Arterioscler Thromb Vasc Biol 41(1): 141-152.
- Marchetti M, Zermatten MG, Bertaggia Calderara D, Aliotta A, Alberio L (2021) Heparin-induced thrombocytopenia: a review of new concepts in pathogenesis, diagnosis, and management. J Clin Med 10(4): 683.
- Onuoha C, Barton KD, Wong EC, Raval JS, Rollins RMA, et al. (2020) Therapeutic plasma exchange and intravenous immune globulin in the treatment of heparin‐induced thrombocytopenia: a systematic review. Transfusion 60(11): 2714-2736.
- Warkentin TE (2019) High-dose intravenous immunoglobulin for the treatment and prevention of heparin-induced thrombocytopenia: a review. Expert rev hematol 12(8): 685-698.
- Berger JS, Marie H (2020) Heparin-induced thrombocytopenia (HIT): Review of incidence, diagnosis, and management. Vasc Med 25(2): 160-173.
- Favaloro EJ, Hellfritzsch M, Hvas AM (2021) Heparin-induced thrombocytopenia: pathophysiology, diagnosis and treatment. Expert Rev Hematol 14(4): 335-346.
- Cines DB, Gowthami MA (2020) Pathogenesis of heparin-induced thrombocytopenia. Transl Res 225: 131-140.
- Warkentin TE (2015) Heparin-induced thrombocytopenia. Curr Opin Crit Care 21(6): 576-585.
- Krzych ŁJ, Nowacka E, Knapik P (2015) Heparin-induced thrombocytopenia. Anaesthesiol Intensive Ther 47(1): 63-76.
- Ortel TL, Gowthami MA (2010) Heparin-induced thrombocytopenia. Annu Rev Med 61: 77-90.
- Solanki J, Shenoy S, Downs E, Palkimas S, Goldman S, et al. (2019) Heparin-Induced Thrombocytopenia and Cardiac Surgery. Semin Thorac Cardiovasc Surg 31(3): 335-344.
- Fabris F, Luzzatto G, Stefani PM, Girolami B, Cella G, et al. (2000) Heparin-induced thrombocytopenia. Haematologica 85(1): 72-81.
- Nilius H, Kaufmann J, Cuker A, Nagler M (2021) Comparative effectiveness and safety of anticoagulants for the treatment of heparin-induced thrombocytopenia. Am J Hematol 96(7): 805-815.
- Warkentin TE (2005) Heparin-induced thrombocytopenia. Dis Mon 51(2-3): 141-149.
- Lo GK, Juhl D, Warkentin TE, Sigouin CS, Eichler P, et al. (2006) Evaluation of pretest clinical score (4 T's) for the diagnosis of heparin-induced thrombocytopenia in two clinical settings. J Thromb Haemost 4(4):759-765.
- Danhof M, De Boer A, Magnani HN, Stiekema JC (1992) Pharmacokinetic considerations on Orgaran (Org 10172) therapy. Haemostasis 22(2): 73-84.
- Davis KA, Davis DO (2017) Direct acting oral anticoagulants for the treatment of suspected heparin-induced thrombocytopenia. Eur J Haematol 99(4): 332-335.
- Kiser TH, Fish DN (2006) Evaluation of bivalirudin treatment for heparin-induced thrombocytopenia in critically ill patients with hepatic and/or renal dysfunction. Pharmacotherapy 26(4): 452-460.
- Levine RL, Hursting MJ, McCollum D (2006) Argatroban therapy in heparin-induced thrombocytopenia with hepatic dysfunction. Chest 129(5): 1167-1175.
- Lobo B, Finch C, Howard A, Minhas S (2008) Fondaparinux for the treatment of patients with acute heparin induced thrombocytopenia. Thromb Haemost 99(1): 208-214.
- Mazzolai L, Hohlfeld P, Spertini F, Hayoz D, Schapira M, et al. (2006) Fondaparinux is a safe alternative in case of heparin intolerance during pregnancy. Blood 108(5): 1569-1570.
- Padmanabhan A, Jones CG, Pechauer SM, Curtis BR, Bougie DW, et al. (2017) IVIg for Treatment of Severe Refractory Heparin-Induced Thrombocytopenia. Chest 152(3): 478-485.
- Snodgrass MN, Shields J, Rai H (2016) Efficacy and Safety of Fondaparinux in Patients With Suspected Heparin-Induced Thrombocytopenia. Clin Appl Thromb 22(8): 712-717.






























