Heavy Metal Toxicity in Ayurvedic Drug Formulated using Tinospora cordifolia (Giloy) – An Approach to Probe the Veracity Behind the Blanket Assertions
Hareendran Nair J and Shan Sasidharan*
Department of Research and Development, Pankaja Kasthuri Herbal Research Foundation, Pankaja Kasthuri Ayurveda Medical College Campus, Kerala, India
Submission: September 27, 2021; Published: October 28, 2021
*Corresponding author: Shan Sasidharan, Department of Research and Development, Pankaja Kasthuri Herbal Research Foundation, Pankaja Kasthuri Ayurveda Medical College Campus, Kerala, India
How to cite this article: Hareendran Nair J, Shan S. Heavy Metal Toxicity in Ayurvedic Drug Formulated using Tinospora cordifolia (Giloy) – An Approach to Probe the Veracity Behind the Blanket Assertions. Open Acc J of Toxicol. 2021; 5(2):555657. DOI: 10.19080/OAJT.2021.05.555657.
Abstract
In Ayurveda, Tinospora cordifolia is considered a nectar plant and has been called “Amruth” in Sanskrit in recognition of its various medicinal properties. But nowadays, this plant is seriously blamed for inducing hepatotoxicity due to the presence of heavy metals in it. Unfortunately, heavy metals accumulate in natural settings due to multiple human activities such as industry, metallurgy, mining, chemical fertilizers containing heavy metals, and traffic activities. Heavy metals released into the air, soil, and environment can be absorbed by plants through their roots and leaves and accumulate in their parts. Furthermore, T. cordifolia has been shown to absorb heavy metals from the environment and store them in its parts, particularly warty tubercles (nodules). Hence, if T. cordifolia is collected for medicine preparation from heavy metal contaminated/industrialized area may lead to toxicity in humans. Therefore, the analysis of toxic metals in the raw material is pivotal before formulation by any pharmaceutical industry to avoid toxicity. Therefore, AYUSH should strictly control and implement quality assurance (QA)/quality control (QC) procedures in raw material handling and processing until converting them into quality finished goods to avoid unnecessary disparagement of the ayurvedic products. Further, before reporting the toxicity of any herbal drug, it is mandatory to conduct a systematic and scientific study to avoid misinterpretation about the outcome of the study. Since Ayurveda lacks systematic and scientific methods to avoid misinterpretation of such reports, the implementation of Pharmacovigilance in Ayurveda is a need of the hour to address this issue and make the system more scientific.
Keywords: AYSUH; Tinospora cordifolia; Urbanization; Heavy metal; Mercury; Arsenic; Herb-induced toxicity; Hepatotoxicity; Contamination; Pharmacovigilance in Ayurveda
Abbreviation: QA: Quality assurance; QC: Quality Control; GOI: Government of India; RCT: Randomised Clinical Trials; SMP: Standard Manufacturing Procedure
Introduction
Herbal medicines have found extensive use in disease treatment, prevention, and management. Due to the immense benefits, herbal medicines bring to bear, the majority of the world’s population in one way or the other, depending on them for various health benefits. According to the world health organization report, an estimated 65 to 80% of the world’s population relies on traditional (alternative) medicine as their primary form of healthcare [1]. A large number of plants are being used in medicine for therapeutic purposes in India. Out of these, Tinospora species play a pivotal role as a major ingredient in several Ayurvedic formulations. The three main Tinospora species are commonly available: Tinospora cordifolia, Tinospora crispa and Tinospora malabarica. Out of these three species, T. cordifolia also known as Guduchi/Giloy is widely used in various traditional Ayurvedic formulations for several decades.
Tinospora cordifolia- a wonder herbal medicinal plant
T. cordifolia commonly called Guduchi (Giloy) is a natural herbaceous creeper belonging to the moonseed family Menispermaceae. This plant is very useful in treating several diseases like jaundice, skin diseases, gout, diabetes etc. which has been established in the history of traditional medicine practices for several centuries. In this perspective, T. cordifolia is considered a nectar plant and has been called “Amruth” in Sanskrit in recognition of its detoxifying, rejuvenating, and immune-boosting properties [2]. The root of T. cordifolia is used as a potent emetic and for bowel obstruction. The starch of this plant serves as a beneficial household remedy for chronic fever, relieves burning sensation, increases energy and appetite. It is useful in treating helminthiasis, heart diseases, leprosy, and rheumatoid arthritis, supporting the immune system, the body’s resistance to infections, and supporting standard white blood cell structure, function, and levels [3]. It also helps in digestive ailments such as hyperacidity, colitis, worm infestations, loss of appetite, abdominal pain, excessive thirst, and vomiting, and even liver disorders like hepatitis [4,5].This pharmacological activities of the plant is due to its chemical constituents like diterpenoid lactones, glycosides, steroids, sesquiterpenoid, phenolics, aliphatic compounds, essential oils, a mixture of fatty acids, and polysaccharides and is present in a different part of the plant body, including root, stem, and whole part [6]. In modern medicine, this medicinal has been evaluated and studied more profoundly and recently the drug has been implemented to mitigate the negative effects of chemotherapy in cancer patients. Some of the imperative formulations prepared from T. cordifolia are: Guduchi taila, Sanjivani vati, Kanta-Kari avaleha, Guduchyadi churna, Guloochyadi kashayam, Chyavnaprasha, Guduchu ghrita, Guduchi satva, Brihat guduchi taila, Amrita guggulu, amritashtaka churna and many more. So, it is clear that T. cordifolia is the most important medicinal herb considered by the ancient rishis in Vedic times with great potential (medicinal qualities) to cure several diseases.
Recent report on Tinospora cordifolia induced toxicity
A recent study published in the Journal of Clinical and Experimental Hepatology reported that the herbal immune booster induced liver injury during COVID-19 may arise from direct and indirect mechanisms through the metabolites of the herb or their interactions with other drugs, including contaminants. The researchers reported that the major culprit behind this toxicity is T. cordifolia, a key herb commonly used in the preparation of herbal immune booster. Further, the consumption of T. cordifolia reportedly surged up during the first wave of the COVID-19 pandemic [7]. Responding to this report, the ministry of AYUSH repelled the claims by supplying substantial data to back up the medicinal benefits of T. cordifolia. Moreover, the ministry also zeroed in on the case study and discovered that the liver injury in six people was due to pre-existing and underlying medical conditions. AYUSH also stated that “liver damage would be misleading and disastrous as it has been used in Ayurveda since long”. The Government of India (GOI) alongside AYUSH also condemned the study and arrayed it as a delusive and incompatible result which is disparaging India’s indigenous medicinal system. In addition to this, GOI highlighted that the researchers have made this oblivious presumption by assessing mere 6 candidates against the demography of billions, and also alleged that the researchers are purportedly trying to make this report prevalent. Karousatos [8] also reported that three patients presented with acute hepatocellular injury and jaundice after taking Ayurvedic supplements for 90–120 days. In this case, the one of the patients consumed Giloy Kwath, a drug solely formulated using T. cordifolia [8].
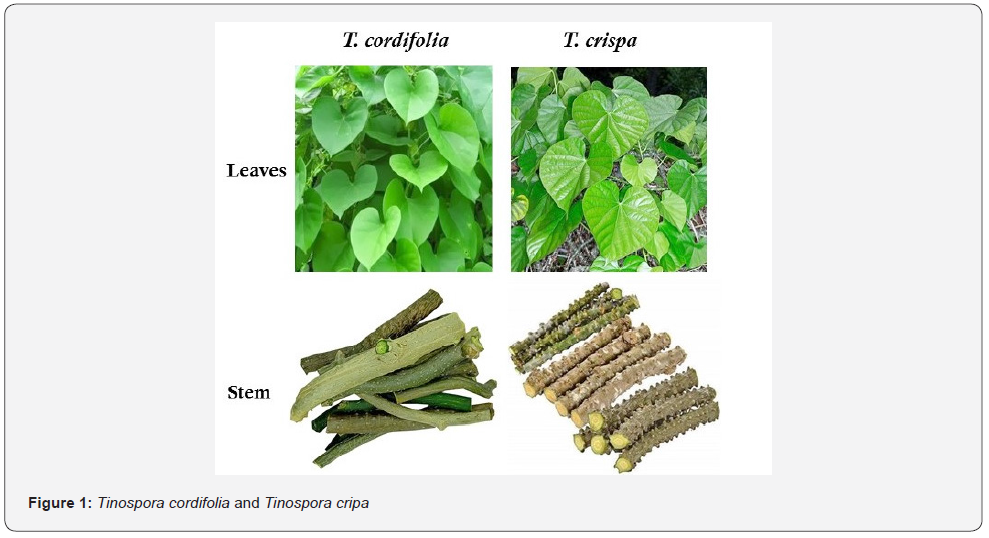
Tinospora crispa is an herbaceous vine that grows extensively in the tropical and subtropical regions of Southeast Asia. The decoction from the stem of T. crispa is used to inhibit inflammation, decrease thirst, enhance hunger, and cool down body temperature as an antipyretic to maintain good health. Since T. crispa or its medicinal potential is not mentioned anywhere in the classics of Ayurveda, no Ayurvedic drugs are available yet on the market based on this formulation. Nonetheless, an increasing number of cases revealed that T. crispa might have an attribute to induce hepatotoxicity [9,10]. Often deceived by T. crispa for T. cordifolia due to its persona and facade (Figure 1), unbeknownst to its hepatotoxicity, people tend to consume T. crispa to enhance and maintain their liver function. In fact, many studies point out that identifying the herb incorrectly could lead to erroneous results. As it said earlier, if we are using a similar-looking herb, T. crispa instead of T. cordifolia may have a worst impact on liver and its functions. Thus, T. crispa may act as a substitution or adulteration for T. cordifolia. Considering this, AYUSH ministry or APl should stipulate and implement precise Pharmacognostic identification of T. cordifolia to avoid the imperilment caused by T. crispa. Hence, before labelling T. cordifolia, drug manufacturers should ensure the integrity of this drug component by following the standard herbal authentication guidelines. In addition to this, if any proprietary or patented medicine manufacturers want to use T. crispa, they should thoroughly purify and decontaminate it by removing the epidermis and warty tubercles (nodules).
Recently, a Cochin-based (Kerala, India) hepatologist/ gastroenterologist published an article in the Elsevier journal named Journal of Clinical and Experimental Hepatology about the death of a 24-year-old woman who had taken herbal supplements from a renowned company. Further, he reported that the examination of these herbal supplements revealed the presence of heavy metals, bacteria and other toxic compounds. However, the drug manufacturer resisted the journal by pointing that the original article was deficient in significant stats and data. They have also figured out that the researcher applied inappropriate analytical methodologies and incomplete investigational protocols to achieve this crafty and crooked conclusion. Comprehending these rational claims and appreciating the serious flaw, Elsevier took extraordinary steps to remove the article from the journal, stating that the scientific methodology, analysis and interpretation of data underlying the article were insufficient for the conclusion drawn by the authors [11]. Adding to this, in an interview with a Kerala-based YouTube channel, the aforesaid hepatologist reiterated this blanket assertion that liver injuries are common in patients who were administered herbs or herbal medicines. Unluckily, his interview went viral across the state which ended up in deceiving a large-scale community. Unfortunately, the horrid thing is, most of his conclusions were drawn based on his studies conducted on just one or two patients. Moreover, he is mainly targeting herbal medicine that comes under AYUSH systems, and his unscientific action has tarnished the image of AYUSH systems.
The conclusion of most of these published articles and videos on human toxicity was based on the study conducted on a few patients (case studies) which are smearing the credibility of Ayurveda, a world-renowned medicinal system that has been practicing for several centuries with significant benefits to people. Subsequently, suppose anyone want to ascertain the toxicity of any drugs, that include herbals, it is mandatory to conduct stringent ADR and Pharmacovigilance studies followed by Randomised Clinical Trials (RCT). Evidence-based studies on the safety of the drug and metaanalysis are also the prerequisites to pronounce the claims. All these analyses should be strictly adhered to make a resultant conclusion. In fact, rigorous verifications of all these claims by the respective researchers and their studies found that they have failed to follow the science to achieve a credible culmination. Hence, we can figure out that all these case studies showcase the contusive behavior of the researchers who are relentlessly trying to do nothing but disrepute the traditional medicinal system of India. In stark contrast, despite some media propagating false news on the side effects of vaccines and certain medicines, no one is literally lending their ears to it. We need not just unprejudiced researchers but earnest studies that abide valid science to manifest such kind of validations. Sadly, some or most of the researchers who published the above-mentioned articles are keeping a derogatory approach in their minds and trying to impose a malicious agenda to disgrace a system for their personal welfare. Since Ayurveda lacks systematic and scientific methods to avoid misinterpretation of such reports, the implementation of Pharmacovigilance in Ayurveda is a need of the hour to address this issue and make the system more scientific.
T. cordifolia induce toxicity as it is well known for hepatoprotective property
There are several scientific reports available for the hepatoprotective property of T. cordifolia. Chavan [12] reported that T. cordifolia could be used as a liver tonic that can help to restore and strengthen liver functions [12]. Another study conducted by Sharma and Pandey reported the importance of T. cordifolia stem and leaves extract as a possible hepatoprotective effect in Swiss albino male mice against lead nitrate-induced toxicity. Their study clearly portrayed that T. cordifolia could afford protection against lead-induced hepatic damage [13]. In addition to this, Baskaran [14] also reported the protective effect of T. cordifolia stem methanolic extract on cadmium-induced hepatotoxicity. Cadmium pollution is of serious concern due to its toxic effects on humans and animals [14]. Recent studies on T. cordifolia have shown that it has protective effects against both hepatotoxic and immunotoxin consequences from carbon tetrachloride intoxication, and it also inhibits hepatocellular carcinoma, induced by diethylnitrosamine [15, 16]. Furthermore, T. cordifolia has been reported to effectively prevent hepatotoxicity induced by antitubercular drugs in rats and humans [17]. Further, the combination of Andrographis paniculata (Kalmegha), T. cordifolia (Guduchi), and Solanum nigrum (Kakmachi) was effective against paracetamol-induced hepatotoxicity [18]. All these studies support the hepatoprotective property of T. cordifolia. Then a question will come about how this plant can cause liver toxicity? So, before coming to any conclusion regarding a plant’s toxicity known for its miracle medicinal properties, the authors should conduct various studies like animal toxicity, clinical research, etc. to confirm its toxicity.
Various factors affecting heavy metal accumulation in the medicinal plants
The geoclimatic/environmental conditions of the region and the modern agricultural practices decide the level of heavy metals that could accumulate in medicinal plants. Thus, the primary factor affecting heavy metal toxicity is from where the medicinal plants are collected. If the medicinal plants are collected from an urbanized or industrial area, the chance of heavy metal contamination is more. This was supported by the research conducted by Barthwal [19], where they reported that lead and cadmium were more in the soil of heavy traffic and indusial area sites, possibly due to heavy vehicular traffic or industrial activity [19]. Plants growing at the roadside may be exposed to high levels of metal pollution, especially vehicle emissions and trace content in the air. Feng [20] suggested heavy metal from traffic emission may accumulate in roadside plants from the soil [20].
Meanwhile, other researchers reported that airborne heavy metals could be deposited and absorbed on the leafy part [21,22]. Cadmium, copper, and lead may originate from tires, engine oil consumption, brake wear, and road surface material [23-25]. Heavy metals from traffic activities could accumulate in the soil before being absorbed by plant roots. Since heavy metals are resistant and stay in the plant for a long time [26], they may be transferred to humans via the food chain [27]. This may be due to different anthropogenic factors such as industrial activities, population and settlement patterns, and differences in traffic density. The levels also depend on the soil concentrations of those heavy metals that are available naturally. Furthermore, heavy metal concentrations in roadside plants were higher than those in the same plant species from the uncontaminated area. This indicates that human activities (traffic) do influence the metal concentration in the plants. Perhaps high-metal concentrations were found at those sampling sites where human activities are more intense [28,29]. So, proper processing and standardization are critical to removing the heavy metals from the raw materials collected from contaminated areas. Therefore, the world health organization recommends that medicinal plants, which form the raw materials for most herbal remedies, should be checked for the presence of heavy metals. The maximum permissible limit of heavy metal as per API is mercury (1 ppm), cadmium (0.3 ppm), lead (10 ppm) and arsenic (3 ppm).
Scientific proof of heavy metal absorption by T. cordifolia
Several scientific reports are available to substantiate that T. cordifolia will absorb the heavy metals from the soil and store them in its parts. Sahu [29] reported that T. cordifolia is a biosorbent used for removing cadmium ions from industrial effluents [30]. Another study conducted by Vyas [30] reported that T. cordifolia effectively removes lead, iron, phosphate, and arsenic from water [31]. In the case of T. Cordifolia, the heavy metals will be accumulated in the warty tubercles seen on the stems. These warty tubercles were absent in the early growth stages of T. cordifolia. When it grows older, the warty tubercles will develop due to these plants’ absorption and accumulation of heavy metals. So, suppose these warty tubercles are not removed during the preparation of Ayurvedic formulations. In that case, the heavy metals accumulated in the warty tubercles will contaminate the formulations and lead to toxicity. Thus, continuous use of heavy metals contaminated Ayurvedic drugs may lead to multiple health hazards in humans, predominantly renal and hepatic toxicity.
How to prepare clean and decontaminate T. Cordifolia - need of a standardized uniform procedure
Non-availability of standard references and lack of Standard Manufacturing Procedures (SMPs) are the prime causes of nonuniformity in production. Standardization implies applying suitable methods and processes by which optimal conditions are ensured for obtaining predictable results and products that conform to a specific set of standards in terms of quality, purity, stability, and safety. Hence, to maintain the uniformity of production at the market label, the study was undertaken to validate that the technical manufacturing process is compliant with traditional principles. Satwa is the essence of the material or drug. About the Satwa of an herbal medication is a waterextractable solid substance. Plants with the medicinal value present in their starchy parts are subjected to a unique method to extract the same. It is an important Kalpana (preparation) among the other pharmaceutical processes used in the general practice of Ayurveda. Nimajjana (sinking/dipping), Manthana (churning or rubbing) and Sosana (drying) are the Samskaras, in which Toya & Agni sarnikarsa (water & heat treatment) are the specific methods of pharmaceutical process or techniques that modify the natural product into a potent dosage form which is easily absorbable in the biological system [32].
Heavy metal contamination and its associated problems in the pharmaceutical industry.
Medicinal plants have been cited as a potential source of heavy metal toxicity to both man and animals. The most common heavy metals implicated in human toxicity include lead, mercury, arsenic, and cadmium, although aluminum and cobalt may also cause toxicity. The contamination of heavy metals in the Ayurvedic plants is a major constraint to the pharmaceutical manufacturers to maintain stringent quality and broader safety profile and deliver standard and safe contaminant free finished products to the consumers. Ultimately, it is becoming a severe health-related social problem leading to several illnesses and serious diseases like kidney, liver damage, and cancer. The contamination process increases throughout the journey of crude drugs from agriculture sites to pharmaceutical industries. Lack of proper quality assurance and quality control mechanism allows all these contaminated crude drugs to enter into the production system and carries all this contaminant to finished products and ultimately to the consumers. The controlling of entrapment of contaminants from agricultural site to industry is not an easy job and it needs coordination and control from both governmental and individual sides, and it is a distant goal in the current scenario.
Conclusion
Contamination in herbal medicines is well demonstrated and clearly poses a serious potential risk to health. The analysis of toxic metals in the raw material is absolutely necessary before going for formulation. Hence, we have already started a study to verify the presence of heavy metals in the Tinospora species collected from various locations. The preliminary results clearly showed that Tinospora species collected from urban areas contain much more heavy metals than those collected from rural areas. Further, before reporting the toxicity of any herbal drug, it is absolutely necessary to conduct a systematic and scientific study to avoid misinterpretation about the outcome of the study. Therefore, AYUSH should strictly control and implement quality assurance /quality control procedures in raw material handling and processing until converting them into quality finished goods to avoid unnecessary disparagement of the ayurvedic products. Further, before reporting the toxicity of any herbal drug, it is mandatory to conduct a systematic and scientific study to avoid misinterpretation about the outcome of the study. Since Ayurveda lacks systematic and scientific methods to avoid misinterpretation of such reports, the implementation of Pharmacovigilance in Ayurveda is a need of the hour to address this issue and make the system more scientific.
Acknowledgments
Authors give adored Pranams to “Aadhyathma Chinthalayesan”, Chinthala Ashram, Pothencode, Trivandrum, Kerala, India for his benevolence and blessings. We sincerely acknowledge Pankajakasthuri Herbal Research Foundation, Kattakada, Thiruvananthapuram, Kerala, India and Pankajakasthuri Herbals India Pvt. Ltd Poovachal, Kattakada, Thiruvananthapuram, Kerala, India for providing necessary support to conduct studies.
References
- Geneva Switzerland (1998) Quality control methods for medicinal plant materials.
- Tiwari P, Nayak P, Prusty SK, Sahu PK (2018) Phytochemistry and Pharmacology of Tinospora cordifolia: A Review. Sys Rev Pharm 9(1): 70-78.
- Sinha K, Mishra NP, Singh J, Khanuja SPS (2004) Tinospora cordifolia (Guduchi) a reservoir plant for therapeutic applications. Indian Journal of Traditional Knowledge 3(3): 257-
- Salkar K, Chotalia C, Salvi R (2017) Tinospora cordifolia: an antimicrobial and immunity enhancer plant. IJSR 6(3): 1603-1607.
- Upreti P, Chauhan RS (2018) Effect of leaf powder of giloy (Tinospora cordifolia) in fish feed on survival and growth of post larvae of Catla catla. ANSF 10(1): 144-148.
- Khan MM, dul Haque MS, Chowdhury MS (2016) Medicinal use of the unique plant Tinospora cordifolia: evidence from the traditional medicine and recent research, Asian J Med Biol Res. 2(4): 508-512.
- Nagral A, Adhyaru K, Rudra OS, Gharat A, Bhandare S (2021) Herbal Immune Booster-Induced Liver Injury in the COVID-19 Pandemic - A case series. J Clin Exp Hepatol.
- Karousatos CM, Lee JK, Braxton DR, Fong TL (2021) Case series and review of Ayurvedic medication induced liver injury. BMC Complement Med Ther 21(1): 91.
- Denis G, Yann Gérard, Sevser Sahpaz, Rémi Laporte, Nathalie Viget, et al. (2007) Malarial prophylaxis with medicinal plants: toxic hepatitis due to Tinospora crispa. Therapie 62(3):271-272.
- Langrand J, Regnault H, Cachet X, Bouzidi C, Villa AF, et al. (2014) Toxic hepatitis induced by a herbal medicine: Tinospora crispa. Phytomedicine 21(8-9): 1120-1123.
- Philips CA, Augustine P, Rajesh S, John SK, Valiathan GC, et al. (2019) Slimming to the Death: Herbalife-Associated Fatal Acute Liver Failure-Heavy Metals, Toxic Compounds, Bacterial Contaminants and Psychotropic Agents in Products Sold in India. J Clin Exper Hepatol 9(2): 268-272.
- Chavan T, Ghadge A, Karandikar M, Pandit V, Ranjekar P, et al. (2017) Hepatoprotective activity of Satwa, an ayurvedic formulation, against alcohol-induced liver injury in rats. Altern Ther Health Med 23(4): 34-40.
- Sharma V, Pandey D (2010) Protective Role of Tinospora cordifolia against Lead-induced Hepatotoxicity. Toxicol Int 17(1): 12-17
- Baskaran R, Priya LB, Sathish KV, Padma VV (2018) Tinospora cordifolia extract prevents cadmium-induced oxidative stress and hepatotoxicity in experimental rats. J Ayurveda Integr Med 9(4): 252-257.
- Bishayi B, Roychowdhury S, Ghosh S, Sengupta M (2002) Hepatoprotective and immunomodulatory properties of Tinospora cordifolia in CCl4 intoxicated mature albinorats. J Toxicol Sci. 27: 139-146.
- Dhanasekaran M, Baskar AA, Ignacimuthu S, Agastian P, Duraipandiyan V (2009) Chemopreventive potential of Epoxy clerodane diterpene from Tinospora cordifolia against diethylnitrosamine-induced hepatocellular carcinoma. Invest New Drugs 27(4): 347-355.
- Manjrekar PN, Jolly CI, Narayanan S (2000) Comparative studies of the immunomodulatory activity of Tinospora cordifolia and Tinospora sinensis. Fitoterapia 71(3): 254-257.
- Singh DP, Awasthi H, Luqman S, Singh S, Mani D (2015) Hepatoprotective effect of a polyherbal extract containing Andrographis Paniculata, Tinospora Cordifolia and Solanum Nigrum against paracetamol induced hepatotoxicity. Phcog Mag 11(Suppl 3): S375-379.
- Barthwal J, Smitha Nair, Poonam Kakkar (2008) Heavy Metal Accumulation in Medicinal Plants Collected from Environmentally Different Sites. Biomedical and Environmental Sciences 21(4): 319-324.
- Feng J, Wang Y, Zhao J, Zhu L, Bian X, et al. (2011) Source attributions of heavy metals in rice plant along highway in Eastern China. J Environ Sci 23(7): 1158-1164.
- Nabulo G, Oryem-Origa H, Diamond M (2006) Assessment of lead, cadmium, and zinc contamination of roadside soils, surface films, and vegetables in Kampala City, Uganda. Environ Res 101(1): 42-52.
- Shahid M, Dumat C, Khalid S, Schreck E, Xiong T, et al. (2017) Foliar heavy metal uptake, toxicity and detoxification in plants: a comparison of foliar and root metal uptake. J Hazard Mater 325: 36-58.
- Chen X, Xia X, Zhao Y, Zhang P (2010) Heavy metal concentrations in roadside soils and correlation with urban traffic in Beijing, China. J Hazard Mater 181(1-3): 640-646.
- Zhang M, Yan X, Zhang F, Zeng C, Devkota LP (2012) Factorial analysis of heavy metal concentration in roadside farmland plants around Kathmandu, Nepal. Appl Mech Mater 181: 1016-1021.
- Ugolini F, Tognetti R, Raschi A, Bacci L (2013) Quercus ilex L. as bioaccumulator for heavy metals in urban areas: effectiveness of leaf washing with distilled water and considerations on trees distance from traffic. Urban for Urban Green 12(4): 576-584.
- Boularbah A, Schwartz C, Bitton G, Aboudrar W, Ouhammou A, et al. (2006) Heavy metal contamination from mining sites in South Morocco: 2. Assessment of metal accumulation and toxicity in plants. Chemosphere 63(5): 811-817.
- Kofi Annan, Rita A Dickson, Isaac K Amponsah, Isaac k Nooni (2013) The heavy metal contents of some selected medicinal plants sampled from different geographical locations. Pharmacognosy Res 5(2): 103-108.
- Sulaiman FR, Hamzah HA (2018) Heavy metals accumulation in suburban Roadside plants of a tropical area (Jengka, Malaysia). Ecological Processes 7: 28.
- Sahu C, Pandey PK, Khan F, Pandey M (2018) Biosorptive Removal of Cadmium by Tinospora cordifolia (Wild Giloy). Water Environ Res 90(6): 554-562.
- Vyas G, Bhatt S, Paul P (2019) Tinospora cordifolia derived biomass functionalized ZnO particles for effective removal of lead(II), iron(III), phosphate and arsenic(III) from water. RSC Adv 59: 34102-34113.
- Shastri SN (1993) Vimansthana, Charak Samhita, Chaukhamba Bharati Academy, Varanasi 21(2): 680.
- Dalai S (2020) Traditional Manufacturing Procedure of Guduchi Satwa; Sedimental Water Insoluble Starchy Extract of Tinospora cordifolia Miers & its Process Validation. Int J Pharma Res Health Sci 8(4): 3207-3213.






























