Impact of Manganese Cobalt Ferro-Magnetite Composite Nanoparticles on Hypothalamic- Pituitary-Gonadal Axis Disturbance Induced by Vanadium Exposure in Adult Male Rat
Mohamed M Rezk* and Tarek F Mohammaden
Department of Isotopes Geology, Nuclear Materials Authority, Egypt
Submission:May 01, 2019;Published:July 11, 2019
*Corresponding author:Mohamed M Rezk, Department of Isotopes Geology, Nuclear Materials Authority Cairo, Egypt
How to cite this article: Md M Rezk, Tarek F M. Impact of Manganese Cobalt Ferro-Magnetite Composite Nanoparticles on Hypothalamic-Pituitary-Gonadal Axis Disturbance Induced by Vanadium Exposure in Adult Male Rat. Open Acc J of Toxicol. 2019; 4(1):555629. DOI: 10.19080/OAJT.2019.04.555629.
Abstract
Vanadium which used in various industrial applications, might lead to pathological and irreversible damage effect in tissues and organs where it can accidently enter. Rats were distributed into four groups discriminated as; control, vanadium (V), manganese cobalt ferro-magnetite composite nanoparticles (MNPs) and V-MNPs. The rats underwent four decapitations after the 3rd, 7th, 10th and 15th day. The oral administration by 20mg/Kg b.wt. of vanadium lead to its accumulation in hypothalamus brain area and tests causing a disturbance in hypothalamic-pituitary-gonadal axis appears in the significant decrease of testosterone, FSH and LH hormone levels and GSH level, also in the significant increase of MDA level which may backed to vanadium ability to inhibit the adenylate cyclase, disturb the mitochondrial function and down-regulated the steroidogenic enzymes. On the other hand, the IP administration of 5% MNPs showed its hormone enhancement ability for testosterone, FSH and LH which appears at the end of the experiment, also it induces a significant decrease in MDA and increase in GSH levels. MNPs effects could be due to the essentiality of the three involved metal oxide to the body. So, The Mn-Co-Fe nanoparticle seems as good candidate performs preventive and/or reactionary roles against vanadium exposure hazards.
Keywords: Vanadium; Ferro-Magnetite; Nanoparticles; Rats, Hypothalamus; Tests
Introduction
Vanadium occupies the 21st rank of the elemental abundance in the Earth’s crust and used in various applications such as; steel fabrication, oil industry, energy storage etc.… [1]. However, receiving high and undesirable doses of vanadium might lead to pathological and irreversible changes in tissues and organs where it can enter the body via the lungs and, more commonly, the stomach. When it reaches the blood, vanadium is distributed to the body tissues and bones leading to some gastrointestinal symptoms e.g. diarrhea, vomiting, general dehydration with weight reduction, intestinal inflammation and the characteristic green tongue [2] Other bio-hazards include; hematological and biochemical alterations [3], loss of body weight and nephrotoxicity [4] and immunotoxicity and behavioral toxicity [5]. Moreover, vanadium has been classified within the group 2B carcinogen [6].
Recently, utilization of metal and metal-oxide magnetic nanoparticles for biohazard and biomedicine treatment attracted more attention particularly they are easily cleaned by the body macrophages which engulf these magnetic nano particles (MNPs) via the phagocytosis [7,8].
The ferromagnetic transition metals oxides (ferrite) are typical magnetic nano-particles (MNPs), its properties can be enhanced by its conjunction with other divalent metallic ions [9]. Iron, cobalt and manganese are essential trace nutrients play an important role in the general health and fertility. Iron (Fe) is a physiological component in the cells and tissues of the male reproductive system and about 70% of the total- body iron is associated with hemoglobin [10]. The major biological activity of cobalt (Co) is synthesizing of vitamin B12 as well as a small number of other cobalt-containing enzymes [11] Manganese (Mn) metal is required for normal mammalian physiological functions, where it is a cofactor for varieties of brain enzymes such as; glutamine synthetase and mitochondrial superoxide dismutase as well as transferases and hydrolases. Also, it is necessary for the normal growth and development of bone, cartilage, connective tissue, and the reproductive system [12].
The present study was designed to investigate the toxic hazards effects of vanadium ions administration (as ammonium metavanadate, Am.V) on the hypothalamic-pituitary-gonadal axis and reproductive system and to demonstrate the attenuation and mitigation effects of Mn, Co, Fe composite nano-particles (MNPs) regarding the hazardous which raised by vanadium administration.
Materials and methods
Materials
a) The employed dose of Am.V was 20mg/kg. rat that meets 1/5 of LD50 [13] and was prepared by dissolving the adequate weight of Am.V (Sigma Co.) in suitable volume of the double distilled water.
b) For ferrite (Co0.5Mn0.5Fe2O4) preparation, the ferric nitrate (Loba Co.), Cobalt nitrate (Merck Co.), Manganese nitrate (Sigma Co.) were mixed in stesiometric amounts, then citric acid solution was addded to the solution (molar ratio of 1:1.5) to synthesiz the desired MNPs using the non-conventional citrate precursor method, where every 5g of the prepared mixture were suspended in 100ml saline to get 5% MNPs solution [14].
Animals grouping and treatment
Rattus rattus rats (110±20g) were purchased from the Research National Center, Giza, Egypt. They were maintained under standard laboratory ambient conditions and housed in cages (six rats/cage) at a constant temperature (approximately 25°C) with 12h light/dark cycle. Food and water were given ad libidum. The ethics-rule of animals using and care was followed in this study.
Rats were randomly grouped into four groups; G1 (the control group), G2 (animals were daily oral ingested by stomach tube with 1ml Am.V by dose 20mg/kg. B.wt. for two weeks), G3 (animals were daily IP injected for two weeks with 1ml of 5% of MNPs), G4 (animals were daily oral ingested by the used dose of Am.V accompanied with IP injection of 5% MNPs for two weeks). The rats of the different groups were decapitated after the 3rd, 7th, 10th and 15th day (6 rats/period) during the treatment process.
The hypothalamus was divided into two hemispheres, also the testes were separated after the rats’ autopsy [15] and each part was separately weighted and stored at -30 °C till using. Blood samples were collected after decapitation and centrifuged after standing for 15 minutes in a water bath at 35°C and finally stored at -30°C till using.
Vanadium Estimation
To estimate vanadium in the desired tissues, one hypothalamus hemisphere and one teste were separately placed in Teflon beakers with equal volumes of nitric acid (90%), perchloric acid (70%) and hydrogen peroxide (32%) then, the mixtures were heated on hot plate at 130°C till complete dryness. After cooling, the contents were transferred into 25ml volumetric flasks and diluted up to volume using double distilled water [16,17]. In the resulted solutions, vanadium was measured by the graphite atomic absorption spectrometer (AAS; Thermal-Jarrel Ash Model 12 Spectrophotometer). Vanadium concentration (in μg/g tissue) was determined using the Fisher Certified Standard diluted to appropriate concentrations in 2% HNO3.
Hormones Estimation
Using the radioimmunoassay (RIA) and according to the method described by [18] the Testosterone, FSH and LH were measured
Estimation of Brain Reduced Glutathione (GSH) And Malondialdehyde (MDA)
The second half of hypothalamus and the other teste were individually homogenized in ice-cold isotonic potassium chloride (1.2%) then centrifuged for 10minutes at 3000rpm where the GSH and MDA were determined in the clear supernatant according to methods described by [19,20] respectively.
Statistical Analysis
The obtained data were statistically analyzed using one-way analysis of variance (ANOVA) by the statistical package for the social science (SPSS) program version 20.
Results
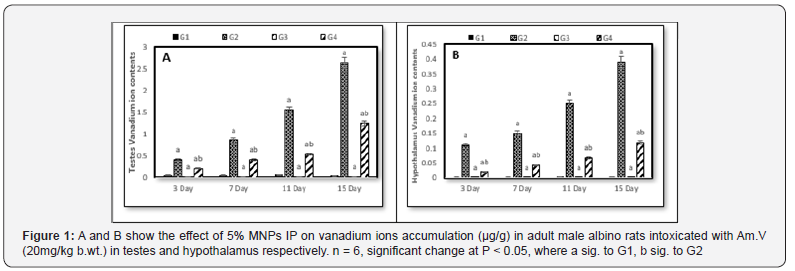
Comparing to the control (G1), vanadium content gradually increased in both G2 and G4 reaching its maximum value at 15 days. On the other hand, vanadium content showed a significant decrease in G4 in relative to G2 at each decapitation that point to the effect of the contemporaneous administration of MNPs with Am.V (Figure 1).
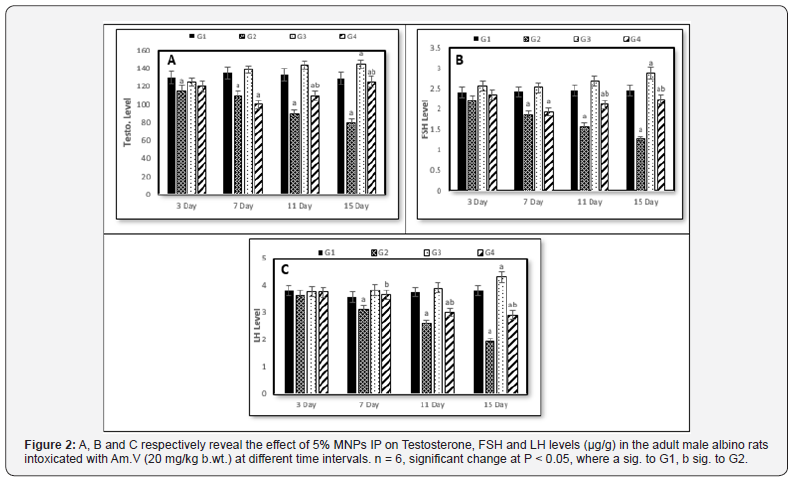
Referring to the testosterone, the continuous Am.V administration (G2) accompanied with gradual decreasing in its level reaching the minimum level on the 15th day. In G3, the testosterone level revealed faint increasing particularly with the 15th day. On the other hand, the testosterone level in G4 went downward till the 7th day then, turned to the gradual increasing until ending of the experiment period (Figure 2A). To great extent, the FSH level similarly behaved as the testosterone except the non-significant change at the 7th day in G3 (Figure 2B). Comparing to control (G1), the LH hormone level showed a similar trend like the FSH in G2 and G3, but in G4 its level continued in decreasing with increasing of the administration time and reached the minimum level on the 15th day (Figure 2C).
In general, the mitigation effect of MNPs on the investigated parameters is clearly observed when we take in account the comparison between G2 and G4.
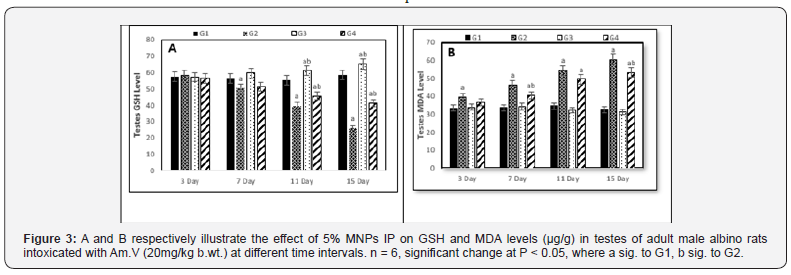
Comparing to control (G1), the GSH level showed a marked decreasing in the 2nd, 3rd, and 4th decapitations of G2 and this decreasing was more significant with the continuous Am.V administration. The same behavior was observed in G4 but with lesser decreasing particularly on the 3rd and 4th decapitations which reasonably attributed to the enhancement effect of MNPs (Figure 3A)., while no significant change was observed on G3 (Figure 3).
On the other hand, the testes MDA level showed significant increasing in both G2 and G4 while approximately no change was observed in G3(Figure 3B). It is worth to mention that increasing of MDA under the effect of parallel Am.V and MNPs administration was lesser than its increasing under the effect of the Am.V administration only.
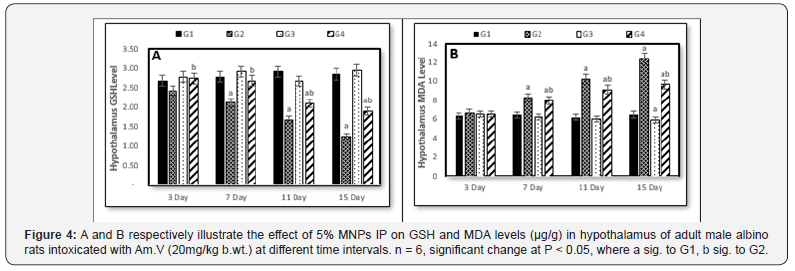
Relative to the control (G1), the hypothalamus GSH level suffered continuous decreasing with the Am. V. administration (Figure 4A) while in G4 (Am. V.+MNPs administration), the GSH restored its level in the 1st and 2nd decapitations approximately as was in the control group but unexpectedly it backed to decreasing in the 3rd and 4th decapitations. However, its level in G4 was always greater than in G2. In G3 (MNPs administration), the hypothalamus GSH level has no significant change comparing to the control over all the experiment period.
Referring to the hypothalamus MDA level, all the administration (G2, G3 and G4) showed no significant changes relative to the control (Figure 4B). While the MDA level was steadily increased from the 2nd to 4th decapitations but its level showed no changes in G3 over the experiment period. Although it did not impose an effect on the MDA level as the unique administration, but the MNPs caused decreasing of MDA level in G4 versus its level in G2 (Figure 4B).
Discussion
With the physiological conditions, a considerable proportion of vanadate (V+5) is reduced to vanadyl (V+4) under glutathione effect until reaching to the equilibrium balance between the two vanadium species [21]. Vanadium can exist as cationic, neutral, and anionic species depending on the initial metal concentration and the medium pH.
In this work the oral administration of vanadium showed its ability to enter and accumulate in the hypothalamus brain area as well as in the testes of the adult male albino rats which agrees with other studies demonstrated the capability of vanadium to access the blood-brain barrier and accumulate in rat’s brain as well as in its testes [22-24]. Also, the ability of the individual magnetite, cobalt and manganese nano particles to access the blood-brain barrier were noticed by many studies [25-29].The significant decreasing of vanadium ions content after the IP injection of MNPs with vanadium gavages could be ascribed to complexation and/or the electrostatic interaction between the adsorbent (MNPs) and the adsorbate vanadium species [30].
It is Known that the hypothalamic-pituitary-gonadal axis is starting by the secretion of gonadotrophin hormone from hypothalamus, which stimulates the anterior pituitary gland to secrete FSH and LH, then LH together with testicular auto- and paracrine factors is responsible for the regulation and the balance of the sex hormone production and gametogenesis [31].
Several reasons can explain the negative effect that imposed on the investigated hormones by vanadium exposure and accumulation. Decreasing of FSH, LH and Testo levels are functions for the hypothalamic-pituitary-gonadal axis disturbance that might be caused by vanadium administration due to its redox reaction leading to tissue damage [32]. Also, the hormones decreasing could be ascribed to ability of vanadium to inhibit Adenylate cyclase which aids in formation of the second messenger cyclic adenosine monophosphate (cAMP) that performers an important role in controlling the cells responsiveness to the hypothalamic hormones [33]. Moreover, declining of FSH, LH and Testo is reasonably happened due to the down-regulation of the steroidogenic enzymes (17b-HSD, 3b-HSD) by the effect of vanadium administration where those enzymes have a major role the hormones formation [23] Finally, vanadium could induce a mitochondrial over oxidation affecting on the cytochrome P450 substrates that have a regulatory function in Leydig cells [34-36].
As a consequence of vanadium accumulation in the hypothalamus and testes, a significant elevation in MDA level together with a significant decrease in GHS level were observed. Such disturbance in the hypothalamic-pituitary-gonadal axis is likely ascribed to the oxidative stress and tissue damage happened due to the effect of vanadium poisonous. One or more mechanism can be involved in this disturbance. Like other heavy metals, poisoning of vanadium results in tissue damage and production of reactive oxygen species (ROS) causing a peroxidation of structural lipids and an alteration of the antioxidative activity of enzymes like SOD, CAT and GPX [37,38]. The high polyunsaturated fatty acids of the brain and the testes are vulnerable to the free radical attacks hence, the hormones levels suffer disturbance [39], Gutteridge [40-42,24]. Moreover, the vanadium IP injection may also be involved in the endocrinological processes and disturbs them [24] as well as affects the thyroid function, growth, gonadal function, adrenal hormones, prolactin, glucose homeostasis, calcium-phosphorus metabolism or thymulin activity [32].
On contrary to the effect of vanadium, the IP injection of MNPs resulted in significant increase in hormones (FSH, LH, Testo) as well as the GSH level in both hypothalamus and testes, while decreasing of MDA level in both organs were observed.
The essential roles of Fe, Mn and Co in several body’s biological functions were the corner stone in their selection in this work. Iron has many diverse biological functions, including reversible binding of gases, enzyme catalysis, and electron transport. Furthermore, iron plays an important role in developing spermatozoa and providing an extra layer of protection to the testicular tissue where it is found in a large quantity in Sertoli and Leydig testes cells [43,44] and enhances the spermatogenesis process [45,46]. Also, as the iron quantified in bovine seminal plasma as the sperm’s motility characteristics getting better [47,48].
Manganese is an essential nutrient necessary for varieties of metabolic functions including those involved in normal human development, activation of certain metalloenzymes (Prasad et al., 2014), nervous system function, reproductive hormone function and the antioxidant enzymes that protect cells from damage due to free radicals [49] IOM(2011) [50]. Also, manganese has the ability to stimulate secretion of the rat LH [25]. On the other hand, cobalt contributes in the cobalamin formation which is the function unit of Vit B12. This vitamin increases the sperm production in men who have low sperm counts, increases the functionality of reproductive organs and decreases homocysteine toxicity [22] and decreases the inflammation-induced semen impairment by controlling nuclear factor-κB activation [51-53].
Conclusion
According to the executed work and the conducting results in this study, we can come to some conclusions.
a) Exposure to vanadium doses greater than the permissible levels disturbs the Hypothalamic-Pituitary-Gonadal Axis and testes leading to verified health risks.
b) The Mn-Co-Fe composite nanoparticles was presented as new effective ferrite (MNPs) able to enter the hypothalamus and testes enhancing their performance and mitigating the disturbance they were suffered due to vanadium pollution.
c) Further researches on this MNP are recommended to investigate its capabilities in treating with other metals poisoning pollutants which could affect the biological functions of the different organs.
The Mn-Co-Fe composite nanoparticle seems as good candidate performs preventive and/or reactionary roles against vanadium exposure hazards for workers in facilities or activities containing vanadium handling.
References
- Perles T (2014) Vanadium market outlook in: 2nd International Vanadium Symposium. Vancouver Canada.
- Venkataraman BV, Sudha S (2005) Vanadium Toxicity. Asian J Exp Sci 19(2): 127-134.
- Mustapha O, Oke B, Offen NS, Siren A, Olopade A (2014) Neurobehavioral and cytotoxic effects of vanadium during oligodendrocyte maturation: A protective role for erythropoietin. Environmental Toxicology and Pharmacology 38(1): 98-111.
- Sarsebekov EK, Dzharbusynov BU, Doskeeva RA (1994) The nephrotoxic action of heavy crude with a high vanadium content and of its refinery products. Urol Nefrol (3): 35-36.
- Domingo JL (2002) Vanadium and tungsten derivatives as antidiabetic agents: a review of their toxic effects. Biol Trace Elem Res 88(2): 97-112.
- IARC (2006) Cobalt in Hard Metals and Cobalt Sulfate, Gallium Arsenide ,Indium Phosphide and Vanadium Pentoxide. IARC Sci Publ 86: 1-294.
- Colombo M, Romero SC, Casula WF, Gutierrez LA, Morales MA, et al. (2012) Biological applications of magnetic nanoparticles. Soc Rev 41(11): 4306-4334.
8. El-Batal AI, El-Sayyad GS, El-Ghamery A, Gobara M (2017) Response surface methodology optimization of melanin production by Streptomyces cyaneus and synthesis of copper oxide nanoparticles using gamma radiation. Journal of Cluster Science 28(3): 1083-1112.
- Yayayuruk AE, Yayayuruk O (2017) Adsorptive performance of nanosized zero-valent iron for V(V) removal from aqueous J Chem Technol Biotechnol 92: 1891-1898.
- Yellepeddi V, Whalen K, Finkel R, Panavelil TA (2015) Lippincott illustrated reviews: pharmacology sixth edition, philadilphia, Baltimora, Principal of drug therapy Newyork, USA, pp. 1-25.
11. Renfrew AK, Neill AS, Hambley TW, New EJ (2018) Harnessing the properties of cobalt coordination complexes for biological application. Coordination Chemistry Reviews 375: 221-233.
13. Llobet JM, Domingo JL (1984) Acute toxicity of vanadium compounds in rats and mice. Toxicol Lett 23(2): 227-231.
- Ramay SM, Saleem M, Atiq S, Siddiq SA, Naseem S, et al. (2011) Influence of temperature on structural and magnetic properties of Co5Mn0.5Fe2O4 ferrites. Bull Mater Sci 34(7): 1415-1419.
- Glowinski LJ, Iversen LL (1966) Regional studies of catecholamines in the rat brain I Disposition of ha-noradrenaline, ha-dopamine and ha-dopa in various regions of the brain. J Neurochem 13(8): 655-669.
- FAO BFood Administration Organization (1992) Committee for Inland fisheries of Africa. Report of the third session of the working party on pollution and Hisheries. Accra, Ghana, pp. 25-29.
- Cicik B, Engin K (2005) The effects of cadmium on levels of glucose in serum and glycogen reserves in the liver and muscle tissues of Cyprinus Carpio (L.., 1758). Turk J Vet Arum Sci 29: 113-117.
- Joshi UM, Shah HP, Sudhama SP (1979) A sensitive and specific enzyme immunoassay for serum testosterone. Steroids 34(1): 35-46.
- Ellman GL (1959) Tissue sulfhydryl groups. Arch Biochem Biophys 82(1): 70-77.
- Ohkawa H, Ohishi N, Yagi K (1979) Assay for lipid peroxidation in animal tissues by thiobarbituric acid reaction. Anal Biochem 95(2): 351-358.
- Peacock LC, Sherman DM (2004) VANADIUM (V) Adsorption onto goethite (α-FeOOH) at pH 1.5 to 12: A Surface Complexation model based on ab initio molecular geometries and EXAFS Spectroscopy. Geochim et Cosmochim Acta 68(8): 1723-1733.
- Chandra AK, Ghosh R, Chatterjee A, Sarkar M (2010) Vanadium-induced testicular toxicity and its prevention by oral supplementation of zinc sulfate. Toxicol Mech Methods 20: 306-315.
- Wilk A, Koziarska BS, Wiszniewska B (2017) The toxicity of vanadium on gastrointestinal, urinary and reproductive system, and its influence on fertility and fetus’s malformations, Postepy Hig Med Dosw 71: 850-859.
- Folarin OR, Adaramoye OA, Akanni OO, Olopade JO (2018) Changes in the brain antioxidant profile after chronic vanadium administration in mice. Metab Brain 33(2): 377-385.
- Pine M, Lee B, Dearth R, Hiney JK, Dees WL (2005) Manganese Acts Centrally to Stimulate Luteinizing Hormone Secretion: A Potential Influence on Female Pubertal Development. Toxicological Sciences 85(2): 880-885.
- Farahani MK, Fareghi-Alamdari R, Kiasat AR (2016) Cooperative Activation in the Synthesis of Flavanone Antioxidants Using a Simple and Highly Efficient Magnetically Recoverable Nano-Cu-CoFe2O4 Polycyclic aromatic compounds.
- Lee K, David AE, Zhang J, Shin MC, Yang VC (2017) Enhanced accumulation of theranostic nanoparticles in brain tumor by external magnetic field-mediated in site clustering of magnetic nanoparticles. Journal of Industrial and Engineering Chemistry 54: 389-397.
- Su L, Zhang B, Huang Y, Zhang H, Xu Q, et al. (2017) Superparamagnetic iron oxide nanoparticles modified with dimyristoylphosphatidylcholine and their distribution in the brain after injection in the rat substantia nigra. Materials Science and Engineering C 18: 400-406.
- Nasir AF, Cameron SF, Hippel FA, Postlethwait J, Niehaus AC, et al. (2018) Manganese accumulates in the brain of northern quolls (Dasyurus hallucatus) living near an active mine. Environmental Pollution 233: 377-386.
- Scibior A (2016) Vanadium (V) and magnesium (Mg)- In vivo interactions: A review Chemico-Biological Interactions 258: 214-233.
- Guyton AG, Hall JE (2006) Reproductive and hormonal function of male. In: Textbook of medical physiology, Philadelphia, Pennsylvania, pp. 996-1011.
- Neve J (1992) Clinical implications of trace elements in endocrinology. Biol Trace Elem Res 32: 173-185.
- Krivanek J (1983) Vanadate and brain adenylate cyclase effect of spreading depression, Neuroscience 10(2): 545-552.
- Payne AH, Hales DB (2004) Overview of steroidogenic enzymes in the pathway from cholesterol to active steroid hormones. Endocr Rev 25(6): 947-970.
- Anwar-Mohamed A, Elbekai RH, El-Kadi AO (2009) Regulation of CYP1A1 by heavy metals and consequences for drug metabolism. Expert Opin Drug Metab Toxicol 5(5): 501-521.
- Zhao Y, Ye L, Liu H, Xia Q, Zhang Y, et al. (2010) Vanadium compounds induced mitochondria permeability transition pore (PTP) opening related to oxidative stress. Journal of Inorganic Biochemistry 104(4): 371-378.
- Stohs SJ, Bagchi D (1995) Oxidative mechanisms in the toxicity of metal ions. Free Radic Biol Med 18(2): 321-336.
- Fatola OI, Olaolorun FA, Olopade FE, Olopade JO (2017) Trends in Vanadium Neurotoxicity. Brain Research Bulletin 145:75-80.
- Schaich KM (1992) Metals and lipid oxidation. Contemporary issues Lipids 27(3): 209- 218.
- Meagher EA, Fitzgerald GA (2000) Indices of lipid peroxidation in vivo: strengths and limitations. Free Radical Biology and Medicine 28(12): 1745-50.
- Dotan Y, Lichtenberg D, Pinchuk I (2004) Lipid peroxidation cannot be used as a universal criterion of oxidative stress. Prog Lipid Res 43(3): 200-227.
- Adebiyi OE, Olopade JO, Olayemi FO (2016) Neuroprotective effect of Grewia carpinifolia extract against vanadium induced behavioral impairment. Folia Veterinaria 60(4): 5-13.
- Toebosch AM, Kroos MJ, Grootegoed A (1987) Transport of transferrin bound iron into rat Sertoli cells and spermatids. Int J Androl 10(6): 753-764.
- Wise T, Lunstra DD, Rohrer GA, Ford JJ (2003) Relationships of testicular iron and ferritin concentrations with testicular weight and sperm production in boars. J Anim Sci 81(2): 503-511.
- Hales KG (2010) Iron testes: sperm mitochondria as a context for dissecting iron metabolism. BMC Biol 8: 79.
- Metzendorf C, Lind MI (2010) Drosophila mitoferrin is essential for male fertility: evidence for a role of mitochondrial iron metabolism during spermatogenesis. BMC Dev Biol 10: 68.
- Kanwal MR, Rehman NU, Ahmad N, Samad HA, Uh-Rehman Z, et al. (2000) Bulk cations and trace elements in the Nili-Ravi buffalo and crossbred cow bull semen. Int J Agric Biol 2: 302-305.
- Tvrda E, Knazicka Z, Lukacova J, Schneidgenova M, Massanyi P, et al. (2012) Relationships between iron and copper content, motility characteristics and antioxidant status in bovine seminal plasma. JMBFS 2: 536-547.
- ATSDR (Agency of Toxic Substances and Disease Registry) (2012) Toxicological Profile for Manganese U.S. Department of Health and Human Services Public Health Service.
- Gordijo CR, Abbasi AZ, Amini MA, Lip HY, Maeda A, et al. (2015) Design of hybrid MnO2-polymer-lipid nanoparticles with tunable oxygen generation rates and tumor accumulation for cancer treatment Adv Funct Mater 25(12): 1858-1872.
- Banihani SA (2017) Vitamin B12 and Semen Quality. Biomolecules 7(2): 42-53.
- Glade MJ, Meguid M (2018) A glance at…antioxidant and anti-inflammatory properties of dietary cobalt. Nutrition 46: 62-66.
- Siegel RC (1978) Lysyl oxidase. International review of connective tissue research 8: 115-118.






























