Drug Induced Kidney Disease
Siddama Amoghimath and Suneel I Majagi*
Department of Pharmacology, Gadag Institute of Medical Sciences, India
Submission: July 28, 2017; Published: September 22, 2017
*Corresponding author: Suneel I Majagi, HOD, Department of Pharmacology, Gadag Institute of Medical Sciences, Gadag-582103, Karnataka, India, Mobile: 9448160742, Email: suneelmajagi@yahoo.co.in
How to cite this article: Siddama A, Suneel I M. Drug Induced Kidney Disease. Open Acc J of Toxicol. 2017;2(1): 555576. DOI: 10.19080/OAJT.2017.02.555576
Abstract
Drug induced kidney disease (DKID) is a frequently noticed adverse event contributing to morbidity and healthcare utilization. Drugs are known to cause nephrotoxicity due to their adverse effects by one or more mechanism. DKID is common among certain patients and inpatients with specific clinical conditions. Measures to prevent drug induced kidney disease require adequate knowledge regarding mechanism of action of renal injury, patient related risk factors, drug related risk factors and need early intervention along with close vigilance.
Keywords: Drug; Kidney; Nephrotoxicity
Abbreviations: CYP: Cytochorome; MDR: Multi Drug Resistance; ACE: Angiotensin Converting Enzyme; TGF: Transforming Growth Factor; CCR: Chemokine Receptor; GFR: Glomerular Filtration Rate; CKD: Chronic kidney disease
Introduction
Drug induced kidney disease (DIKD) is a significant contributor of acute kidney injury (AKI) and chronic kidney disease (CKD). The incidence of drug induced nephrotoxicity is 14-26% in adults and 16% in paediatric cases [1]. Nephrotoxicity is defined as 0.5mg/dl or 50% rise in serum creatinine over 2472 hour time frame and a minimum of 24-48h drug exposure [2]. But 50% increase in serum creatinine may not be highly specific. DIKD is a significant contributor to AKI and CKD. DIKD can be categorised as Type A- Dose dependent and Type B-Idiosyncratic reactions. Dose dependent reactions are predictable which are based on the pharmacological properties of the drug, whereas the idiosyncratic reactions are unpredictable as they are based on peculiarities of the patient. The Kidney Disease Improving Global Outcomes (KDIGO) classify DIKD into acute (1-7 days), sub-acute (8-90 days) and chronic (>90 days) [3, 4]. Nephrotoxicity caused due to administration of various drugs can be explained by their different mechanisms like, [5-7]
A. By altering the Intraglomerular hemodynamics: Interfere with the kidney's ability to auto regulate glomerular pressure, decrease in pressure and cause dose dependent vasoconstriction of afferent arterioles.
Examples: NSAID's, ACE inhibitors, ARB's, Calcineurin inhibitors like Cyclosporine and Tacrolimus.
B. Renal tubular toxicity: Interfere with the mitochondrial function by increasing the oxidative stress and forming free radicals.
Examples: Aminoglycosides, Amphotericin B, Antiretrovirals (Adefovir, Cidofovir), Cisplatin, Contrast dye and Zoledronate.
C. Due to inflammation in glomerulus, renal tubular cells, and surrounding interstitium:
a) Glomerulonephritis: Inflammatory condition due to immune mechanism associated with proteinuria in nephrotic range.
a. Examples: Gold, Hydralzine, Interferon alpha, Lithium, NSAID's, Propylthiouracil, Pamidronate.
b) Acute Interstitial Nephritis: due to non-dose dependent idiosyncratic response.
a. Examples: Allopurinol, Antibiotics (Beta lactam, Quinolones, Sulphonamides and Vancomycin), Anti virals (Acyclovir, Indinavir), Diuretics (Loop and Thiazide), NSAID’s, Phenytoin, Proton pump inhibitors (Omeprazole, Pantoprazole, Lansoprazole, and Ranitidine)
c) Chronic interstitial nephritis: Due to hypersensitivity reactions.
a. Examples: Calcineurin inhibitors (Tacrolimus, Cyclosporin), Lithium, Aspirin, Acetaminophen
D. Crystal Nephropathy: Use of drugs which produce crystals that are insoluble in urine. These crystals precipitate within the distal tubular lumen, obstructing the urine flow and eliciting the interstitial reaction.
Examples: Antimicrobial agents (Ampicillin, Ciprofloxacin, Sulphonamides), Antivirals (Acyclovir, Foscarnet, Ganciclovir, Indinavir), Methotrexate, Triamterene.
DKID presents in one of the four phenotypes. At least one criterion must be met for all the drugs which are suspected of causing DKID. Following are the Bradford-Hill criteria which are used for defining each phenotype [3,8]
a. The drug exposure must be at least 24h prior to the event.
b. There should be biological plausibility for the causal drug effect, metabolism and immunogenicity.
c. Complete data (including but not limited to comorbidities, additional nephrotoxic exposures, exposure to contrast agents, surgical procedures, blood pressure, and urine output) surrounding the period of exposure is required to account for concomitant risk and exposure to other nephrotoxic agents.
d. Strength of the relationship between the attributable drug and phenotype should be based on the drug exposure duration, extent of primary and secondary criteria met as well as the time course of the injury.
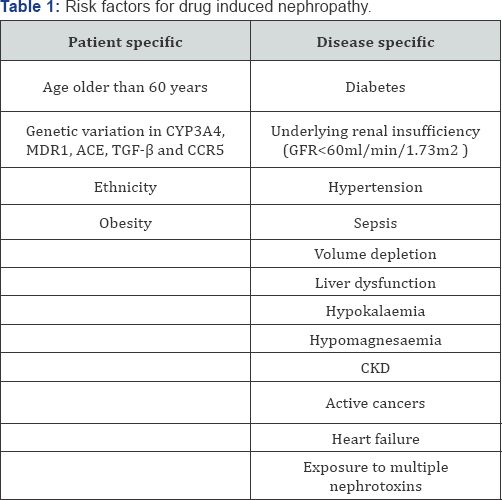
Drug induced kidney disease can occur due to patient related factors and in specific disease (Table 1).
Management
Treatment of nephrotoxicity is dependent on phenotype, severity of the injury, underlying condition for which the medications were prescribed and patients risk factors. The decision to stop or reduce the dosage depends on careful observation of risk versus benefit. In type A reaction dose reduction may be sufficient to mitigate the injury. While in Type B reactions which is idiosyncratic will require the discontinuation of the offending drug and careful observation.
Preventive measures of DKID include: use of equally therapeutic effective drugs which are non nephrotoxic, correction of the risk factors for nephrotoxicity, before starting the therapy assess the base line renal function, avoid nephrotoxic drug combinations. Various formulas can be used to assess the renal function. Adequate perfusion is important to maintain the renal perfusion and to avoid renal impairment. In patients associated with multiple risk factors, serum creatinine level has to be monitored after starting the treatment and while increasing the dosage of a drug. A systematic approach towards the electronic medical record for automated monitoring of patients at risk of nephrotoxicity is also required.
References
- Hoste EA, Bagshaw SM, Belloma R, Cely CM, Colman R, et al. (2015) Epidemiology of acute kidney injury in critically ill patients: the multinational AKI-EPI study. Intensive Care Med 41(8): 1411-1423.
- Awdishu L, Mehta RL (2017) The 6R's of drug induced nephropathy. BMC Nephrology 18: 124.
- Mehta RL, Awdishu L, Davenport A, Murray PT, Macedo E, et al. (2015) Phenotypr standardization for drug induced kidney disease. Kidney Int 88(2): 226-232.
- Group KDIGO KAKIW (2012) KDIGO clinical practice guideline for acute kidney injury. Kidney Inter 2(1): 1-138.
- Palmer BF (2002) Renal dysfunction complicating the treatment of hypertension. N Engl J Med 347(16): 1256-1261.
- Markowitz GS, Perazella MA (2005) Drug-induced renal failure: a focus on tubulointerstitial disease. Clin Chim Acta 351(1-2): 31-47.
- Rossert J (2001) drug-induced acute interstitial nephritis. Kidney Int 60(2): 804-817.
- Leblanc M, Kellum JA, Gibney RT, Lieberthal W, Tumlin J, et al. (2005) Risk factors for acute renal failure: inherent and modifiable risks. Curr Opin Crit Care 11(6): 533-536.






























