Development of Respiratory Mucosal Irritation and Toxicity Screening Methods: Comparison of MTT and Colorimetric Resazurin-Based Assay
Chibueze Peter Ihekwereme1,3, Di Shao3, Charles Okechukwu Esimone2 and Remigius Uchenna Agu3*
1Department of Pharmacology and Toxicology, Nnamdi Azikiwe University, Nigeria
2Department of Pharmaceutical Microbiology and Biotechnology, Nnamdi Azikiwe University, Nigeria
3Biopharmaceutics and Drug Delivery Laboratory, Dalhousie University, Canada
Submission: June 30, 2016 ; Published: August 05, 2016
*Corresponding author: Remigius Uchenna Agu, Biopharmaceutics and Drug Delivery Laboratory, College of Pharmacy, Dalhousie University, Halifax, NS B3H 3J5, Canada.
How to cite this article: Chibueze PI, Di Shao, Charles OE, RemigiusUchenna A.Development of Respiratory Mucosal Irritation and Toxicity Screening Methods: Comparison of MTT and Colorimetric Resazurin-Based Assay. Open Acc J of Toxicol. 2016;1(2): 555557. DOI: 10.19080/OAJT.2016.01.555557
Abstract
Availability of reliable and reproducible assay methods that use simple tools will facilitate research especially in resource-poor settings. The purpose of this study is to evaluate colorimetric resazurin reduction assay (CRA) as a possible tool in respiratory mucosal cell irritation and toxicity screening. It compared CRA with another well-accepted colorimetric cell viaility assay method (3-[4, 5-Dimethylthiazol-2-yl]-2, 5-diphenyl tetrazolinium bromide {MTT} assay). Cell viability results of treated cells were obtained using both assay methods and used to determine Z' factor, signal-to-background (S/B) ratio and IC50 values. The results show all the Z ' values obtained were positive except values from CRA for cells treated with triton-X 100 incubated for 30min and 2h. All the Z ' values for MTT shows the method is optimized (0.5< Z'<1.0) while most values for CRA were outside the acceptable range (Z'<0, Z'>1). MTT demonstrated large S/B ratio at both 30min and 2h for all the compounds. The range of values obtained for the S/B ratio is 12-57 for MTT and 1-3 for CRA. Furthermore, CRA failed to estimate IC50 for some of the compounds (BKC, 2h; SDS, 2h; BKC, 30min). The values obtained for Z' factor, S/B ratio and IC50 for both assays were at variance. This result demonstrates that CRA performed below acceptable assay standard and therefore may not be an appropriate method for human respiratory cell viability studies. Consequently, we conclude that CRA is not useful in human respiratory mucosal irritation and toxicity studies.
Keywords: Calu-3 cells; Colorimetric resazurin assay; Assay performance measures; Respiratory mucosal irritation; Toxicity screening; MTT
Abbreviations: CRA: Colorimetric Resazurin Reduction Assay; DST: Drug Susceptibility Testing; REMA: Resazurin Microtiter Assay; FRA: Fluorimetric Resazurin-Based Assays; RA: Resazurin-Based Assay; APM: Assay Performance Measures; HTS: High-Throughput Screening
Introduction
There seems to be a conflict between the reported sensitivity of colorimetric resazurin-based assays (CRA) and the wide clinical use of Resazurin Microtiter Assay (REMA). REMA, a liquid culture- based CRA has been endorsed by the World Health Organization for drug susceptibility testing (DST) for Mycobacterium tuberculosis [1]. Though CRA is not popular in respiratory cell culture as fluorimetric resazurin-based assays (FRA) [2,3], its use in DST of M. tuberculosis has been compared with similar assay methods and reported to be accurate [4,5]. Furthermore, the method has been used as a reference method in the evaluation of other assay methods [5]. The inherent advantage of resazurin- based assay (RA) that cells may be re-used (since the dye is not lethal) makes the method more suitable in resource-poor settings where acquisition and sustenance of some cell types poses a challenge. There is need to assess the extent of deficiency of the method as well as determine its relative usefulness especially in resource-poor countries.
Though, CRA have previously been judged less sensitive than FRA, supporting data is scarce [2,3,6]. Outcome measure of resazurin-based assay (RA) is taken either by colorimetric or fluorimetric determination [7-11]. Fluorimetric reduction assay have earlier been compared with other assay methods [7-9]. One of such studies demonstrated that FRA is comparable with MTT [12]. Beyond DST of M. tuberculosis, few studies use CRA [13-15]. Consequent upon the successes recorded with CRA, as well as the understanding that the rate at which resazurin are reduced changes from cell to cell [3,16,17], there arises a need to explore the usefulness of CRA in respiratory mucosal cell viability.
Assay performance measures (APM) may quantify the amount of separation in measured signals between positive and negative controls in an assay, while accounting for the observed variability [18]. APMs are used in the evaluation of assay methods. Commonly used APMs include Z' factor, signal-to-background ratio, and assay variability ratio. The Z' factor is a dimensionless, simple statistical characteristic for high-throughput screening (HTS) assay. It has been used widely in several assay measures for quality assessment, optimization, comparison of instrumentation quality, and validation [19-21]. High-quality assays compatible with HTS should have an "excellent ”Z' factor value (between 0.5 and 1.0). Z' factor takes into consideration most factors necessary for HTS assay characterization and is preferred to other APMs like signal- to-background ratio [22,23] that lack this feature. Consequently, Z' factor has become an accepted parameter for assessment of performance of assays and has been used widely in several assay measures [19-21]. IC50 comparison, even though not an APM, has been used severally in assay performance comparison [12].
The purpose of this study is to evaluate CRA as a possible tool in respiratory mucosal cell irritation and toxicity screening. Since a previous study reported that FRA and MTT produce similar results, our approach was to observe whether values obtained using a respiratory mucosal cell line for Z' factor, signal-to- background ratio, and IC50 for CRA and MTT were similar. There are justifications for this study. Absorbance measuring equipment is commonly available in most laboratories unlike fluorescence measuring devices. To overcome the limitation imposed by lack of fluorescence measuring equipment, there is need to develop CRA for respiratory mucosal irritation and toxicity screening. Furthermore, CRA can easily replace MTT with the added benefit that cells can be re-used (a feature MTT does not have). In addition, this study will quantitatively provide data on the performance of CRA in a mammalian cell.
Materials and Methods
Chemicals
Triton X-100 (TX) and benzalkonium chloride (BKC) were supplied by Sigma (St. Louis, MO, USA). Sodium dodecyl sulphate (SDS) USP was from Fisher Scientific (Ottawa, ON, CA). Resazurin was obtained as resazurin sodium salt powder (Acros Organic NV). Tissue culture materials including Dulbecco's modified eagle's medium-Ham's F-12 nutrient (D-MEM/F-12), fetal bovine serum (FBS), phosphate buffered saline (PBS), penicillin/streptomycin, Glutamax, and phosphate-buffered saline-trypsin were purchased from Invitrogen (Burlington, ON Canada). Human bronchial/subbronchial gland cell line (Calu-3) was purchased from American Type Culture Collection (Manassas, VA, USA).
Cell culture
The Calu-3 cells were grown according to a standard protocol. Cells were cultured in 96-well plates (Fisher Scientific, ON, Canada) in 1:1 D-MEM/F-12 supplemented with 10% FBS, 1% Glutamax, 100U/ml penicillin, and 100 mg/ml streptomycin. They were fed every other day with a DMEM-F12 containing 10% FBS,, 1% Glutamax, 1% 10,000 units/mL penicillin and 1%, 100 mg/ml streptomycin and maintained at 95% O2 and 5% CO2.
Cell viability studies
Compounds known to be toxic or irritating to respiratory cells were selected. The test compounds (benzalkonium chloride, sodium dodecyl sulphate, triton X-100) were applied as solutions in DMEM/F-12 at 8 different concentrations in wells (n=4) incubated for 30 min and 2 h. The concentrations of the test solutions used were determined through a range finding procedure. Blank DMEM/F-12 was used as control. Cells used for the experiments were within 8-15 passages and at 70- 80 percent confluency. Before the experiments, cells were washed with DMEM/F-12, pH 7.4, followed by 30 min equilibration in the buffer. Subsequently, the supernatant was completely discarded from each well followed by addition of 200 μl of test compoundor control and incubated. One batch of cells was used for each test compound to eliminate the effect of batch variation on the result. The test compounds and control were discarded after incubation and 100 μl of DMEM/F-12, pH 7.4 used to wash each well and subsequently discarded before cell viability assessment. The viability of the cells post-incubation was determinedusing separate wells for MTT and resazurin assay methods. The Z' factor, signal-to-background ratio and IC50 were determined for each time frame. We calculated the IC50 of the test compounds after the cell viability assay. think that comparable assay methods should have comparable inhibitory curves with similar IC50 values. Both assays were done on submerged cells.
Resazurin reduction assay (CRA)s
Resazurin stock solution 0.01% was prepared by dissolving resazurin powder (Sigma, St. Louis, MO, USA) in distilled water, filtered with a sterile 0.20 μm-pore filter (Corning Inc., MA, USA) and stored in the dark at 4°C for up to 1 week. An aliquot of the stock solution is diluted (1:10) before use. After treatment of cells, 200 μl of 0.001% resazurin solution was added to each well and incubated in 5% CO2 at 370C for 2 h. Subsequently, 100 μl of 3.0% SDS was added directly to each well to stop the resazurin reaction. The absorbance of the reduced dye was read at 570 nm [24-26] using 96-well plate reader (ABI systems).
3-[4, 5-Dimethylthiazol-2-yl]-2, 5-diphenyl tetrazolinium bromide (MTT) assay
After treatment of cells, 100 μl of MTT solution (5.0 mg/ml) prepared in distilled water was added to each well and incubated for 3 hin 5% CO2 at 37°C. Subsequently, the supernatant was discarded and replaced with 100 μl isopropyl alcohol per well. The plate was then sealed in an aluminium foil and incubated for 2 h to extract formazan produced by cellular mitochondrial dehydrogenase [27]. The absorbance of the solution was read within an hour after extraction on an ELISA plate reader (ABI systems) at 570 nm. One batch of cells was used on the plate for each test compound with control to eliminate the effect of batch variation on the result.
Data analysis
The Z'-factor, signal-to-background ratio (S/B) and IC50 values were determined [12,22]. The optical density values was used to determine the viability per well. The mean absorbance of the control (blank DMEM/F-12) was considered equivalent to 100 % cell viability. Mean absorbance of treated samples in each assay was expressed as a percentage of the mean absorbance of control. The mean cell viability was used to calculate the Z'-factor [22] using the formula
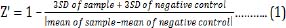
The signal-to-background ratio was calculated using the equation
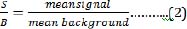
Where Z' means Z' factor value and SD is standard deviation.
Mean signal is the mean cell viability obtained from the negative control corresponding to 100 % cell viability or mean of the top (maximum) signal. Mean background is the mean cell viability obtained from the test sample corresponding to the least cell viability obtained or mean of the bottom (minimum) signal. The concentration of the test solutions causing 50% reduction in cell viability was calculated using values spread between maximum and minimum cell viability. The data was fitted into GraphPad Prism 5.0 (GraphPad Software, San Diego, CA, USA) and used to generate a sigmoidal dose-response curve. In comparing both values, we computed the ratio (IC50 CRA/ IC50 MTT). Comparable IC50 values should be close to 1.0.
Results
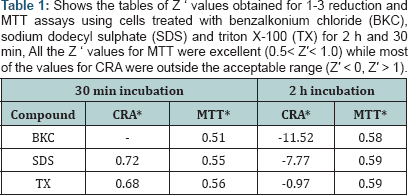
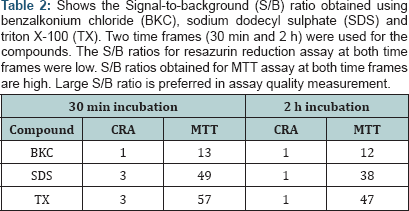
The evaluation of CRA and MTT was based on the determination of the Z'-factor, S/B ratio and IC50 using the percent viability of sample compounds. The result of values for Z’-factor is presented in ( while most values for CRA were outside the acceptable range. On comparison, the Z’ values for MTT from 2 h incubations have higher values than their corresponding values in 30 min incubations. All the Z' values obtained for MTT (30 min; 2 h) was positive while all the values for CRA at 2 h exposure was negative. Nevertheless, the values for CRA for SDS (0.72) and TX (0.68), both at 30 min exposure were excellent. The Z' values obtained for MTT were within a narrow range (0.51 - 0.59) unlike CRA. MTT demonstrated a large S/B ratio both at 30 min and at 2 h for all the compounds as seen in (Table 2). The range of values obtained for the S/B ratio is 12-57 for MTT and 1-3 for CRA. A common trend for MTT at both incubation periods shows the order of decreasing S/B ratios as BKC < SDS < TX.
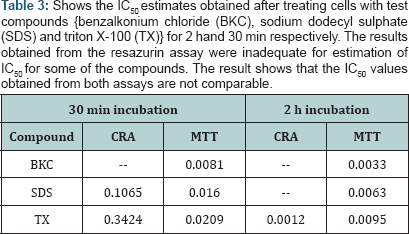

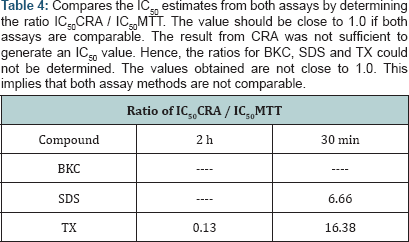
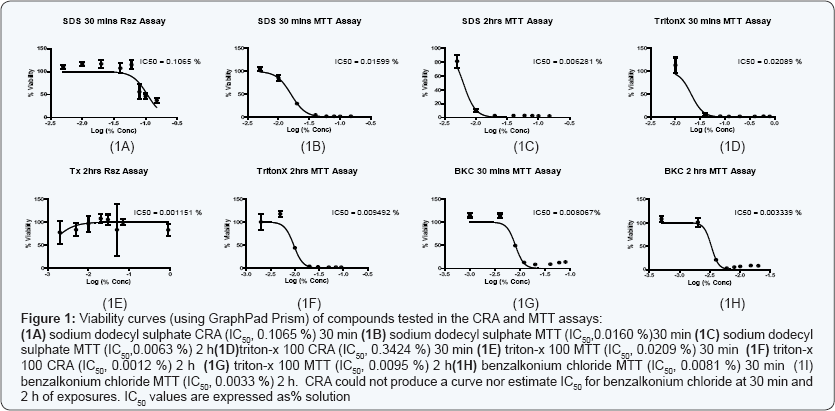
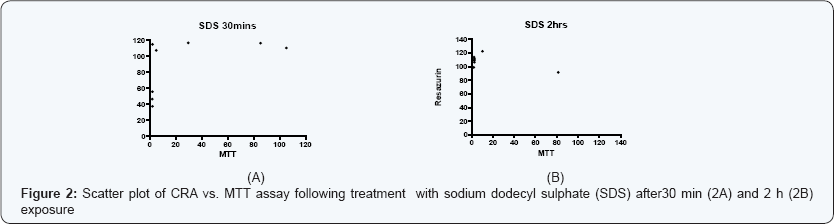
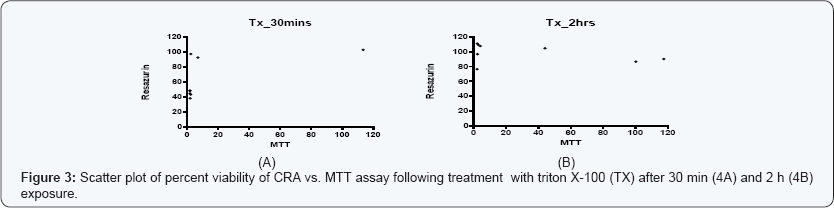
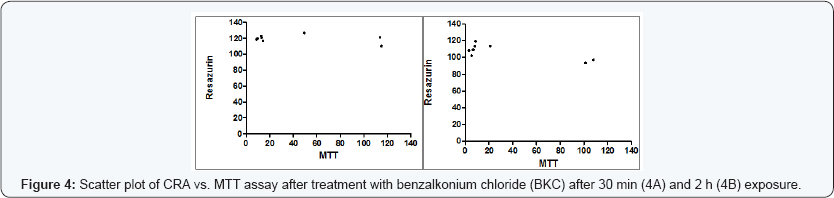
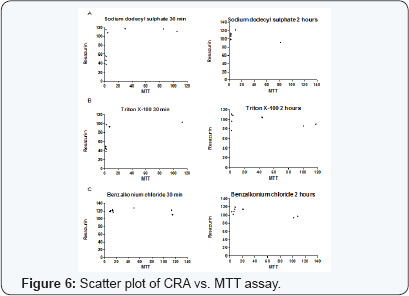
The estimation of IC50 from viability curves after treatment with SDS, triton X-100 and benzalkonium chloride is presented in Table 3 and Figure 1. The order of decreasing value of IC50 both at 30 min and at 2h treatment for MTT is BKC < SDS < TX. In addition, the values for 30 min incubation are consistently higher than its corresponding value in 2 h incubation. The scatter plots for SDS, triton X-100 and benzalkonium chloride are presented in Figures 2-4 respectively. Figure 3A shows that only1 point has value more than 80 % for both assay methods while the other points contrast sharply. Few of the values of the points for resazurin are similar to the values of negative control. All of the values of the points for resazurin are above 35 % while all of the points for MTT (except one) are less than 20 %. Figure 3B shows that only 2 points has value more than 80 % for both assay methods while the other points contrast sharply. Majority of the values of the points for resazurin are similar to the values of negative control while majority of the values for MTT are less than 50 %. The result demonstrates that except for a few cases, the percent viability reported by each assay is not comparable. In Figure 4, both plots show that only 2 points demonstrates cell viabilities more than 80 % for both assay methods while the other points contrast sharply. The values of all the points in Figure 4A for resazurin are similar to the values of negative control while majority of the values for MTT are less than 60%. Figure 4B shows that majority of the values of the points for resazurin are similar to the values of negative control while majority of the values for MTT are less than 30%. The results from Figure 4A and Figure 4B demonstrate that except for a few cases, the percent viability reported by each assay is not comparable.. Scatter plots for BKC at 30 min and 2 h are similar. All the points of CRA for TX (30 min) show cell viabilities > 35% while most of the points for MTT were < 20%. Majority of points for CRATX (2 h) were similar to values of negative control while majority of points for MTT had viabilities < 50%. CRA failed to estimate IC50 for some compounds (BKC, 2 h; SDS, 2 h; BKC, 30 min) (Table 3 &Figures 2- 4). The ratio (IC50 CRA/ IC50 MTT) showed a wide disparity between the IC50 estimates obtained for both assays (Table 4).
Discussion
There is no specific HTS assay for irritation or toxicity of respiratory mucosal cells. RA-based assay has been used for decades on different cell types and for various purposes [28]. As previously stated, CRA is less commonly used and data supporting this practice is scarce. The popularity enjoyed by FRA in cell viability assessment over CRA is due to its superior sensitivity which has been reported in literature [2,3]. Recently, an assay that uses Belgian slugs was developed for mucosal irritation [29].

Our result show that the excellent Z' values for MTT indicates a quality HTS assay for respiratory mucosal cell irritation and toxicity. In contrast, Z ' values from CRA for BKC (30 min, 2 h) and SDS (2 h) are null as they had values >1.0 and Z' values cannot be >1.0 [22]. Of all the 6 values for CRA, only one (SDS 30 min) is within the acceptable range. Negative Z' value of CRA for Triton-X 100 means that there is no separation band between signal variation of sample and control. Negative Z' value or values close to zero may result if the assay conditions are not optimized. The consequence of band overlap is that it is essentially impossible to use this assay for HTS screening. Since large S/B ratio is preferred in assay quality measurement, the excellent Z ' values for MTT is corroborated by the large S/B ratio of MTT [30]. Both assay methods are not comparable since the inhibitory curves and IC50 values are at variance. This opinion is further strengthened as we could not obtain IC50 estimate of CRA for some of the compounds. The values of the ratio (IC50 CRA/ IC50 MTT) as seen in (Table 4) shows a difference in performance between both assays. This difference is further confirmed by the scatter plots (Figures 5,6). The Z' factor, signal-to-background (S/B) ratio and IC50 demonstrates that both CRA and MTT are not comparable.
This finding agrees with previous reports that CRA is less sensitive than FRA. The Draizeeye test, which is a whole animal test has been the standard for mucosal irritation and toxicity assay. Perrot et al. [26] previously used isolated pig cornea to demonstrate that FRA can be a substitute for Draize test for some class of compounds. Another study compared FRA and MTT and demonstrated that even though FRA was slightly more sensitive than MTT assay, the Z'-factor and EC50 values were comparable in both assays [12].
It appears that CRA performs well in cell viability assays involving bacteria, and poorly in human cells. A multicenter study of MTT assay and CRA for testing drug susceptibility to antituberculosis drugs revealed both assays as comparable, rapid, low-cost methods [31]. A similar study which compared nitrate reduction assay, MTT assay and Colorimetric Resazurin Microtitre Assay (REMA) showed that area under the curve (AUC) values obtained for the test drugs for MTT and REMA were similar [32]. That study further revealed that sensitivity and specificity for MTT and CRA were good and comparable [32]. These results may explain why CRA thrives in Mycobacterial culture studies [4,5]. To further show its usefulness in bacterial studies, CRA has been found reliable in HTS of bacteria for radiation sensitivity [28]. From our work and other previously published literature, it does appear that CRA has adequate sensitivity for bacterial but not for human cells. The dissimilarity in cellular constituents might be responsible for this variance. This study further agrees with previous reports that cell type may affect RA results [33].
Conclusion
This study evaluated CRA as a possible tool inhuman respiratory mucosal cell irritation and toxicity screening and found it less sensitive than MTT. The dissimilarity between the cellular constituents of human and bacterial cells may be affecting the sensitivity of CRA. Furthermore, we conclude that CRA is deficient as a tool in human respiratory mucosal cell studies.
References
- Katawera V, Siedner M, Boum Ii Y (2014) Evaluation of the modified colorimetric resazurin microtiter plate-based antibacterial assay for rapid and reliable tuberculosis drug susceptibility testing. BMC Microbiol 14: 259.
- Riss TL, Moravec RA, Niles AL, Benink HA, Worzella TJ, et al. (2004) Cell Viability Assays. In: Sittampalam GS, et al. (Eds.,) Assay Guidance Manual. Bethesda (MD): Eli Lilly & Company and the National Center for Advancing Translational Sciences.
- O Brien J, Wilson I, Orton T, Pognan F (2000) Investigation of the Alamar Blue (resazurin) fluorescent dye for the assessment of mammalian cell cytotoxicity. Eur J Biochem 267(17): 5421-5426.
- Miyata M, Pavan FR, Sato DN, Marino LB, Hirata MH, et al. (2013) Comparison of resazurin microtiter assay performance and BACTEC MGIT 960 in the susceptibility testing of Brazilian clinical isolates of Mycobacterium tuberculosis to four first-line drugs. Braz J Microbiol 44(1): 281-285.
- Sanchotene KO, von Groll A, Ramos D, Scholante AB, Honscha G, et al. (2008) Comparative evaluation of the Nitrate Reductase Assay and the Resazurin Microtitre Assay for drug susceptibility testing of Mycobacterium tuberculosis against first line anti-tuberculosis drugs. Braz J Microbiol 39(1): 16-20.
- Czekanska EM (2011) Assessment of Cell Proliferation with Resazurin- Based Fluorescent Dye. In: Stoddart MJ (Ed). Mammalian Cell Viability. Methods Mol Biol 740: 27-32.
- Heredero-Bermejo I, Copa-Patino JL, Soliveri J, Gômez R, de la Mata FJ, et al. (2013) In vitro comparative assessment of different viability assays in Acanthamoeba castellanii and Acanthamoeba polyphaga trophozoites. Parasitol Res 112(12): 4087-4095.
- Werner M, Biss K, Jérôme V, Hilbrig F, Freitag R, et al. (2013) Use of the mitochondria toxicity assay for quantifying the viable cell density of microencapsulated jurkat cells. Biotechnol Prog 29(4): 986-993.
- Mao W, Chen X, Yang T, Yin Y, Ge M, et al. (2012) A rapid fluorescent screening method for cellular sensitivity to anti-cancer compound. Cytotechnology 64(4): 451-457.
- Cui Z, Wang J, Lu J, Huang X, Zheng R, et al. (2013) Evaluation of methods for testing the susceptibility of clinical Mycobacterium tuberculosis isolates to pyrazinamide. J Clin Microbiol 51(5): 1374-1380.
- Bwanga F, Joloba ML, Haile M, Hoffner S (2010) Evaluation of seven tests for the rapid detection of multidrug-resistant tuberculosis in Uganda. Int J Tuberc Lung Dis 14(7): 890-895.
- Hamid R, Rotshteyn Y, Rabadi L, Parikh R, Bullock P (2004) Comparison of alamar blue and MTT assays for high through-put screening. Toxicol In Vitro 18(5): 703-710.
- Tritten L, Braissant O, Keiser J (2012) Comparison of novel and existing tools for studying drug sensitivity against the hookworm Ancylostoma ceylanicum in vitro Parasitology 139(3): 348-357.
- Lutfi AN, Kannan TP, Fazliah MN, Jamaruddin MA, Saidi J (2010) Proliferative activity of cells from remaining dental pulp in response to treatment with dental materials. Aust Dent J 55(1): 79-85.
- Carter RA, Ericsson SA, Corn CD, Weyerts PR, Dart MG, et al. (1998) Assessing the fertility potential of equine semen samples using the reducible dyes methylene green and resazurin. Int J Oncol 3(3): 473476.
- Page B, Page M, Noel C (1993) A new fluorometric assay for cytotoxicity measurements in-vitro. Int J Oncol 3(3): 473-476.
- Nakayama GR, Caton MC, Nova MP, Parandoosh Z (1997) Assessment of the Alamar Blue assay for cellular growth and viability in vitro. J Immunol Methods 204(2): 205-208.
- Iversen PW, Eastwood BJ, Sittampalam GS, Cox KL (2006) A comparison of assay performance measures in screening assays: signal window, Z' factor, and assay variability ratio. J Biomol Screen 11(3): 247-252.
- Sherman SP, Pyle AD (2013) Small molecule screening with laser cytometry can be used to identify pro-survival molecules in human embryonic stem cells. PLoS One 8(1): e54948.
- Ozawa M, Shimojima M, Goto H, Watanabe S, Hatta Y, et al. (2013) A cell-based screening system for influenza A viral RNA transcription/ replication inhibitors. Sci Rep 3: 1106.
- Shun TY, Lazo JS, Sharlow ER, Johnston PA (2011) Identifying actives from HTS data sets: practical approaches for the selection of an appropriate HTS data-processing method and quality control review. J Biomol Screen 16(1): 1-14.
- Zhang JH, Chung TD, Oldenburg KR (1999) A Simple Statistical Parameter for Use in Evaluation and Validation of High Throughput Screening Assays. J Biomol Screen 4(2): 67-73.
- Zhang JH, Chung TD, Oldenburg KR (2000) Confirmation of primary active substances from high throughput screening of chemical and biological populations: a statistical approach and practical considerations. J Comb Chem 2(3): 258-265.
- Larson EM, Doughman DJ, Gregerson DS, Obritsch WF (1997) A new, simple, nonradioactive, nontoxic in vitro assay to monitor corneal
- Al-Nasiry S, Geusens N, Hanssens M, Luyten C, Pijnenborg R (2007) The use of Alamar Blue assay for quantitative analysis of viability, migration and invasion of choriocarcinoma cells. Hum Reprod 22(5): 1304-1309.
- Perrot S, Dutertre-Catella H, Martin C, Rat P, Warnet JM (2003) Resazurin metabolism assay is a new sensitive alternative test in isolated pig cornea. Toxicol Sci 72(1): 122-129.
- Mosmann T (1983) Rapid colorimetric assay for cellular growth and survival: application to proliferation and cytotoxicity assays. J Immunol Methods 65(1-2): 55-63.
- Hudman DA, Sargentini NJ (2013) Resazurin-based assay for screening bacteria for radiation sensitivity. Springerplus 2(1): 55.
- Lenoir J, Bachert C, Remon JP, Adriaens E (2013) The Slug Mucosal Irritation (SMI) assay: a tool for the evaluation of nasal discomfort. Toxicol In Vitro 27(6): 1954-1961.
- Van der Vorst JR, Schaafsma BE, Verbeek FPR, Hutteman M, Mieog JSD, et al. (2012) Randomized comparison of near-infrared fluorescence imaging using indocyanine green and 99(m) technetium with or without patent blue for the sentinel lymph node procedure in breast cancer patients. Ann Surg Oncol 19(13): 4104-4111.
- Martin A, Morcillo N, Lemus D, Montoro E, Teiles MA, et al. (2005) Multicenter study of MTT and resazurin assays for testing susceptibility to first-line anti-tuberculosis drugs. Int J Tuberc Lung Dis 9(8): 901906.
- Montoro E, Lemus D, Echemendia M, Martin A, Portaels F, et al. (2005) Comparative evaluation of the nitrate reduction assay, the MTT test, and the resazurin microtitre assay for drug susceptibility testing of clinical isolates of Mycobacterium tuberculosis. J Antimicrob Chemother 55(4): 500-505.
- Rodriguez Antona C, Donato MT, Boobis A, Edwards RJ, Watts PS, et al. (2002) Cytochrome P450 expression in human hepatocytes and hepatoma cell lines: molecular mechanisms that determine lower expression in cultured cells. Xenobiotica 32(6): 505-520.






























