Toxicity of Acacia Nilotica (Garad) to Nubian Goats
Medani AB*, Samia MAE2, Ahmed EA2
1Department of Pharmacology & Toxicology, Nile College, Sudan
2Department of Pharmacology & Toxicology, University of Khartoum, Sudan
Submission: March 10, 2016 ; Published: March 24 2016
*Corresponding author: Medani AB, Assistant Professor of Pharmacology & Toxicology, Nile College, Khartoum, Sudan, Tel: +249912369706; E-mail: amna_medani@ yahoo.com
How to cite this article: Medani AB, Samia M, Ahmed E, . Toxicity of Acacia Nilotica (Garad) to Nubian Goats. Open Acc J of Toxicol. 2016;1(2): 555555. DOI: 10.19080/OAJT.2016.01.555555
Abstract
Variable plants present in nature are used by simple rural and urban people, researchers and drug manufacturers for medicinal purposes. Garad is one of the most commonly used in Sudan for both treatment and prophylaxis of infections in the respiratory and urinogenital tracts and the skin. Water extracts from Acacia Nilotica bods were used in this very experiment to test for their toxicity to Nubian goats at two dose rates under proper experimental conditions. The clinical, pathological, hematological and biological changes in Nubian goats given daily oral doses of 1 and 5 g/kg body weight of Acacia Nilotica to two groups of goats. The goats of the control group were not dosed with Acacia Nilotica. Other than the dose co-related mortality rates, the clinical signs were observed to be salivation, staggered gait, intermittent loss of voice and low appetite. On histopathological testing, the main lesions were hepatic centrolobular necrosis and fatty changes associated with the significant changes in GGT and ALP were indicating hepatic dysfunction .Renal malfunction is indicated by hemorrhages in addition to the change in urea concentration. The congested, hemorrhagic, emphysematous, edematous and cyanotic lungs may contribute to the development of dyspnea. Acacia Nilotica poisoning may lead to an immuno suppression pointed out by the lymphocyte infiltration. On evaluation of the above results Acacia Nilotica was considered toxic to Nubian goats at the above mentioned doses. Future work for Acacia Nilotica was forwarded and practical implications of the result were highlighted.
Keywords: Acacia Nilotica; Toxicity Data; Nubian Goats; Garad
Abbreviations: RBC: Red Blood Cells; GGT: Gamma Glutamyl Transferase; AST: Aspartate Amino Transferase; ALP: Alkaline Phosphatase
Introduction
Acacia Nilotica (Mimosaceae) is a tree found in the central and Northern parts of the Sudan and in Egypt. It is known in Sudanese folk medicine by the common name 'Garad' (Figure 1). Some 160 Acacia species are natives of Africa and the Near East. They are reported to be utilized for fuel, timber, forage, gum, tannins, fiber, medicine, food, handicrafts, domestic utensils, environmental protection, soil fertility, shade and shelter, game refuge, amenity and ornamental plantings, and agro-forestry [1]. Tannin extracts of Acacia Nilotica exhibited algicidal properties and molluscidal activity against the fresh water snails Bulinus truncatus and Biomphalaria pfeifferi which transmit schistosomiasis in Sudan [2-4]. The gum, stem bark, leaves and fruits of Acacia Nilotica have been used medicinally for colds, bronchitis, pneumonia, ophthalmic, diarrhea and hemorrhage [5]. The fruit juice and the stem bark are used as a haemostatic agent [6]. The fruit and the stem bark are regarded as tonics and astringents and are used internally to treat diarrhea and dysentery [7]. A decoction of the fruits, which is considered to be a febrifuge, is used internally as a remedy for sore throat, chest complaints, dysentery and leprosy. The water extract is used externally to treat syphilitic lesions and other venereal diseases [8-11]. In the Sudanese traditional medicine an infusion of about 5 g of the fruits of Acacia Nilotica in 200 ml cold water overnight is used to treat sore throat and common cold. In a report on the antibacterial effect of Acacia Nitotica fruit extracts by Abd M, et al. [12], 25.6 g were suspended in 250 ml water at different but constant temperatures. The filtrate was found active against some bacteria and fungi. On a study involved the evaluation of anti-inflammatory activity of Acacia Nilotica pods in different experimental models of inflammation, it was suggested that the mechanism of action of the methanolic extract of Acacia Nilotica may be related to inhibition of prostaglandin synthesis. The methanolic extract of Acacia Nilotica pods was found to possess a significant anti-inflammatory activity. The tannin fraction of the methanolic extract was found to be more potent than the methanol extract alone. This was supported by the literature that indicates that the major categories of compounds that modulate the inflammatory pathways are polyphenolics [6]. Aqueous extracts of Acacia Nilotica pods from a commercial source were tested for its anti-inflammatory, analgesic and antipyretic properties in rat and mice models ,it inhibited the development of carrageenan induced paw edema and yeast-induced pyrexia in rats [13]. The decoction of the stem bark and root has been claimed to have an intoxicating effect as a nerve stimulant [14]. It was used traditionally for the treatment of sore throat, colds, pneumonia, ophthalmic, dysentery, leprosy ,venereal diseases and hemorrhages due to its tonic ,astringent and stimulant properties .The aqueous extract of fruits showed activity against Candida albicans, gram positive and gram negative bacteria [12].

Materials and Methods
Animals
Nine 5-7 month old male Nubian goats' kids were purchased from a local livestock market in the vicinity of Omdurman and housed in pens within the premises of the Veterinary Teaching Hospital, University of Khartoum. Animals were clinically adapted to proper health conditions for a period of two weeks.
Administration of the plant fruits
At the end of the adaptation period, the animals were weighed and allotted into three groups each of three. (Group 1) were the un-dosed control group. Acacia pods were ground and given by drench at 1 g/kg/day to (Group 2) goats and at 5 g/kg/ day to (Group 3) goats.
Parameters
Clinical signs and mortality rates were recorded. Blood samples were obtained from the jugular vein before the start of the experiment and thereafter at a week interval for hematological investigations (Hb count, packed cell volume PCV, RBC ,mean corpuscular volume MCHC ) and serum analysis (for activity of gamma glutamyl transferase (GGT), Aspartate amino transferase (AST), Alkaline phosphatase (ALP) and concentration of creatinine ,urea, total protein albumin, calcium and phosphorus) .
Statistical Methods
The difference between mean values of data was analyzed by the un-paired students- t-test [15].
Results
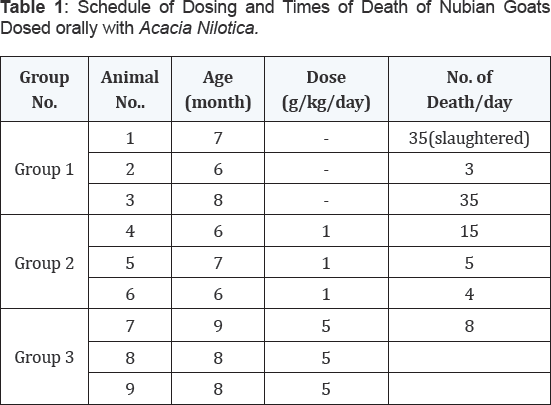
The experimental plan, doses and times of death are given in Table 1.
Clinical signs
In goats of group (2) receiving oral doses of 1 g/kg/day of Acacia Nilotica, the clinical signs observed included salivation, intermittent loss of voice and in coordination in movement .One goat form this group died after 3 days of dosing ,another died on day 15 and the last goat in this group died on day 35.Goats of group (3) which received oral doses of 5 g/kg/day of Acacia Nilotica pods, the prominent signs observed from day one of dosing was the thick saliva (Figure 2), loss of voice, staggered movement, recumbence (Figure 3), loss of appetite and all goats of this group died between day 4 and day 8 of the experiment. The un-dosed control goats showed no abnormality.


Post-mortuum findings
The principal lesions in goats of group (2) that received oral doses of 1g/kg/day of Acacia Nilotica were congested lung and kidney, fatty liver and congested intestine. In goats of group (3) which received the fruit extract at a dose of 5g/kg/day, the lesions were almost the same, but more severe.
Histopathological signs
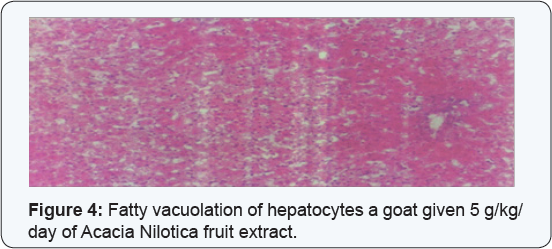
On microscopy, in goats of both dosed-groups were fatty vacuolations of the hepatocytes with generalized necrosis (Figure 4) congested pulmonary blood vessels, necrotic renal convoluted tubules, congested cardiac blood vessels and catarrhal enteritis. The control goats in group (1) showed normal histopathological picture (Table 2A, 2B).
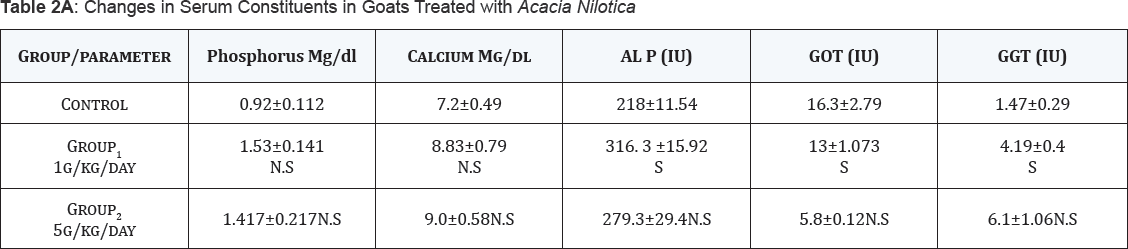
According to Table 2A, 2B, goats of group (2) dosed with the extract of Acacia Nilotica fruit at 1g/kg/day, the concentration of creatinine and the activity of alkaline phosphatase (ALP) were higher (P<0.05-0.01) than the control group. Urea concentration and gamma Glutamyl transferase (GGT) activity were found to show significant increase (P<0.05-0.01) in goats of group (2) dosed with the extract of Acacia Nilotica fruit at 1g/kg/day and those of group (3) dosed with Acacia Nilotica extract at 5g/kg/day in comparison with the values of the same parameters showed by the normal un-dosed control group. There were no changes in the concentrations of phosphorus, albumin and calcium nor in the activity of aspartate amino-transferase (AST) in under-tested goats of both groups compared versus the control group.
Haematological changes
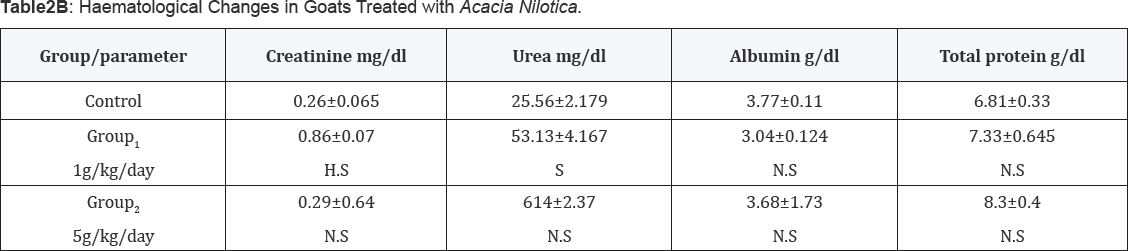
These are given in Table 3, goats dosed orally with Acacia Nilotica at 1g/kg/day or 5 g/kg/day showed no changes in mean corpuscular volume (MCV), packed cell volume (PCV) and mean corpuscular hemoglobin concentration (MCHC) values. The hemoglobin concentration (Hb) and the red blood cells (RBC) counts were lower (P<0.05-0.01) than those normal values manifested by the un-dosed control group.

Discussion
Oral daily dosing with 1 g/kg/day of Acacia Nilotica showed low appetite, excessive salivation which may be due to the bitter taste of the fruit extract, intermittent loss of voice as a result of the effect of tannin and staggered gait and ataxia as an initial sign of cerebral palsy in form of polymyositis including muscle weakness and joint pain clarified in obvious clinical signs induced by Acacia Nilotica as a neurotoxin due to its high carbon and sulphur content [16]. Although Acacia Nilotica was claimed by Kannan, et al. [17] to have a protective effect against acetaminophen induced hepato cellular damage in wester rats, oral daily dosing with 5 g/kg/day of Acacia Nilotica caused a centrilobular necrosis accompanied by fatty change in the liver as suggestive indication of hepatic dysfunction which is also shown by the change in the activity of GGT and ALP [18]. This may target the liver as a biotransformation and detoxification engine for Acacia Nilotica [19]. On the other hand this may target Acacia Nilotica as an antioxidant drug against free radicals associated with cancer treatment in a future research recommendation [20]. The hemorrhagic kidney in addition to the change in urea concentration are functional biomarkers for renal dysfunction [21,22]. Marked pulmonary lesions were found in the undertest goats manifested by congestion, hemorrhages, emphysema, cyanosis and sometimes edema which are all biomarkers for pulmonary dyspnea [23]. The obvious lymphocyte infiltration is pointing out to the development of a cardiopulmonary complication induced by the under test herb [24] immuno suppression [25]. The high mortality rate may be explained as an ischemic sequel of dosing with Acacia Nilotica to tested goats [26].
References
- Wickens GE, Seif El Din AG, Sita G, Nahal I (1995) FAO Cooperate Document Repository, Forestry Department, Food and Agriculture Organization of United Nations.
- Hussein Ayoub SM (1984) Effect of the galloyl group on the molluscicidal activity of tannins. Fitoterapia 5: 343-345.
- Selestin DS, Juliette K, Faustin D, Sylviane S, Barnabe LN, et al. (2013) Acute and chronic anti-inflammatory effects of the aqueous extract of Acacia Nilotica (L.) Del. (Fabaceae) pods. Acad J Med Plants 1(1): 001005.
- Schaufelberger D, Hostettmann K (1983) On the molluscicidal activity of tannin containing plants. Planta Med 48(2): 105-107.
- Umalkar GV, Begum S, Nehemiah KMA (1977) Inhibitory effect of Acacia Nilotica extracts on pectolytic enzyme production by some pathogenic fungi. Indian Phytopath 29(4): 469-470.
- Tabassum KA, Anjaria JK, Dedhia V, Gohel AK (2015) Bioactivity Guided Fractionation and Anti-inflammatory Activity of Acacia Nilotica Pods. Int J Pharm Pharm Sci 7(7): 380-383.
- Ali A, Akhtar N, Ali Khan B, Shoaib Khan M, Rasul A, et al. (2012) Acacia Nilotica: A plant of multipurpose medicinal uses. Journal of Medicinal Plants Research 6(9): 1492-1496.
- Bachaya HA, Iqbal Z, Khan MN, Sindhu ZD, Jabbar A (2009) Anthelmintic activity of Ziziphus nummularia (bark) and Acacia Nilotica (fruit) against Trichostrongylid nematodes of sheep. J. Ethnopharmacol 123(2): 325-329.
- El-Tahir A, Satti GMH, Khalid SA (1999) Antiplasmodial Activity of Selected Sudanese Medicinal Plants with Emphasis on Acacia Nilotica. Phytother Res 13(6): 474-478.
- Mouhssen Lahlou (2004) Study of the Molluscicidal Activity of Some Phenolic Compounds: Structure-Activity Relationship. Pharmaceutical Biology 42(3): 258-261.
- Venkataswamy R, Sukumar M, Doss H, Mubarack HM (2010) Phytochemical, HPTLC finger printing and antibacterial activity of Acacia Nilotica (L.) Delile. J Drug Med 2(2): 38-42.
- Abd Al-Nabi, Reisinger OM, Reinthaler EC, Still FF U, Krejs,GJ (1992) Antimicrobial activity of Acacia Nilotica (L) Wild. Exdel Var Nilotica Journal of Ethnopharmacology 37: 77.
- Dafallah AA, al-Mustafa Z (1996) Investigation of the anti-inflammatory activity of Acacia Nilotica and Hibiscus sabdariffa. Am J Chin Med 24(3- 4): 263-269.
- Watt JM, Breyer-Brandwijk MB (1962) The medicinal and poisonous plants of southern and eastern Africa. (2nd edn), Livingstone ES, Edinburgh & London, United Kingdom, pp. 1457.
- Snedecor GW, Cochran WG (1989) Statistical Methods, 8th edn. Lowa State University Press, USA.
- Kumar NJI, Patel K, Kumar RN, Bhoi RK (2009) An assessment of Indian fuel wood with regards to properties and environmental impact. As J Energy Env 10(2): 99-107.
- Narayanan K, Sakthivel KM, Guruvayoorappan C (2013) Protective Effect of Acacia Nilotica (L.) against Acetaminophen-Induced Hepato cellular Damage in Wistar Rats. Advances in Pharmacological Sciences (2): 987692 .
- Mohan S, Thiagarajan K, Chandrasekaran R, Arul J (2014) In vitro Protection of Biological Macromolecules against Oxidative Stress and in vivo Toxicity Evaluation of Acacia Nilotica (L.) and Ethylgallate in Rats. BMC Complement Altern Med 14: 257.
- Cemek M, Aymelek F, Buyukokuroglu ME, Karaca T, Buyukben A, et al. (2010) Protective potential of Royal Jelly against carbon tetrachloride induced-toxicity and changes in the serum sialic acid levels. Food and Chemical Toxicology 48(10): 2827-2832.
- Singh R, Singh B, Singh S, Kumar N, Kumar S, et al. (2008) Anti-free radical activities of kaempferol isolated from Acacia Nilotica (L.) Willd. Ex. Del. Toxicol in Vitro 22(8): 1965-1970.
- De Geus HR, Betjes MG, Bakker J (2012) Biomarkers for the Prediction of Acute Kidney Injury: A Narrative Review on Current Status and Future Challenges. Clin Kidney J 5(2): 102-108.
- Paragas N, Qiu A, Zhang Q, Samstein B, Deng SX, et al. (2011) The Ngal reporter mouse detects the response of the kidney to injury in real time. Nat Med 17(2): 216-222.
- Matthay MA, Zimmerman GA (2005) Acute lung injury and the acute respiratory distress syndrome: four decades of inquiry into pathogenesis and rational management. Am J Respir Cell Mol Biol 33(4): 319-327.
- Butler J, Rocker GM, Westaby S (1998) Inflammatory response to cardiopulmonary bypass. Ann Thorac Surg 55(2): 552-559.
- Nickerson P (2009) Post-transplant monitoring of renal allografts: are we there yet? Curr Opin Immunol 21(5): 563-568.
- Mishra J, Mori K, Ma Q, Kelly C, Yang J, et al. (2004) Amelioration of ischemic acute renal injury by neutrophil gelatinase-associated lipocalin. J Am Soc Nephrol 15(12): 3073-3082.






























