Is the Ventriculo-Peritoneal Shunt Failure Predictable? A Novel Approach
Mohammed Bangash*, Rana Moshref and Mohamed Mosaad Alfiky
Division of Neurosurgery, Department of Surgery, Faculty of Medicine, King Abdulaziz University, Saudi Arabia
Submission: December 20, 2021; Published: January 18, 2022
*Corresponding author: Mohammed Bangash, MD, FRCSC, Division of Neurosurgery, Department of Surgery, Faculty of Medicine, King Abdulaziz University, PO Box 80215, Jeddah 21589, Saudi Arabia
How to cite this article: Mohammed Bangash*, Rana Moshref and Mohamed Mosaad Alfiky. Is the Ventriculo-Peritoneal Shunt Failure Predictable? A Novel Approach. Open Access J Neurol Neurosurg 2021; 16(4): 555944. DOI: 10.19080/OAJNN.2022.16.555944.
Abstract
Hydrocephalus is a neurosurgical emergency that results from dysfunction of cerebrospinal fluid (CSF) flow, whether through increasing synthesis or decreasing flow with or without an absorption problem. Shunt failure predisposes the patient to serious morbidity and possible mortality, so prompt detection of malfunction can be lifesaving. We propose a scoring system that may help in the prediction of the shunt procedure outcome and in early detection of shunt failure. This is a retrospective cohort study that included data from a 10-year period (January 2010–December 2020) at a single academic center in Jeddah, Saudi Arabia. A scoring system determined mortality and morbidity factors related to ventriculoperitoneal (VP) shunt placement. The scoring system was designed based on the significant odds ratio (OR) and the correlation of the different variables. Risk factors scored a minimum of 0, a maximum of 3, and a total of 9. A total number of 404 patients were screened, and a total of 303 patients were included in the study. The scoring system was graded as low (score 0–1), medium (score 2–3), and high (score >4), with risk for mortality with a specificity of 59.72% and sensitivity of 46.42%. There is no available scoring system to predict mortality. The current study suggests using a scoring system that can predict and identify high-risk patients, allowing close observation and, it is hoped, a reduction in the mortality rate.
Keywords: ventriculoperitoneal shunt; failure; scoring; mortality
Introduction
Hydrocephalus is a neurosurgical emergency that results from dysfunction of cerebrospinal fluid (CSF) flow, whether through increasing synthesis or decreasing flow with or without an absorption problem [1]. It can present acutely or in a chronic form, such as normal-pressure hydrocephalus [2]. Hydrocephalus has an incidence range of 79–124 per 100,000 births in high-income and low-income countries [2,3]. Clinical presentation includes symptoms of increased intracerebral pressure from cerebrospinal fluid accumulation, such as nausea, vomiting, headache, and visual problems, followed later by sensory and motor loss; it will lead eventually to cardiac and respiratory failure from brain stem compression [4]. A plain brain CT is often utilized in diagnosing hydrocephalus, followed by MRI [5]. The treatment of hydrocephalus consists mainly of cerebrospinal fluid diversion, which has different modalities, including diversion of cerebrospinal fluid from ventricles to the peritoneal cavity, atrium, or pleura. The diversion device consists of a proximal ventricular catheter, a valve, and distal peritoneal catheters [6]. The first surgical treatment, performed by Hippocrates in the fifth century BC, involved insertion of a catheter into the lateral ventricle [7]. The most commonly used modality for managing hydrocephalus is ventriculoperitoneal (VP) shunt insertion, which was initially described in 1908 and introduced to practice in 1967 with a silastic catheter and open laparotomy. It involved connecting a valve percutaneously with a distal catheter in the right upper abdominal quadrant [8].
However, shunt failure is reported with causes that include infection, obstruction, over-drainage, and distal catheter failure [9]. Shunt failure has been reported in up to 50% of patients, especially in those patients where hydrocephalus was associated with hemorrhage and congenital etiologies [10]. Risk of complications can be decreased by proper sterilization and timely antibiotic prophylaxis [11], limitation of operating room personnel, shorter operative time [12], and uniform technicality in procedures [13]. Shunt failure predisposes the patient to serious morbidity and possible mortality [14], so prompt detection of malfunction can be lifesaving. A diagnosis of shunt failure is initially studied by CSF examination [15,16] and neuroimaging [17]. However, to our knowledge, there is no tool available in the literature that measures different factors that may facilitate early anticipation of failure. In this article, we study the different factors associated with shunt failure, with morbidity and mortality as a primary outcome. In addition, we propose a scoring system that may assist in prediction of the shunt procedure outcome and early detection of VP shunt failure.
Methods
This is a retrospective cohort study that included data from a 10-year period (2010–20) from a tertiary center in Jeddah, Saudi Arabia. Participants were selected by convenience sampling, as inclusion criteria involved patients who underwent VP shunt insertion and the exclusion criteria involved patients who underwent unrelated abdominal surgeries. Data were collected from assessing patients’ medical records via an electronic system at King Abdulaziz University Hospital within a 10-year interval (January 2010–December 2020), including patients’ profile (age, sex, date of admission), diagnosis, mortality, CSF infection/ colonization, VP shunt date, duration of surgery, valve type, abdominal incision location, antibiotics, shunt revision date, and reason. The data were stored in a Microsoft Excel spreadsheet and were accessible only by authors within a 1-year period before disposal.
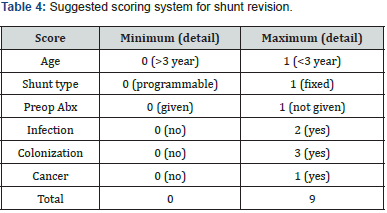
Shunt infection was defined according to Overturf’s criteria: shunt dysfunction with fever 38°C or higher, peritoneal symptoms, or purulent discharge along the shunt track and positive cultures from the ventricular catheter, shunt reservoir, and/or distal catheter; shunt-associated ventriculitis was indicated by ventricular CSF containing more than five leukocytes and with the same microorganism growing in the shunt device and CSF cultures [18]. Data were analyzed in SPSS version 20 (IBM SPSS Statistics for Windows, version 20.0, Armonk, NY). Both descriptive and inferential statistics were done. Pearson’s chi-square test, odds ratio (OR), and 95% confidence interval (CI) were calculated. All p-values < 0.5 were considered statistically significant. A scoring system determined mortality and morbidity factors related to VP shunt placement. Spearman’s correlation was used to investigate the relationship between variables. The scoring system was designed based on the significant OR and the correlation of the different variables. Risk factors scored a minimum of 0, a maximum of 3, and a total of 9. They were then categorized into low, medium, and high risk factors. The study followed the ethical standards of the Helsinki Declaration. Approval was granted by the Unit of Biomedical Ethics of King Abdulaziz University Hospital (KAUH; reference no. 5896321).
Results
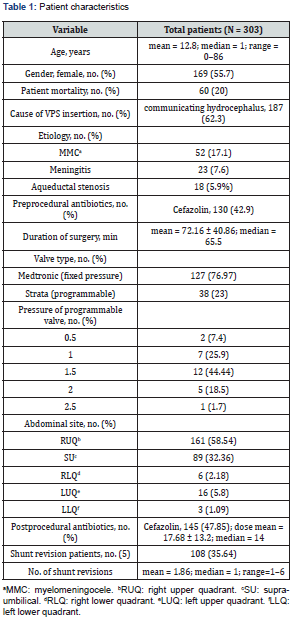
A total of 404 patients were screened for a 10-year period (January 2010–December 2020) in a single academic center in Jeddah, Saudi Arabia. The data were collected from the hospital information system (HIS); 101 patients were excluded (35 had unrelated abdominal surgeries, and 66 had no VP shunt surgeries). A total of 303 patients were included in the study. The median age was 1 year, range 0–86 years, and 55.77% (n = 169) were female. There was 19.8% (n = 60) mortality in patients who underwent VP shunt surgery. Patients’ characteristics are listed in Table 1.
Determinants of Shunt Revision
Shunt revision is affected by multiple factors. Most of the patients (64.35%; n = 195) did not have shunt revisions. Patients who underwent shunt revision had a mean number of revisions 1.86, with a range 1–6. There was no correlation between shunt malfunction and either comorbidities (diabetes mellitus, hypertension, seizures, hypothyroid, cancer, metastasis) in the adult age group or anomalies in the pediatric age group. It was found that 62.3% (n = 187) of patients had communicating hydrocephalus. The causes of shunt insertion are listed in Table 2, followed by causes of shunt revision in Table 3. The most commonly used preoperative antibiotic was cefazolin in 130 patients (42.9%), followed by no antibiotics in 90 patients (30%), not mentioned in 30 (10%), vancomycin in 15 (4.9%), cefuroxime in 14 (4.6%), ceftriaxone in 10 (3.3%), meropenem in 8 (2.6%), gentamycin in 6 (1.9%), cloxacillin in 5 (1.6%), ampicillin in 4 (1.3%), cefotaxime in 2 (0.06%), clindamycin in 2 (0.06%), ceftazidime in 1 (0.03%), and cephalexin in 1 (0.03%).
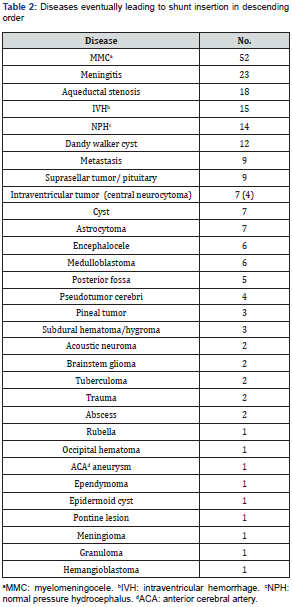
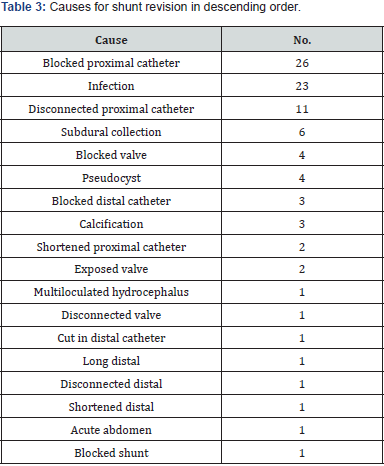
Length in time of surgery was correlated with shunt revision. Higher numbers of revisions were associated with timings of more than 180 min, while a timing of 70–180 min had the lowest risk of revision (p = 0.04).
Number of shunt revisions was highest in the group aged younger than 3 years. Most of these patients had a standard medium pressure valve (76.97%; n = 127), followed by a programmable valve (23%; n = 38).
It was found that the most common location for peritoneal catheter insertion was the right upper quadrant, in 161 patients (58.54%), followed by midline incision in 89 patients (32.36%). Abdominal incision site location did not show a significant correlation with shunt revision (p = 0.5). The most common postprocedural antibiotic was cefazolin in 145 patients (47.85%), followed by vancomycin in 34 patients (11.22%), no antibiotics in 30 patients (10%), cefuroxime in 20 patients (6.6%), meropenem in 19 patients (6.3%), ceftriaxone in 18 patients (5.9%), gentamycin in 8 patients (2.6%), ampicillin in 7 patients (2.3%), and cloxacillin in 6 patients (1.9%); other antibiotics were administered in less than 1% of patients, including cloxacillin, ceftazidime, pyridoxine, isoniazide, cefotaxime, pipracillin tazosin, metronidazole, pyrazinamide, ciprofloxacin, clindamycin, fluconazole, doxycycline, amikacin, rifampin, and acyclovir. The mean number of postoperative doses was 17.68 ± 13.2. It was found that patients who received six doses of antibiotics postoperatively had the lowest risk of shunt revision. On the other hand, those who received three doses of antibiotics had a 1.6-fold increased risk of shunt revision (p = 0.052).
Scoring System
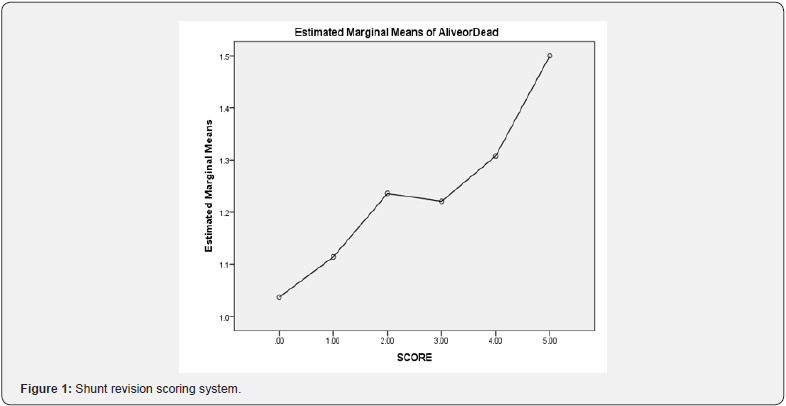
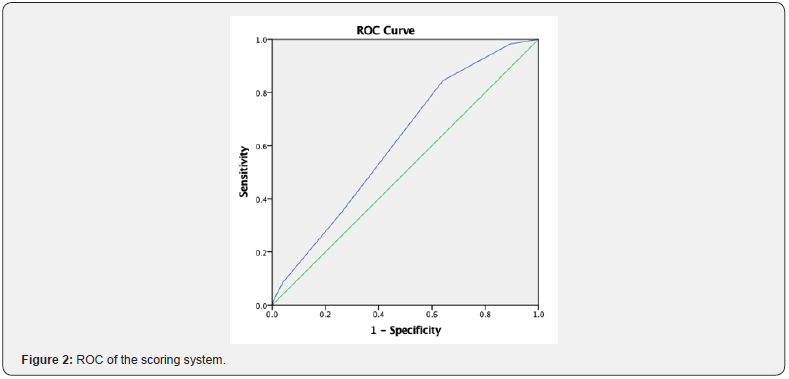
The scoring system was determined by age, shunt type, preoperative antibiotics, infection, colonization, and cancer with Cronbach’s alpha 0.612 (p = 0.042), which is shown in Table 4. The scoring system was graded as low, medium, and high, with risk of mortality with specificity of 59.72% and sensitivity of 46.42%, with a low-risk score of 0–1, a medium-risk score of 2–3, and a high-risk score of ≥4. Low, medium, and high risk had 1-, 1.3-, and 1.5-fold increased risk of mortality, respectively (Figures 1 & 2). The area under the curve was 0.61 (p = 0.007; CI 0.53–0.68).
Discussion
In our article, we included 303 patients with an average age of 12 years. Shunt revision was 36% with a mean number of 1.86. The data are quite comparable to Indian and Turkish studies, where the range of patients 20%–23% underwent a shunt redo [18–20]. However, Reddy et al. [21] showed that pediatric-onset hydrocephalus had an incidence of 82.9% shunt failure that could be explained by prolonged follow-up of nearly 20 years. There was an 80% survival rate, which is the same in Ghritlaharey et al. [14,22], who included 40 pediatric patients and found a mortality post–shunt placement of nearly 20% and 4% in revision. As most of our patients were of pediatric age, there was no correlation with chronic disease. The most common cause of shunt insertion was communicating hydrocephalus. We found that the most commonly used antibiotic was cefazolin, followed by no antibiotics. The lack of preoperative antibiotic administration can be related to overlocking by the medical team or the administration’s lack of documentation. No studies indicated the type and dosage of antibiotics to use preoperatively, but most studies mentioned that the most common organism causing infection is Staphylococcus aureus at 82% and that the authors followed culture and sensitivity [14].
Administration of six doses of antibiotics was linked to a decreased infection rate in our study. The most common causes of revision are blockage or disconnection of the proximal catheter at 40%, which is also similar to previous studies, which found the most common cause to be shunt blockage and migration at 20%– 30% [18,23]. Various risk factors affect morbidity and mortality with a VP shunt. Researchers have found that interventricular hemorrhage, low birth weight, and infections were linked to decreased survival in neonates [24,25]; however, we found no studies that linked the mortality to children and adults. Several studies linked meningitis, use of steroids, previous cranial surgery, and brain tumors to shunt revision [19,23,26], whereas antibiotic impregnated catheter and frontal burr hole were protective factors [27].
The Scoring System
The studies available to predict the outcome of VP shunt in children are limited [28,29]. There is no available scoring system to predict mortality. The current study suggests using a scoring system that can predict and identify high-risk groups, allowing closer observation and, it is hoped, a reduction in mortality rate. The best setting for using this scoring system is upon discharge of the patient after insertion of the VP shunt, then in outpatient follow-up. Those who are not at low risk of mortality need to be more closely followed and seen more frequently than those in low-risk groups.
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Conflict of Interest statement
The authors declare that the article content was composed in the absence of any commercial or financial relationships that could be considered as a potential conflict of interest.
Acknowledgment
The authors extend their thanks to colleagues in the neurosurgery section: Prof. Saleh Baessa, Dr. Bassam Addas, Dr. Abdulrahman Sabbagh, Dr. Soha Alomar, and Dr. Mohammed Alyousef.
References
- Kahle KT, Kulkarni AV, Limbrick DD Jr, Warf BC (2016) Hydrocephalus in children. Lancet 387 (10020): 788-799.
- Leinonen V, Vanninen R, Rauramaa T (2017) Cerebrospinal fluid circulation and hydrocephalus. Handb Clin Neurol 145: 39-50.
- Dewan MC, Rattani A, Mekary R, Glancz LJ, Yunusa I, et al. (2018) Global hydrocephalus epidemiology and incidence: systematic review and meta-analysis. J Neurosurg 1:1–15.
- Freeman WD (2015) Management of intracranial pressure. Continuum 21(5): 1299–323.
- Langner S, Fleck S, Baldauf J, Mensel B, Kuhn JP, et al. (2017) Diagnosis and differential diagnosis of hydrocephalus in adults. Rofo 189(8): 728–739.
- Fowler JB, De Jesus O, Mesfin FB (2021) Ventriculoperitoneal shunt. Treasure Island, FL: StatPearls.
- Lifshutz JI, Johnson WD (2001) History of hydrocephalus and its treatments. Neurosurg Focus 11(2): E1.
- Phan S, Liao J, Jia F, Maharaj M, Reddy R, et al. (2016) Laparotomy vs minimally invasive laparoscopic ventriculoperitoneal shunt placement for hydrocephalus: a systematic review and meta-analysis. Clin Neurol Neurosurg 140: 26-32.
- Hanak BW, Bonow RH, Harris CA, Browd SR (2017) Cerebrospinal fluid shunting complications in children. Pediatr Neurosurg 52(6): 381–400.
- Tervonen J, Leinonen V, Jaaskelainen JE, Koponen S, Huttunen TJ (2017) Rate and risk factors for shunt revision in pediatric patients with hydrocephalus: a population-based study. World Neurosurg 101: 615–622.
- Dawod J, Tager A, Darouiche RO, Al Mohajer M (2016) Prevention and management of internal cerebrospinal fluid shunt infections. J Hosp Infect 93(4): 323–328.
- Wong JM, Ziewacz JE, Ho AL, Panchmatia JR, Bader AM, et al. (2012) Patterns in neurosurgical adverse events: cerebrospinal fluid shunt surgery. Neurosurg Focus 33(5): E13.
- Rekate HL 1991) Shunt revision: complications and their prevention. Pediatr Neurosurg 17(3): 155–162.
- Ghritlaharey RK, Budhwani KS, Shrivastava DK, Srivastava J (2012) Ventriculoperitoneal shunt complications needing shunt revision in children: a review of 5 years of experience with 48 revisions. Afr J Paediatr Surg 9(1): 32–39.
- Khalil A, Mandiwanza T, Zakaria Z, Crimmins D (2016) Routine cerebrospinal fluid analysis during “de novo” ventriculoperitoneal shunt insertion: single institution experience. Br J Neurosurg 30(4): 427–428.
- Sood S, Canady AI, Ham SD (2000) Evaluation of shunt malfunction using shunt site reservoir. Pediatr Neurosurg 32(4): 180–86.
- Shahi MV, Noorbakhsh S, Zarrabi V, Nourozi B, Tahernia L (2018) The neuroimaging studies in children with ventriculoperitoneal shunt complications: a 10 years descriptive study in Tehran. Open Neuroimag J 12: 1-9.
- Overturf GD (2005) Defining bacterial meningitis and other infections of the central nervous system. Pediatr Crit Care Med 6(3): S14–18.
- Bawa M, Dash V, Mahalik S, Rao KLN (2019) Outcome analysis of patients of congenital hydrocephalus with ventriculoperitoneal shunt at a tertiary care hospital in north India. Pediatr Neurosurg 54(4): 233–236.
- Khan F, Shamim MS, Rehman A, Bari ME (2013) Analysis of factors affecting ventriculoperitoneal shunt survival in pediatric patients. Childs Nerv Syst 29(5): 791–802.
- Khan F, Rehman A, Shamim MS, Bari ME (2016) Ventriculoperitoneal (VP) shunt survival in patients developing hydrocephalus after cranial surgery. Turk Neurosurg 26(3): 369–377.
- Reddy GK, Bollam P, Caldito G, Guthikonda B, Nanda A (2012) Ventriculoperitoneal shunt surgery outcome in adult transition patients with pediatric-onset hydrocephalus. Neurosurgery 70(2): 380–388.
- Kumar V, Shah AS, Singh D, Loomba PS, Singh H, et al. (2016) Ventriculoperitoneal shunt tube infection and changing pattern of antibiotic sensitivity in neurosurgery practice: alarming trends. Neurol India 64(4): 671–676.
- Broggi M, Zattra CM, Schiariti M, Acerbi F, Tringali G, Falco J, et al. (2020) Diagnosis of ventriculoperitoneal shunt malfunction: a practical algorithm. World Neurosurg 137: e479–e86.
- Orrego-Gonzalez E, Enriquez-Marulanda A, Ravindran K, Celin-Varcalcel D, Parrado-Sanchez L, et al. (Factors associated with ventriculoperitoneal shunt failures in the first 30 postoperative days in pediatric patients. World Neurosurg S1878-8750(18): 32948-32946.
- Bir SC, Konar S, Maiti TK, Kalakoti P, Bollam P, et al. (2016) Outcome of ventriculoperitoneal shunt and predictors of shunt revision in infants with posthemorrhagic hydrocephalus. Childs Nerv Syst 32(8):1405–1414.
- Iglesias S, Ros B, Martin A, Carrasco A, Segura M, Ros A, et al. (2017) Factors related to shunt survival in paediatric hydrocephalus. Could failure be avoided? Neurocirugia (Astur) 28(4): 159–166.
- Meier U, Lemcke J (2010) Co-morbidity as a predictor of outcome in patients with idiopathic normal-pressure hydrocephalus. Acta Neurochir Suppl 106: 127–130.
- Yang YC, Yin CH, Chen KT, Lin PC, Lee CC, et al. (2021) Prognostic nomogram of predictors for shunt-dependent hydrocephalus in patients with aneurysmal subarachnoid hemorrhage receiving external ventricular drain insertion: a single-center experience and narrative review. World Neurosurg 150: e12–e22.






























