Ischemic Stroke and Ferroptosis
Lie Xiong1,2, Pufan Zhou1,2, Hanqiang Shi1,2 and Yanbo Shi1,2*
1Central Laboratory of Molecular Medicine Research Center, Jiaxing Traditional Chinese Medicine Hospital Affiliated to Zhejiang Chinese Medical University, China
2Jiaxing Key Laboratory of diabetic angiopathy, China
Submission: December 29, 2021; Published: January 04, 2022
*Corresponding author: Yanbo Shi, Jiaxing Key Laboratory of diabetic angiopathy Central Laboratory of Molecular Medicine Research Center, Jiaxing Traditional Chinese Medicine Hospital Affiliated to Zhejiang Chinese Medical University Jiaxing City, Zhejiang Province, 314000 P.R. China
How to cite this article: Borbala Mikos, Fanni Szenasi, Timea Bodo, Anita Gergely, Rita Jakus, et al. Ischemic Stroke and Ferroptosis. Open Access J Neurol Neurosurg 2021; 16(4): 555942. DOI: 10.19080/OAJNN.2022.16.555942.
Abstract
In recent years, a large number of studies have found that the ferroptosis signaling pathway mediates the death of brain cells in the process of ischemic stroke. Ferroptosis is controlled by regulators involved in many aspects of iron metabolism and lipid and amino acid metabolism pathways. During ischemic stroke, the BBB is destroyed, excessive iron enters the brain parenchyma, meanwhile, the iron metabolism of nerve cells is imbalanced, which increases intracellular free iron. The up-regulation of ACSL4 and LOX promote the conversion of PUFAs to lipid peroxides. The abnormal expression of System Xc- and GPX4 leads to excessive accumulation of lipid peroxides. These three interrelated pathways ultimately lead to the ferroptosis of brain cells. Here, we summarized the potential link between ischemic stroke and ferroptosis, provide new ideas for the diagnosis and treatment of ischemic stroke.
Keywords: Ischemic stroke; Ferroptosis; Iron metabolism; Lipid metabolism; Amino acid metabolism
Introduction
Stroke refers to the interruption of blood supply to the brain due to cerebral vascular hemorrhage or ischemia, ischemic stroke accounts for about 85% of stroke. Restoration of blood circulation and reconstruction of blood reperfusion are commonly used treatments for ischemic stroke [1]. On the one hand, reperfusion can save brain cells and promote the recovery of brain function; on the other hand, it will produce a rapid injury cascade, further aggravating brain injury [2]. During the process of stroke, brain cells are affected by inflammation, apoptosis, excitotoxicity, oxidative and nitrifying stress [3-6], etc. Recent studies have found that the ferroptosis pathway is closely related to the death of brain cells in ischemic stroke. The concept of Ferroptosis was first proposed by Dixon in 2012 to describe the mode of cell death induced by earstin [7]., the ferroptosis process is controlled by strict and complicated regulatory mechanisms, including cystine/glutamate antiporter (System Xc-) inhibition, glutathione (GSH) depletion and glutathione peroxidase 4(GPX4) inactivation, mainly manifested as the accumulation of intracellular iron ions and lipid peroxides. Different from cell apoptosis, autophagy, and necrosis, ferroptosis is a form of programmed cell death mediated by iron ions.
In 1988, although there was no concept of ferroptosis, researchers had discovered the phenomenon of iron deposition in the basal ganglia, thalamus, periventricular and subcortical white matter regions during the recovery period of ischemic stroke [8,9]. Subsequently, Kondo et al. found the up-regulation of lipid peroxides in the rat cerebral ischemia reperfusion model and speculated that it is related to the intracellular iron level, indicated the correlation between iron and cerebral ischemia injury [10]. In recent years, Tuo et al. found that ferroptosis occurred in neuron in cerebral ischemia reperfusion mice, and ferroptosis inhibitors Ferrostatin-1 or Liproxstatin-1 could alleviate cerebral ischemic damage [11]. The ferroptosis provides a new research direction for the mechanism of cerebral ischemia damage, that is helpful to broaden the treatment methods. Therefore, this article summarizes the relationship between ischemic stroke and ferroptosis on iron, lipid and amino acid metabolism pathway.
Ischemic stroke and Iron metabolism
As the most abundant metal in the brain [12], iron is strictly regulated by the blood-brain barrier (BBB) [13]. The normal structure and function of the BBB is essential for brain iron homeostasis, the endothelial cells of the BBB are a regulatory site for brain iron uptake. Transferrin/transferrin receptor (Tf/TfR) is the major pathway for iron uptake in endothelial cells, secreted from ferroportin (Fpn) to the brain parenchyma in the form of Fe2+[14] (Figure 1). Next, iron enters various types of brain cells through multiple pathways, but still mainly through the Tf/TfR1 pathway [15]. Iron accumulation is an important factor for ferroptosis. Cerebral ischemia increases the permeability of BBB, large amounts of iron enter the brain parenchyma, which become a prerequisite for ferroptosis in brain cells [16]. Before the concept of ferroptosis appeared, researchers already found that iron accumulation in brain of patients with different grades of ischemic stroke [8,9]. Later, it was found that the Tf and TfR were both up regulated in brain of patients got stroke [16,17].
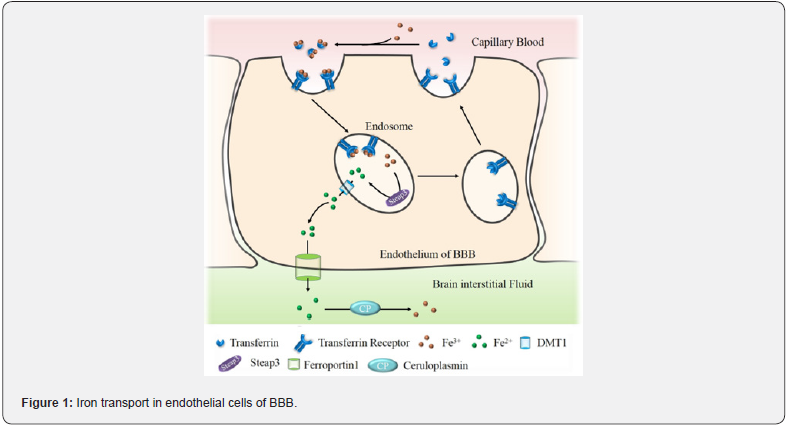
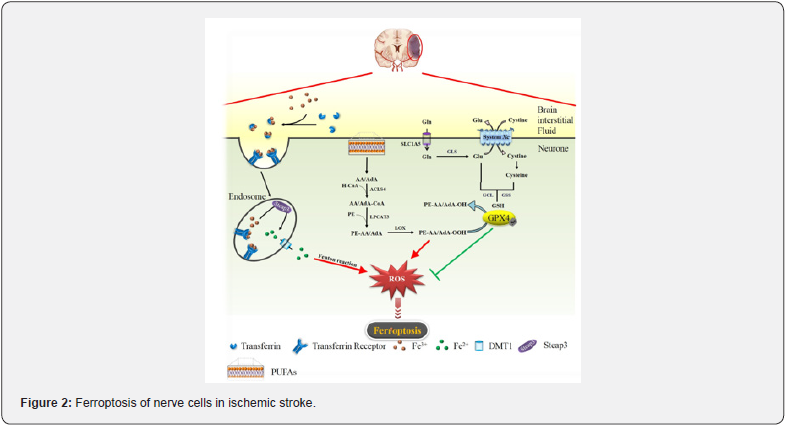
In addition, Tou et al. found that cerebral ischemia-reperfusion injury inhibits tau protein expression severely [11], which is closely related to iron efflux in nerve cells, subsequently inhibit the normal iron excretion function of FPN [18]. These lead to an increase in iron intake of brain cells, but cannot be effectively eliminated, clinically manifested as iron accumulation in the brain ischemic injury area. Excessive intracellular free Fe2+ activates the ferroptosis pathway, promotes the fenton reaction to produce hydroxyl radicals; on the other hand, it participates in the synthesis of lipid peroxides by lipoxygenase (LOX) [19] (Figure 2). Iron chelating agents such as 2,2-bipyridine [20] and deferoxamine [21] could inhibit damage in cerebral ischemia rats. These studies imply that iron and ferroptosis play an important role in nerve cell damage, and iron chelators could inhibit ferroptosis signaling pathways. such studies point out new research directions for the treatment of cerebral ischemia-reperfusion, but clinical evidence is still insufficient.
Ischemic stroke and lipid metabolism
Polyunsaturated fatty acids (PUFAs) such as arachidonic acid (AA) and adrenic acid (ADA) could form PE-AA/ADA with phosphatidyl ethanolamine (PE) under the catalysis of Acyl-CoA synthetase long-chain family member 4(ACSL4) and lysophosphatidylcholine acyltransferase 3(LPCAT3). lipoxygenase(LOX) oxidizes PE-AA/ADA to lipid peroxides mediated by iron, leading to ferroptosis [22] (Figure 2). Gubern et al. first reported the association between ACSL4 and cerebral ischemia, but not confirm this is related to the ferroptosis [23]. In recent years, it has been reported that ACSL4 is up-regulated and participates in ferroptosis signaling pathway during ischemiareperfusion of the intestine [24], heart [25] and other organs. However, the relationship between ACSL4 and ferroptosis pathway of cerebral ischemia is not clear, further research is needed. For the LOX study, it is clear that the expression of 12/15-LOX in the brain of cerebral ischemia is up-regulated [26,27], related to the increase of lipid peroxide levels and the damage of neuronal cells. Furthermore, Stockwell et al. clarified that 12/15-LOX is involved in the ferroptosis pathway [28]. this phenomenon might be due to the 12/15-LOX is regulated by GSH, the decrease of GSH helps to activate 12/15-LOX [29]. Therefore, ferroptosis can be inhibited by regulating LOX and ACSL4. Li et al. used rosiglitazone to inhibit ACSL4 expression and reduce ferroptosis during intestinal ischemia-reperfusion [24], which provides a new strategy for treatment of ischemic stroke. Yigitkanli et al. [30] and Rai et al. [31] reported LOX inhibitors LOXBlock-1 and ML351 could protect brain cells from 12/15-LOX-induced oxidative damage after cerebral ischemia stroke, respectively. The pharmacokinetic study of these inhibitors may become a hotspot in the future.
Ischemic stroke and amino acid metabolism
To avoid ferroptosis, cells were protected by System Xcand GPX4. System Xc- regulated by intracellular glutamate, transfer into cystine and out glutamate without consuming ATP. Intracellular cystine is broken down into cysteine, then GSH is formed with glutamate. Then, GPX4 consumes GSH to reduce lipid peroxides to lipid alcohols and inhibit ferroptosis [32]. This is the most critical pathway for cells to resist ferroptosis (Figure 2). There is a certain controversy in the current research on System Xc- after cerebral ischemia. Dixon et al. mentioned in the study that the inhibition of System Xc- caused the decrease of intracellular cystine, then GSH depleted and GPX4 inactivated [7]. P53 is a pro-apoptotic gene up-regulated after ischemic stroke, which inhibit System Xc-, eventually leads to ferroptosis -mediated brain damage [32]. But there are also studies reported that cerebral ischemic damage could be alleviated through downregulating the System Xc- [33]. The study of Hsieh et al. further reported that hypoxia inducible factor-1α(HIF-1α) promoted System Xc- upregulation during cerebral ischemia and the increase in extracellular glutamate activates N-methyl-D-aspartic Acid Receptor (NMDAR), which promote the uptake of iron by nerve cells, implies the beginning of ferroptosis [34,35]. The different reports of System Xc- may be related to the different stages of ferroptosis. It is currently speculated that System Xc- up-regulated in the early stage of ferroptosis, followed by inhibiting by excess extracellular glutamate [36]. By contrast, the research on GPX4 is not contradictory. The experimental study of Guan et al. clarified the relationship between GPX4, ferroptosis and ischemic stroke [36]. The expression of GPX4 was down-regulated in cerebral ischemia-reperfusion gerbils, when the GPX4 was up-regulated by drug, the lipid peroxide was inhibited, and brain damage was alleviated.
System Xc- and GPX4 play a key role in the process of ferroptosis. Therefore, regulating System Xc- and GPX4 may have a positive effect on ischemic stroke. N-acetylcysteine and ceftriaxone sodium down-regulate the expression of System Xcin cerebral ischemia rats to enhance the tolerance of nerve cells to ferroptosis [33]. Meanwhile, selenium is significance to the biological activity of GPX4, which could promote the expression of GPX4, to inhibit ferroptosis [37].
Discussion
Compared with other human tissues and organs, brain tissue and nerve cells are rich in polyunsaturated fatty acids and iron ions, which are more sensitive to ferroptosis [38]. generally, the BBB stabilize the iron content of the brain within an appropriate range. But cerebral ischemia increases the permeability of the BBB, promotes iron into the brain parenchyma, which become a trigger for ferroptosis in brain cells [16]. At present, the mechanism of ferroptosis of ischemic stroke is gradually being clarified, iron chelators and some related drugs have also achieved certain effects in the treatment of cerebral ischemia. The in-depth study of the different stages of cerebral ischemia involved in ferroptosis will help us to understand the mechanism of occurrence and progression of cerebral ischemic injury, provides a new strategy for the treatment of cerebral ischemia.
References
- Zhang XX, Fang Q (2020) Research progress of circadian rhythm and reperfusion therapy in wake-up stroke. Chin J Neurol 53(1): 55-56.
- Zhang YQ, LU M, Hou JK (2019) Effects of different degrees of reperfusion after endovascular therapy on prognosis in patients with acute ischemic stroke. Chin J Neurol 52(12): 1031-1032.
- Wang L, Deng S, Lu Y, Y Zhang, L Yang, et al (2012) Increased inflammation and brain injury after transient focal cerebral ischemia in activating transcription factor 3 knockout mice. Neuroscience 220: 100-108.
- Pandya JD, Sullivan PG, Pettigrew LC (2011) Focal cerebral ischemia and mitochondrial dysfunction in the TNFalpha-transgenic rat. Brain Res 1384: 151-160.
- Arranz AM, Gottlieb M, Perez-Cerda F, Carlos Matute (2010) Increased expression of glutamate transporters in subcortical white matter after transient focal cerebral ischemia. Neurobiol Dis 37(1): 156-165.
- Tajes M, Ill-Raga G, Palomer E, Eva Ramos-Fernández, Francesc X Guix, et al. (2013) Nitro-oxidative stress after neuronal ischemia induces protein nitrotyrosination and cell death. Oxid Med Cell Longev 2013: 826143.
- Dixon SJ, Lemberg KM, Lamprecht MR, Rachid Skouta, Eleina M Zaitsev, et al (2012) Ferroptosis: an iron-dependent form of nonapoptotic cell death. Cell 149(5): 1060-1072.
- Dietrich RB, Bradley WJ (1988) Iron accumulation in the basal ganglia following severe ischemic-anoxic insults in children. Radiology 168(1): 203-206.
- Baenziger O, Martin E, Steinlin M, R Largo, R Burger, et al. (1993) Early pattern recognition in severe perinatal asphyxia: a prospective MRI study. Neuroradiology 35(6): 437-442.
- Kondo Y, Asanuma M, Nishibayashi S, et al (1997) Late-onset lipid peroxidation and neuronal cell death following transient forebrain ischemia in rat brain. Brain Res 772(1-2): 37-44.
- Tuo QZ, Lei P, Jackman KA, X-L Li, H Xiong, et al (2017) Tau-mediated iron export prevents ferroptotic damage after ischemic stroke. Mol Psychiatry 22(11): 1520-1530.
- Ward RJ, Zucca FA, Duyn JH, Robert R Crichton, Luigi Zecca, et al (2014) The role of iron in brain ageing and neurodegenerative disorders. Lancet Neurol 13(10): 1045-1060.
- Duck KA, Neely EB, Simpson IA, James R Connor, et al (2018) A role for sex and a common HFE gene variant in brain iron uptake. J Cereb Blood Flow Metab 38(3): 540-548.
- Leitner DF, Connor JR (2012) Functional roles of transferrin in the brain. Biochim Biophys Acta 1820(3):393-402.
- Zhongming Qian, Ya ke (2019) Brain iron transport. Biological Reviews 94(5): 1672-1684.
- DeGregorio-Rocasolano N, Marti-Sistac O, Gasull T (2019) Deciphering the Iron Side of Stroke: Neurodegeneration at the Crossroads Between Iron Dyshomeostasis, Excitotoxicity, and Ferroptosis. Front Neurosci 13: 85.
- Park UJ, Lee YA, Won SM, Jin Hwan Lee, Seung-Hee Kang, et al (2011) Blood-derived iron mediates free radical production and neuronal death in the hippocampal CA1 area following transient forebrain ischemia in rat. Acta Neuropathol 121(4): 459-473.
- Lei P, Ayton S, Finkelstein D I, Loredana S, Giuseppe DC, et al (2012) Tau deficiency induces parkinsonism with dementia by impairing APP-mediated iron export. Nat Med 18(2): 291-295.
- Shintoku R, Takigawa Y, Yamada K, Chisato K, Yuhei Y, et al. (2017) Lipoxygenase-mediated generation of lipid peroxides enhances ferroptosis induced by erastin and RSL3. Cancer Sci 108(11): 2187-2194.
- Van Hoecke M, Prigent-Tessier A, Bertrand N, Laurent P, Christine M, et al (2005) Apoptotic cell death progression after photothrombotic focal cerebral ischaemia: effects of the lipophilic iron chelator 2,2'-dipyridyl. Eur J Neurosci 22(5): 1045-1056.
- Hanson LR, Roeytenberg A, Martinez PM, Valerie GC, Donald CS, et al (2009) Intranasal deferoxamine provides increased brain exposure and significant protection in rat ischemic stroke. J Pharmacol Exp Ther 330(3): 679-686.
- Zhou J, Jin Y, Lei Y, Tianyi L, Zheng W, et al (2020) Ferroptosis Is Regulated by Mitochondria in Neurodegenerative Diseases. Neurodegener Dis 20(1): 20-34.
- Gubern C, Camos S, Ballesteros I, Rocío R, Víctor G R, et al. (2013) miRNA expression is modulated over time after focal ischaemia: up-regulation of miR-347 promotes neuronal apoptosis. FEBS J 280(23): 6233-6246.
- Li Y, Feng D, Wang Z, Yan Z, Ruimin S, et al. (2019) Ischemia-induced ACSL4 activation contributes to ferroptosis-mediated tissue injury in intestinal ischemia/reperfusion. Cell Death Differ 26(11): 2284-2299.
- Li W, Li W, Leng Y, Yonghong X, Zhongyuan X, et al. (2020) Ferroptosis Is Involved in Diabetes Myocardial Ischemia/Reperfusion Injury Through Endoplasmic Reticulum Stress. DNA Cell Biol 39(2): 210-225.
- Van Leyen K, Kim HY, Lee SR, Guang Jin, Ken Arai, et al. (2006) Baicalein and 12/15-lipoxygenase in the ischemic brain. Stroke 37(12): 3014-3018.
- Jung JE, Karatas H, Liu Y, Ayfer Yalcin, Joan Montaner, et al. (2015) STAT-dependent upregulation of 12/15-lipoxygenase contributes to neuronal injury after stroke. J Cereb Blood Flow Metab 35(12): 2043-2051.
- Stockwell BR, Friedmann AJ, Bayir H, Ashley I B, Marcus Conrad, et al (2017) Ferroptosis: A Regulated Cell Death Nexus Linking Metabolism, Redox Biology, and Disease. Cell 171(2): 273-285.
- Zheng Y, Liu Y, Karatas H, Kazim Y, Theodore RH, et al. (2019) Contributions of 12/15-Lipoxygenase to Bleeding in the Brain Following Ischemic Stroke. Adv Exp Med Biol 1161: 125-131.
- Yigitkanli K, Pekcec A, Karatas H, Stefanie P, Emiri M, et al. (2013) Inhibition of 12/15-lipoxygenase as therapeutic strategy to treat stroke. Ann Neurol 73(1): 129-135.
- Rai G, Joshi N, Jung JE, Yu Liu, Lena Schultz, et al. (2014) Potent and selective inhibitors of human reticulocyte 12/15-lipoxygenase as anti-stroke therapies. J Med Chem 57(10): 4035-4048.
- Murphy ME (2016) Ironing out how p53 regulates ferroptosis. Proc Natl Acad Sci U S A 113(44): 12350-12352.
- Krzyzanowska W, Pomierny B, Bystrowska B, Lucyna P, Małgorzata F, et al. (2017) Ceftriaxone- and N-acetylcysteine-induced brain tolerance to ischemia: Influence on glutamate levels in focal cerebral ischemia. PLoS One 12(10): e186243.
- Hsieh CH, Lin YJ, Chen WL, Yen-CH, Chi-WC, et al. (2017) HIF-1alpha triggers long-lasting glutamate excitotoxicity via system Xc- in cerebral ischaemia-reperfusion. J Pathol 241(3): 337-349.
- Cheah JH, Kim SF, Hester LD, Kathleen WC, Stanley EP, et al. (2006) NMDA receptor-nitric oxide transmission mediates neuronal iron homeostasis via the GTPase Dexras1. Neuron 51(4): 431-440.
- Guan X, Li X, Yang X, Junwei Y, Pilong S, et al. (2019) The neuroprotective effects of carvacrol on ischemia/reperfusion-induced hippocampal neuronal impairment by ferroptosis mitigation. Life Sci 235: 116795.
- Ingold I, Berndt C, Schmitt S, Sebastian D, Gereon P, et al. (2018) Selenium Utilization by GPX4 Is Required to Prevent Hydroperoxide-Induced Ferroptosis. Cell 172(3): 409-422.
- Cobley JN, Fiorello ML, Bailey DM (2018) 13 reasons why the brain is susceptible to oxidative stress. Redox Biol 15: 490-503.






























