A Significant Presence of Pyrogen in Blood Bags was Revealed in a Pilot Study
Moses Dabah Lugos1*, Kabang Klingret Anthony1, Umanka Yelpoji Polit1, Mary-Jane Nkechi Ofojekwu1, Elisha Markus Rumji2, James Garba Damen1 and Obadiah Dapus Damulak2
1 Department of Medical Laboratory Science, University of Jos, Nigeria
2 National Blood Transfusion Service, North Central Zonal Centre, Nigeria
Submission: November 30, 2019; Published: January 08, 2020
*Corresponding author: Moses D Lugos, Department of Medical Laboratory Science, Faculty of Health Sciences & Technology, University of Jos, Nigeria
How to cite this article: Moses D L, Kabang K A, Umanka Y P, Mary J N O, Elisha M R, et al. A Significant Presence of Pyrogen in Blood Bags was Revealed in a Pilot Study. Open Acc Blood Res Trans J. 2020; 3(2): 555610. DOI: 10.19080/OABTJ.2020.03.555610
Abstract
Pyrogens are substances that are typically produced by bacteria, which, when introduced into the bloodstream, can cause pyrexia (fever). The presence of pyrogens in injectable fluids such as anticoagulant solutions in blood bags and water for injection could cause unexplained fever in patients transfused or injected with such fluids. We designed this study to screen for the presence of pyrogen in anticoagulant solutions in blood bags. Chemical method was used to test for the presence of pyrogen (endotoxin) in anticoagulant solutions of 40 blood bags. 1% potassium permanganate was prepared using the sample to be tested, 500 microliters of the 1% potassium permanganate were mixed with 10ml of the samples to be tested and boiled for 30 minutes. Colour change was used as an indicator for measuring the pyrogenic activity. Data were analyzed using simple percentage calculations. The result revealed a significant presence of pyrogen in blood bags. Fifteen out of the 40 anticoagulant solutions in blood bags, representing 3 out of 8 types of blood bags (37.5%) were found to contain pyrogen. Earlier data and this demonstrate that some of the fevers reported in patients following blood transfusion may be caused by the presence of pyrogen in the blood bags, not necessarily due to serological mismatched. Therefore, a full-scale study in this area will help to provide the basis for adequate quality control measures to be put in place to verify the sterility of blood bags for routine use in blood transfusion.
Keywords:Pyrogen; Anticoagulant solutions; Potassium permanganate; Fever; Therapeutic Benefits; Serological; Cryoprecipitate; Morbidity; Staphylococcus
Introduction
Blood transfusion is an essential routine life-saving medical procedure in which a compatible blood or blood product is given to patients that require such a form of medical care [1,2]. This procedure provides remarkable therapeutic benefits, especially to anemic medical and surgical patients [1,3]. Although blood transfusion is used in various medical conditions to help in replacing whole blood loss or blood products, such as, red blood cells concentrate, platelets concentrate, fresh frozen plasma and cryoprecipitate or plasma derivatives such as albumin, coagulation factors and immunoglobulins, the intervention is not without the potential hazards of acute or delayed complications such as acute hemolytic transfusion reaction and transfusion-transmissible infections respectively [4,5]. Notable signs of reactions following blood transfusion may include fever, chills, pruritus, or urticaria as well as severe shortness of breath, red urine, and even loss of consciousness [6]. More severe and potentially fatal acute or delayed transfusion reactions have been reported [7].
Pyrogen refers to substances, typically of biological origins such as bacteria (basically gram-negative endotoxin), viruses, and fungi, that can cause pyrexia when injected into the body via parenteral routes; for instance, the intravenous (IV) [8-10]. Some characteristics of pyrogens include a very low molecular weight that allows them to pass through a semi-permeable membrane; they are also thermostable and filterable. When sufficient quantity of pyrogen is present in the body, it can lead to severe signs of inflammation, including fever, shock, multiorgan failure, and sometimes even death in humans [8]. The best-studied pyrogen is the lipopolysaccharide found in the membrane of gram-negative bacteria. However, bacteria are not the only sources of pyrogen; they can also be derived from plants and animal tissues [11]. Pyrogenic contamination of solutions for parenteral use, such as in blood transfusion anticoagulant preservatives and injection water has posed serious threats to medical care [8]. The major contaminants which contribute to pyrogenic response and complications used in vivo or in vitro are endotoxins. These endotoxins are not easily inactivated by heat as such heat treatment of toxins contaminated products does not help in combating the threats they pose [12].
Transfusion pyrexia (fever) is one of the important clinical symptoms which may occur as an isolated event or as part of complications associated with blood transfusion [13,14]. Fever has been reported as one of the principal causes of morbidity and maybe an essential sign of life-threatening complications of blood transfusion [13]. Fever is often a reason why blood transfusion may be discontinued abruptly, and unfortunately, adequate evaluation of this has remained a thing of concern to the clinicians and other healthcare providers [13].
It is, speculated that the presence of pyrogen in blood bags could be responsible for some of the transfusion pyrexias and idiopathic fevers that could be life-threatening to the patient. This pilot study, therefore, aimed to screen for the presence of pyrogen in anticoagulant solutions in blood bags sold in some pharmaceutical stores within Jos metropolis. It hoped that the outcome of this study might provide some useful insights that can help in the design of a largescale study that will employ both the chemical and biological methods to screen for the presence of pyrogen in blood bag anticoagulant preservatives. The proposed full-scale study will cover more pharmaceutical stores in Jos metropolis, and wider makes of blood bags will be analyzed.
Materials and Methods
Study location
This study was carried out in Jos metropolis in Jos North Local Council of Plateau State. Jos is the capital city of Plateau State, which is located in the Middle Belt region of Nigeria. The city has a population of about 900,000 residents based on the 2006 census. Plateau state has a size of 26,899 km2, with a population of 3.5million and the twelfth largest state in Nigeria. The State lies at latitude 9°55’N and longitude 8°55’E. The highland rises from 1,200 at the lowlands to a peak of 1,829metres above sea level. It has a near temperate climate with an approximate mean temperature of 22°C and the lowest temperature of 18°C. The mean annual rainfall varies from 131.8 to 146 cm [15,16].
Experimental design
Blood bags were purchased randomly from pharmaceutical stores in Jos metropolis. A pack of 5 Citrate-Phosphate-Dextrose (CPD) Blood bags from different vendors/companies were purchased from pharmaceutical stores code numbered 1-5, in Jos metropolis. We screened the anticoagulant solutions of the five bags each of make coded A, B C, D, E, F, G and H for the presence of pyrogen. A controlled study was carried out prior to the commencement of this study where five positive and five negative samples were boiled at 100°C for at different time intervals of 20, 30, 40, 50 and 60 minutes respectively. A colony was obtained using the McFarland’s standard from an overnight incubation of Staphylococcus aureus and introduced into the five positive control tubes before heating in pair with the negative control for the various time intervals.
Inclusion and exclusion criteria
All the blood bags screened were inspected at the point of purchase to ensure that there were no breakages or damages of any sort. Any blood bag that physical integrity could not be ascertained or expired was excluded from this study.
Study sample size
This is a pilot study, aimed at conducting a small-sized experiment that can help in planning and provide justification for a future large-scale study in this subject area [17]. Therefore a sample size of 40 was considered adequate for this work.
The anticoagulant solutions & materials
Each CPDA (Citrate-Phosphate-Dextrose-Adenine) blood bag contains about 63ml of anticoagulant solution for a final anticoagulant: blood ratio of approximately 1:7 in a 450ml wholeblood unit. We used pyrogen-free glass khan tubes, Nickel-Electro 4litre unstirred water bath with a thermometer and a plastic lid. Other materials used included a mechanical analogue stop clock, 100ml measuring cylinder, automatic pipettes and five-milliliter sterile plain tubes.
The laboratory experiment & principle of the test
Chemical method was adopted to screen for the presence of pyrogen in the anticoagulant solutions using potassium permanganate (KMn04). The principle of the experiment is based on the fact that potassium permanganate is a potent oxidizing agent, which is reduced from its tinge purple colour to colorless in the presence of organic matter such as bacteria endotoxin (pyrogen) and plants by-products, after boiling for 30 minutes. If the colour remains tinge purple after boiling, it indicates a negative result, but when it turns colorless, it is an indication that pyrogen may be present [18].
Preparation Of 0.5% Mcfarland standard
A 1.2% of Barium chloride (BaCl2) was prepared by dissolving 1.2g of BaCl2 salt in 100ml of water. 1% sulphuric acid (H2SO4) was prepared by adding 1ml of sulphuric acid to 100ml of water. From the solutions prepared, 99ml of the diluted sulphuric acid was dispensed into a clean beaker, and 1ml of BaCl2 solution was added to the 99ml of H2SO4, and the optical density was read. This solution was stored and used as a reference standard for picking a colony of Staphylococcus aureus being introduced into the positive control in each batch of the samples tested, as shown in (Figure 1). A 0.5 McFarland standard provides an optical density comparable to the density of a bacterial suspension 1.5×108 colony-forming unit (CFU)/ml [19,20]. In other words, 1.5×108 cells suspension was used in each of the positive controls.

The test procedure
One per cent (1%) potassium permanganate was prepared by dissolving 0.1g of the crystalline KMn04 in 10ml of the anticoagulant to be tested. From the solution of 1% KMNO4 prepared, 500 microliters were dispensed into a sterile test tube, 10ml of the anticoagulant preservative solution to be tested was added to the solution above, and the mixture was boiled for 30 minutes. The boiled mixture was observed for colour change. This same procedure was used to test the remaining blood bags.
Data analysis
The prevalence of pyrogen in the total number of blood bags screened and in the types of blood bags screened was determined using simple percentage calculations.
Results
Eight different types of blood bags were purchased from some Pharmaceutical vendors and stores in Jos metropolis as depicted in (Table 1). A pack of five blood bags was purchased from each Vendor and a total of 40 blood bags were screened for the presence of pyrogen.
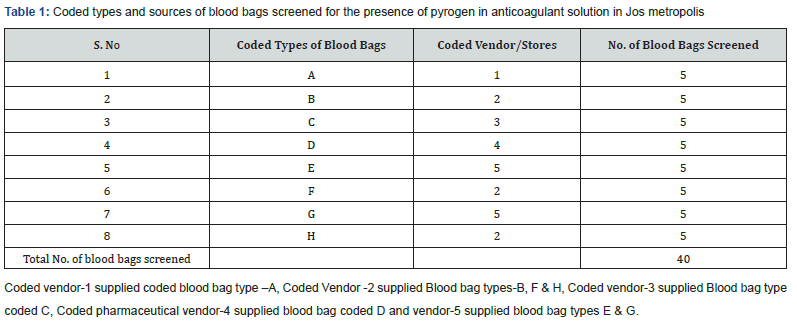
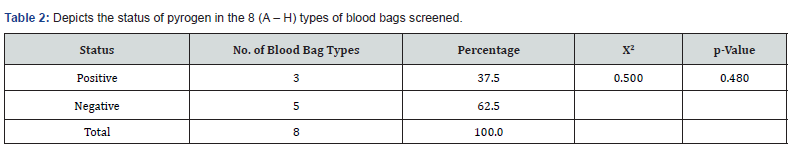
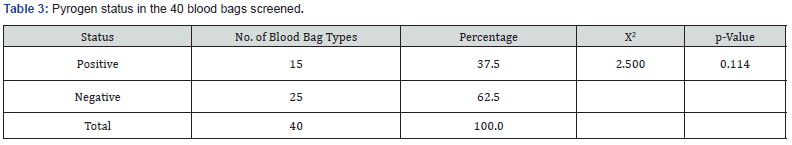
As shown in (Table 2) of the eight types of blood bags coded A – H that were collected, 37.5% (representing 3 types of blood bags) tested positive for pyrogen while62.5% tested negative. Even though there was no statistically significant difference between the number of the types of blood bags that tested positive to those that tested negative (ꭓ2 = 0.500; p = 0.480), data showed that close to half of the types of blood bags tested positive. Also, of the 40 blood bags that were collected, 37.5% tested positive for pyrogen 62.5% tested negative as shown in (Table 3). However, there was no significant difference between the total number of blood bags that tested positive for pyrogen and those that were negative statistically (ꭓ2 = 2.500; p = 0.114).
Discussion
This experiment was designed to evaluate for the presence of pyrogen in the anticoagulant solutions in CPDA blood bags. The pilot study was aimed at testing the hypothesis which states that, the presence of pyrogen in anticoagulant solutions in blood bags could contribute to the increased cases of fevers (blood transfusion reactions) following blood transfusion. The data presented in tables 2 & 3 revealed a markedly increased presence of pyrogen in the anticoagulant solutions in blood bags of 37.5%. The study further showed that only 62.5% of blood bags in circulation in Jos metropolis were pyrogen-free. The finding of this study further suggests that the presence of pyrogen in anticoagulant preservatives could contribute significantly to febrile blood transfusion reactions. Kenedy and colleagues in their study, which comprised of 90% of patients who had total knee replacement surgery reported that the risk of developing fever increases four times for each unit of blood transfused [21]. The study by Kennedy [21]. did not point to the factors that pyrogens are associated with increased fever following the transfusion of each unit of blood. From the finding of this pilot study, the presence of pyrogens in the blood bags may be responsible for such increased in fevers reported.
The study by Payne and Rolfs believed that febrile nonnonhemolytic reaction was mainly due to antileucocytes antibodies directed against HLA antigens or granulocytes- specific antigens [22]. Contrarily to the beliefs by Payne and Rolfs, a group of researchers found that there was no significant impact of leucodepletion of red cells on the incidence of febrile nonhemolytic transfusion reaction [23]. Transfusion pyrexia could be a sign or symptoms of the Systemic Inflammatory Response Syndrome (SIRS) complication associated with transfusion episode due to bacterial contamination of blood for transfusion [24]. This study correlates with the finding our work that observed the significantly high presence of pyrogen in blood bags tested; these both underscore the need for increased sterility surveillance during the preparation of blood bags especially the fluid use for the anticoagulant storage and transportation.
Another related study carried out by Kindergarten, which evaluated the air-borne pyrogens based on human whole blood cytokine response, reported that the product identity was pyrogen-free, and there was no statistical difference. Also, in another similar study conducted by Hindman. It was shown that most of the injection water they tested show no statistical significance pyrogen presence (p value>0.05) [25]. This study is not free from limitation as the experiment was only designed as a pilot study in preparation for a large-scale study. Therefore, there is a need to fund further works in this field in order to generate data that could significantly improve the quality of blood transfusion services for better management of patients.
Conclusion and Recommendation
Blood transfusion services are an integral part of medicine, therefore quality control mechanism needs to be put in place to guarantee pyrogen-free blood and blood product transfusion for effective management of patient requiring such services. Every health facility producing and or using blood products should ensure that quality control systems are in place to ascertain the sterility of such blood products before they are being transfused. A full-scale study on the subject, covering regions and increased varieties of blood bags in routine use, will provide an insight into the roles of pyrogens in complications of blood transfusion. As a part of the future study, blood bag makes that are associated with fever in patients during or soon after transfusion will be investigated for pyrogen.
References
- Harmening DM (2018) Modern blood banking & transfusion practices FA Davis.
- English M, Ahmed M, Ngando C, Berkley J, Ross A (2002) Blood transfusion for severe anemia in children in a Kenyan hospital. Lancet 359(9305): 494-495.
- Sazama K, DeChristopher PJ, Dodd R, Harrison CR, Shulman IA, et al. (2000) Practice parameter for the recognition, management, and prevention of adverse consequences of blood transfusion. Archives of pathology & laboratory medicine 124(1): 61-70.
- Nabajyoti Choudhury (2010) Transfusion transmitted infections: How many more? Asian journal of transfusion science 4(2): 71-72.
- Clevenger B, A Kelleher (2013) Hazards of blood transfusion in adults and children. Continuing Education in Anaesthesia, Critical Care & Pain 14(3): 112-118.
- Tinegate H, Birchall J, Gray A, Haggas R, Massey E, et al. (2012) Guideline on the investigation and management of acute transfusion reactions Prepared by the BCSH Blood Transfusion Task Force. British journal of haematology 159(2): 143-153.
- Bolton Maggs PH (2016) SHOT conference report 2016: serious hazards of transfusion–human factors continue to cause most transfusion‐related incidents. Transfusion Medicine 26(6): 401-405.
- Boucher P (2017) A Framework for Evaluating Nonclinical Safety of Novel Adjuvants and Adjuvanted Preventive Vaccines, in Immunopotentiators in Modern Vaccines Elsevier, Netherlands, pp. 445-476.
- Sandle T (2016) Endotoxin and pyrogen testing. Pharmaceutical Microbiology. (80th), Kidlington: Woodhead Publishing, UK, pp. 131-145.
- John Durkee (2006) Management of industrial cleaning technology and processes.
- Ding JL, Ho B (2001) A new era in pyrogen testing. TRENDS in Biotechnology 19(8): 277-281.
- Bhandari Aakanksha , Bhandari Monika, Aakanksha Bhandari (2010) An Overview On Pyrogen Reduction Methods.
- Oladimeji P Arewa (2012) Evaluation of transfusion pyrexia: A review of differential diagnosis and management. ISRN hematology.
- Jolee T Suddock, Kendall PCrookston (2019) Transfusion Reactions, in StatPearls [Internet]. StatPearls Publishing.
- Esther S Yiltok, Sunday D Pam, Stephen Oguche, Edmund B Banwat, Stephen Yohanna, et al. (2014) Intestinal parasites and human immunodeficiency virus (HIV) status of children in Jos, Nigeria 6(3): 72-78.
- Temitope Emmanuel Alonge, Dada Emily Bola, S Sunday Ajewole (2018) Determination of Heavy Metals in Soil of High Traffic Road in Jos, Plateau State, Nigeria.
- In J (2017) Introduction of a pilot study. Korean journal of anesthesiology 70(6): 601-605.
- Tui C, M Schrift (1942) A tentative test for pyrogen in infusion fluids. Proceedings of the Society for Experimental Biology and Medicine 49(3): 320-323.
- Kralik P, V Beran, I Pavlik (2012) Enumeration of Mycobacterium avium subsp. paratuberculosis by quantitative real-time PCR, culture on solid media and optical densitometry. BMC research notes 5(1): 114.
- Betty Forbes, Daniel Sahm, Alice Weissfeld (2007) Study guide for Bailey & Scott's diagnostic microbiology. Mosby USA.
- Kennedy JG, Rodgers WB, Zurakowski D, Sullivan R, Griffin D, et al. (1997) Pyrexia after total knee replacement. A cause for concern? American journal of orthopedics (Belle Mead, NJ) 26(8): 549-552, 554.
- Payne R , MR Rolfs (1960) Further observations on leucoagglutinin transfusion reactions: with special reference to leucoagglutinin transfusion reactions in women. The American journal of medicine 29(3): 449-458.
- Uhlmann EJ, Isgriggs E, Wallhermfechtel M, Goodnough LT (2001) Prestorage universal WBC reduction of RBC units does not affect the incidence of transfusion reactions. Transfusion 41(8): 997-1000.
- Pittman M (1953) A study of bacteria implicated in transfusion reactions and of bacteria isolated from blood products. The Journal of laboratory and clinical medicine 42(2): 273-288.
- Hindman SH, Favero MS, Carson LA, Petersen NJ, Schonberger LB, et al. (1975) Pyrogenic reactions during haemodialysis caused by extramural endotoxin. The Lancet 2(7938): 732-734.






























