Waldenstrom’s Macroglobulinemia: An In-depth Review
Sabry A Allah Shoeib1, Essam Abd El Mohsen2, Mohamed A Abdelhafez1, Heba Y Elkholy1 and Mostafa F Fouad3*
1 Internal Medicine Department , Faculty of Medicine, Menoufia University
2El Maadi Armed Forces Institute, Egypt
3 Specialist of Internal Medicine at Qeft Teatching Hospital, Qena, Egypt
Submission: March 14, 2019; Published: May 20, 2019
*Corresponding author: Mostafa Fahmy Fouad Tawfeq MBBCh, Adress: Elzaferia, Qeft, Qena, Egypt
How to cite this article: Sabry A A S, Essam Abd El Mohsen, Mohamed A A, Heba Y E, Mostafa F F. Waldenstrom’s Macroglobulinemia: An In-depth Review. Open Acc Blood Res Trans J. 2019; 3(1): 555603. DOI: 10.19080/OABTJ.2019.03.555603
Abstract
Objective: The aim of the work was to through in-depth lights on new updates in waldenstrom macroglobulinemia disease.
Data sources: Data were obtained from medical textbooks, medical journals, and medical websites, which had updated with the key word (waldenstrom macroglobulinemia ) in the title of the papers.
Study selection: Selection was carried out by supervisors for studying waldenstrom macroglobulinemia disease.
Data extraction: Special search was carried out for the key word waldenstrom macroglobulinemia in the title of the papers, and extraction was made, including assessment of quality and validity of papers that met with the prior criteria described in the review.
Data synthesis: The main result of the review and each study was reviewed independently. The obtained data were translated into a new language based on the need of the researcher and have been presented in various sections throughout the article.
Recent Findings: We now know every updated information about Wald Enstrom macroglobulinemia and clinical trials. A complete understanding of the Wald Enstrom macroglobulinemia will be helpful for the future development of innovative therapies for the treatment of the disease and its complications.
The main conclusion of the presented in the current research paper is that Wald Enstrom macroglobulinemia disease remains to be an incurable disease with heterogeneous clinical course and genetics enhanced our understanding of its pathogenesis .
Keywords: Chemotherapy; Macroglobulinemia; Lymphoma; Viscosity; Wald Enstrom
Abbreviations: WM: Waldenström’s macroglobulinemia; LPL: lymphoplasmacytic lymphoma IGM Immunoglobulin M; MGUS: Monoclonal gammopathy of undetermined significance; DRC: dexamethasone, rituximab and cyclophosphamide; POEMS: Polyneuropathy; Organomegaly; Endocrinopathy; Muliple Myloma and Skin Changes
Introduction
Waldenström’s macroglobulinemia (WM), described in 1944 by Jan Gösta Waldenström, is a lymphoplasmacytic lymphoma (LPL) characterized by Immunoglobulin M (IGM) monoclonal hypergammaglobulinemia with bone marrow infiltration [1]. Its etiology is unknown, but several studies suggest a possible relationship with autoimmune diseases, exposure to environmental factors with chronic antigenic stimulation, such as infection with the hepatitis C virus (HCV). Despite the high incidence of HCV infection in these patients, a statistically significant association between HCV infection and WM was not founded [2]. The physical manifestations of WM are hepatomegaly (20%), splenomegaly (15%), and lymphadenopathy (15%) [3]. And the most common presenting symptom was fatigue related to a normocytic anemia. The median hemoglobin value at diagnosis is 10 g/dL [4]. We have also to know that Hyperviscosity syndrome is a clinical feature in 10-30% of patients with Waldenstrom’s macroglobulinemia (WM), sometimes as its presenting manifestation [5].
Neuropathy is the most common organ-specific complication of WM and is frequently the presenting feature. It was estimated that neuropathy occurs in 40% of patients [6]. And Bing-Neel syndrome is a disease without a clear definition but involves central nervous system invasion by WM cells or the deposit and activity in the CNS of immunoglobulin released by them [7]. In a large series of patients with Waldenstrom macroglobulinemia and amyloidosis, the kidney was identified as the most commonly involved organ and failure to recgnize was a major cause of irreversible organ damage. It was suggested that patients with IgM Monoclonal gammopathy of undetermined significance (MGUS) be serially monitored with urine assessments for albumin and measurements of natriuretic peptides to diagnose early development of cardiac failure [8].
All or part of the monoclonal Ig can deposit in various structures of the skin. Non-organized deposits are rare and mostly represented by the so-called cutaneous macro globulins, characterized by localized or coalescent papules by amorphous intradermal deposits made of monoclonal IgM molecules [9]. Coagulation defects included as disturbance in coagulation pathway is a wellknown complication of IgM gammopathy. It comprises procoagulant activity with thrombosis [10].
Discussion
Differential Diagnosis
It is fundamental to distinguish WM from other disorders that could be clinically confused with this disease. Differential diagnosis is important for the exclusion of neoplasms potentially secreting monoclonal IgM and which can also present lymphocytes with lymphoplasmacytic differentiation in the bone marrow, which includes marginal zone lymphomas : Chronic lymphocytic leukemia (CD5+, CD23+), mantle cell lymphoma (CD5+, CD23-), follicular lymphoma (CD10+) and multiple myeloma (CD138+, CD38+, CD56+) [11].
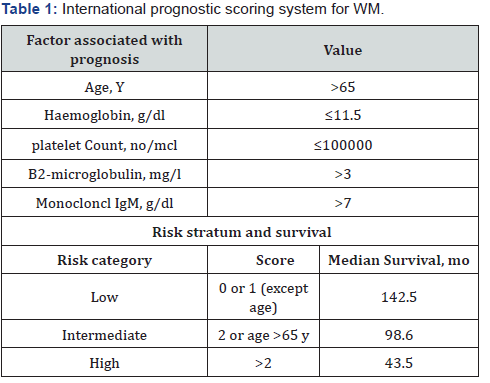
The International Prognostic Staging System for WM
The International Prognostic Staging System for Waldenström Macroglobulinemia (Table1) adopts five variables that correlate with poor survival of patients under treatment: age>65 years, β2-microglobulin concentration >3 mg/L, platelets count 100x109/L, monoclonal IgM concentration >7000 mg/dL, and hemoglobin concentration ≤11.5g/dL. The absence or presence of one or more prognostic factors categorizes the patient into 3 risk levels: low(0-1 risk factor, excluding age), intermediate (2 risk factors and age > 65 years) or high (more than 3 risk factors) [12]. There is a higher incidence in individuals aged between 63 and 68 years. we have to know that Approximately 60% of patients are men, and it is more common in Caucasian induvial .The average survival is 5 years .And as the disease is mainly diagnosed in old age, about 50% of patients die due to comorbidities not related directly to WM [13 ].
Classification of IgM related disorders patients are usually divided into four groups:
A. Patients defined as having IgM MGUS have an IgM level <3g/dL and a bone marrow infiltration with lymphoplasmacytic lymphoma of <10%
B. Patients with smoldering Waldenström macroglobulinemia either have >10% lymphoplasmacytic lymphoma in the bone marrow or an M spike >3g/dL and, by definition, cannot have any symptoms of tumor infiltration or IgM mediated symptoms
C. Another group in which there are patients whose symptoms are directly related to immunologic effects of the IgM monoclonal protein and not to the tumor mass of lymphoplasmacytic lymphoma. These include patients with type 2 mixed cryoglobulinemia, Cold agglutinin hemolytic disease , peripheral neuropathy associated with IgM monoclonal gammopathy , IgM amyloidosis and IgM Polyneuropathy, Organomegaly, Endocrinopathy, Multiple Myeloma and Skin Changes (POEMS) syndrome
D. The final group is those patients who have symptoms that are due to marrow, liver, spleen, and lymph nodal infiltration with lymphoplasmacytic lymphoma causing anemia, hyper viscosity, hepatosplenomegaly, and significant lymphadenopathy, and these patients whom we define them as Waldenström macroglobulinemia group [14].
Molecular Basis
The presence of MYD88L265P in the precancerous IgM-MGUS suggests that this mutation represents an early oncogenic event in WM pathogenesis [15]. Studies of signaling pathways demonstrated that the mutant protein encoded by the MYD88L265P triggers tumor growth through the activation of nuclear factor kappa lightchain enhancer of activated B cells (NF-κB) by Bruton’s tyrosine kinase and IRAK1/IRAK4 [16]. The frequency of MYD88L265P mutation is much lower in other related indolent β-cell lymphomas such as splenic marginal zone β-cell lymphoma, chronic lymphocytic leukemia (<10%), and multiple myeloma (0%). Further studies on this mutation could reveal a very useful diagnostic marker to distinguish WM from other B cell-related disorders [17]. Also, CXCR4 is the next most commonly mutated gene in WM and can be observed in 29% of patients [18]. So, the clinical presentation of WM patients at diagnosis is partly determined by MYD88 and CXCR4 mutation status [19]. We have to know that IgM monoclonal (M) protein, regardless of concentration, cannot be considered indicative of a diagnosis of WM as they may be demonstrable in a proportion of patients with all B-cell LPD, with an overlap in serum concentrations [20]. Detailed morphologic and immunophenotypic assessment of the bone marrow along with close clinical correlation is required if a definitive diagnosis of WM is to be made. It is good practice that a trephine biopsy be examined in addition to bone marrow aspirate cytology as the pattern of infiltration is important to assess, and it will provide a better overall assessment of the degree of infiltration [21]. In WM it is generally possible to demonstrate both monotypic β-cells and monotypic plasma cells, but extended phenotyping is usually only performed on the B-cell component. A recent study has shown that the majority of WM patients have a characteristic immunophenotype. In addition to the almost universal expression of the pan B-cell antigens CD19, CD20 and CD79, approximately 90% of cases have a CD22weak CD25+ CD27+ IgM+ phenotype but lack expression of CD5, CD10, CD11c, CD23 and CD103 [22].
There are a number of disorders where symptoms develop not due to tumor mass but due to unusual immunologic properties of the IgM protein. And the most common among these would be immunoglobulin light chain amyloidosis, in which 5% have an IgM monoclonal protein [23]. In Waldenström macroglobulinemia, a 50% reduction in the IgM protein can result in dramatic reversal of anemia, lymphadenopathy, and hyper viscosity [24]. Examination of the peripheral blood can also be informative in WM. Red cell agglutination and rouleaux formation may be seen although an overt lymphocytosis is rare. Recent studies, using allele- specific polymerase chain reaction PCR for the MYD88 L265P mutation, have demonstrated the presence of low-level peripheral blood involvement in the majority of patients with untreated WM but also a significant proportion of patients with IgM MGUS [25].
Management
In Waldenström macroglobulinemia, the lack of comparative trials amongst regimens makes it difficult to provide high-quality recommendations based on level an evidence. And the long survival with the age range of affected individuals requires longterm follow-up to assess full therapeutic benefit. A significant proportion of patients with Waldenström macroglobulinemia die of large-cell transformation [26]. Therefore, for patients where therapy is indicated due to progressive marrow infiltration or for symptoms for hyper viscosity, multi-agent chemotherapy is preferred over single-agent rituximab. The treatment of asymptomatic patients does not improve the quality of life and survival, biannual clinical observation is the recommended option in these cases if hematologic function is preserved .In fact, the choice of treatment is a critical option and should not be taken so as to limit future options, since all patients will inevitably present relapses after initial treatment, requiring treatment [27].
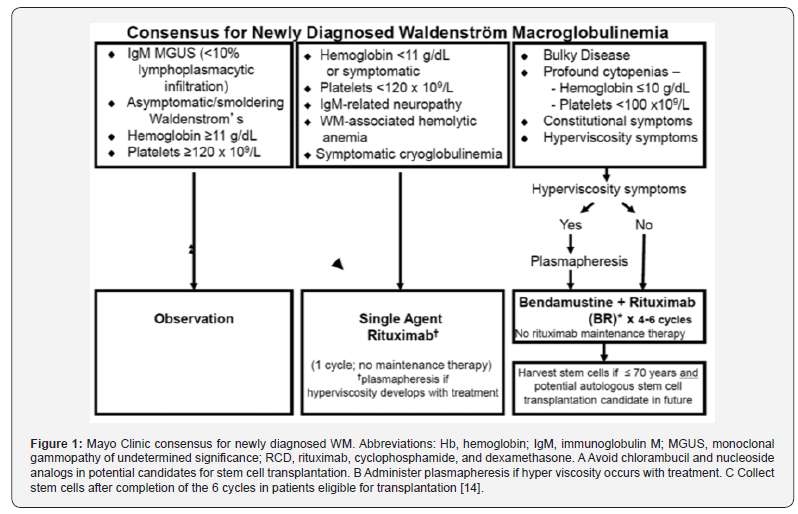
First-line therapy includes alkylating agents, purine analogs and monoclonal anti-cluster-of-differentiation CD20 antibodies [28]. We have to avoid chlorambucil and nucleoside analogs in potential candidates for stem cell transplantation. And administer plasmapheresis if hyper viscosity occurs with treatment. Collect stem cells after completion of 6 cycles in patients eligible for transplantation [14]. Most symptomatic patients are treated with Rituximab as monotherapy or combined with chemotherapy (Figure 1). Monotherapy is recommended in symptomatic patients with moderate hematological impairment, in patients with neuropathy associated with the IgM autoantibody, and in cases of hemolytic anemia resistant to corticosteroids [27].
When we start rituximab treatment, some patients have a paradoxical and often transient increase in serum concentrations of IgM (IgM flare), which can persist for up to 4 months and is not indicative of treatment failure [29]. But the underlying mechanism remains unclear, but two hypotheses have been proposed - release of intracellular IgM resulting from rituximab-mediated cell death and cell signaling mediated by binding to CD20 [29]. Plasmapheresis is indicated in patients requiring urgent control of the disease if they have clinical manifestations of moderate to severe hyper viscosity, cryoglobulinemia and cytopenia’s caused by the action of the monoclonal IgM autoantibody .Usually 2 to 3 plasmapheresis sessions are necessary to reduce the concentration of IgM from 30 to 60%. The sessions should be repeated daily until symptoms subside or until normalization of serum viscosity. And subsequent treatment should be started quickly, because the concentration of IgM will return to its initial level after 4 to 5 weeks [18]. These patients should be treated with the dexamethasone, rituximab and cyclophosphamide (DRC) combination regimen. The main reasons for choosing this regimen in these patients are the good treatment tolerance, reduced myelosuppression and the lack of toxicity for stem cells [28].
We have to know that the concentration of monoclonal IgM is one of the parameters most commonly used among the criteria for assessing response to treatment. However, this biomarker is not always reliable, as its concentration can be affected by the treatment itself [18]. In patients with relapses or who are refractory to therapy, the choice of treatment depends on the first-line treatment already utilized, the quality/duration of the response and other variables, such as age, tolerance to initial treatment, and also the possibility of the patient being a candidate for stem cell transplantation .In patients with short-term remission or resistance to initial treatment, therapy with a drug of different pharmacological class as monotherapy or combined is recommended. In association therapy, a regime using rituximab, fludarabine and cyclophosphamide is highlighted; however, cyclophosphamide should be avoided in younger patients and candidates for autologous stem cell transplantation [28].
Autologous transplantation is associated with improved survival and long periods without disease progression, and all candidate patients presenting relapse should be considered to do autologous transplantation [13]. The concentration of monoclonal IgM is one of the parameters most commonly used among the criteria to assess response to treatment. However, this biomarker is not always reliable, as its concentration can be affected by the treatment itself [29].
Conclusion
Wald Enstrom macroglobulinemia is a lymphoplasmacytic lymphoma characterized by IgM monoclonal hypergammaglobulinemia and bone marrow infiltration and many patients who fulfill the criteria of WM do not require immediate therapy because they are asymptomatic. Wm most commonly presented with anemia .the etiology is unknown. , MYD88 mutation has a prevalence in Waldenström macroglobulinemia of 87 to 100% and CXCR4 is the next most common. Clinical features may occur as a consequence of the physico-chemical and immunological properties of the M protein rather than disease burden. Most symptomatic patients are treated with Rituximab as monotherapy or combined with chemotherapy. Monotherapy is recommended in symptomatic patients with moderate hematological impairment, in patients with neuropathy associated with the IgM autoantibody, and in cases of hemolytic anemia resistant to corticosteroids. Transplantation of hematopoietic stem cells is indicated in younger patients with multiple recurrences or who have been refractory to previous treatments.
Conflicts of Interest
There are no conflicts of Interest.
References
- Campo E, Swerdlow S, Harris N, Pileri S, Stein H, et al. (2011) The 2008 WHO classification of lymphoid neoplasms and beyond: evolving concepts and pratical applications. Blood 117: 5019-5032.
- Leleu X, O Connor K, Ho AW, Santos DD, Manning R, et al. (2007) Hepatitis C viral infection is not associated with Waldenström's macroglobulinemia. Am J Hematol 82: 83-84.
- Dimopoulos MA, Anagnostopoulos A (2005) Waldenstrom's macroglobulinemia. Best Pract Res Clin Haematol 18: 747-765.
- Bjorkholm M, Johansson E, Papamichael D (2003) Patterns of clinical presentation, treatment, and outcome in patients with Waldenstrom's macroglobulinemia: a two-institution study. Semin Oncol 30 (2): 226-230.
- Mehta J, Singdahl S (2003) Hyperviscosity syndrome in plasma cell dyscrasias. Semin Thromb Hemost 29(5): 467-471.
- Klein CJ, Moon JS, Mauermann ML, Zeldenrust SR, Wu Y, et al. (2011) The neuropathies of Waldenstrom’s macroglobulinemia (WM) and IgM-MGUS. Can J Neurol 38(2): 289–295.
- Ly KI, Fintelmann F, Forghani R, Schaefer PW, Hochberg EP, et al. (2011) Novel diagnostic approaches in Bing-Neel syndrome. Clin Lymphoma Myeloma Leuk 11(1): 180-183.
- Palladini G, Merlini G (2013) Diagnostic challenges of amyloidosis in Waldenstrom macroglobulinemia. Clin Lymphoma Myeloma Leuk 13(2): 244-246.
- Camp BJ, Magro CM (2012) Cutaneous macroglobulinosis: a case series. J Cutan Pathol 39(10): 962-970.
- Massengo S, Riffaud L, Morandi X, Bernard M, Verin M (2003) Nervous system lymphoid infiltration in Waldenstrom’s macroglobulinemia. A case reports. J Neurooncol 62(3): 353-358.
- Pangalis G, Kyrtsonis MC, Kontopidou F, Siakantaris M, Dimopoulou M, et al. (2005) Differential diagnosis of Waldenström's macroglobulinemia and other B-Cell disorders. Clin Lymphoma 5: 235-240.
- Morel P, Duhamel A, Gobbi P, Dimopoulos MA, Dhodapkar MV, et al. (2009) International prognostic scoring system for Waldenstrom macroglobulinemia. Blood 113: 4163-4170.
- Ansell SM, Kyle RA, Reeder CB, Fonseca R, Mikhael JR, et al. (2010) Diagnosis and management of Waldenstrom macroglobulinemia: Mayo stratification of macroglobulinemia and risk-adapted therapy (mSMART) guidelines. Mayo Clin Proc 85(9): 824-833.
- Gertz MA (2018) Waldenström macroglobulinemia: 2018 update on diagnosis, risk stratification, and management. Am J Hematol 1-11.
- Xu L, Hunter ZR, Yang G (2014) Detection of MYD88 L265P in peripheral blood of patients with Waldenstrom’s macroglobulinemia and IgM monoclonal gammopathy of undetermined significance. Leukemia. 28(8): 1698-1704.
- Yang G, Liu X, Chen J (2015) Targeting IRAK1/IRAK4 signaling in Waldenstrom’s Macroglobulinemia. Blood 126(23): 4004.
- Varettoni M, Arcaini L, Zibellini S (2013) Prevalence and clinical significance of the MYD88 (L265P) somatic mutation in Waldenstrom’s macroglobulinemia and related lymphoid neoplasms. Blood 121(3): 2522‐2528.
- Treon SP, Cao Y, Xu L, Yang G, Liu X, et al. (2014) Somatic mutations in MYD88 and CXCR4 are determinants of clinical presentation and overall survival in Waldenstrom’s macroglobulinemia. Blood 123(18): 2791-2796.
- Xu L, Hunter ZR, Yang G, Zhou Y, Cao Y, et al. (2013) MYD88 L265P in Waldenstrom’s macroglobulinemia, IgM monoclonal gammopathy, and other B-cell lymphoproliferative disorders using conventional and quantitative allele-specific PCR. Blood 121(11): 2051-2058.
- Lin P, Hao S, Handy BC (2005) Lymphoid neoplasms associated with IgM paraprotein: a study of 382 patients. Am J Clin Pathol 123: 200-205.
- Ng AP, Wei A, Bhurani D (2006) The sensitivity of CD138 immunostaining of bone marrow trephine specimens for quantifying marrow involvement in MGUS and myeloma, including samples with a low percentage of plasma cells. Haematologica 91: 972-975.
- Paiva B, Montes MC, Garcia-Sanz R (2014) Multiparameter flow cytometry for the identification of the Waldenstrom’s clone in IgM-MGUS and Waldenstrom’s Macroglobulinemia: new criteria for differential diagnosis and risk stratification. Leukemia 28(1): 166‐173.
- Cao XX, Meng Q, Mao YY, Su W, Zhen JF, et al. (2016) The clinical spectrum of IgM monoclonal gammopathy: a single center retrospective study of 377 patients. Leuk. Res 46: 85-88.
- Gertz MA, Abonour R, Heffner LT, Greipp PR, Uno H, et al. (2009) Clinical value of minor responses after 4 doses of rituximab in Waldenstrom macroglobulinaemia: a follow-up of the eastern cooperative oncology group E3A98 trial. Br J Haematol 147: 677-680.
- Xu L, Hunter ZR, Yang G (2014) Detection of MYD88 L265P in peripheral blood of patients with Waldenstrom’s macroglobulinemia and IgM monoclonal gammopathy of undetermined significance. Leukemia.; 28(8): 1698-1704.
- Durot E, Tomowiak C, Michallet AS, Dupuis J, Hivert B, et al. (2017) Transformed Waldenstrom macroglobulinaemia: clinical presentation and outcome. A multi-institutional retrospective study of 77 cases from the French Innovative Leukemia Organization (FILO) Br J Haematol 179(3): 439-448.
- Dimopoulos MA, Gertz MA, Kastritis E, Garcia-Sanz R, Kimby EK, et al. (2009) Update on treatment recommendations from the Fourth International Workshop on Waldenström's macroglobulinemia. J Clin Oncol 27(1): 120-126.
- Ghobrial IM, Fonseca R, Greipp PR, Blood E, Rue M, et al. (2004) Initial immunoglobulin M 'flare' after rituximab therapy in patients diagnosed with Waldenstrom macroglobulinemia. Cancer 101(11): 2593-2598.
- Treon SP, Branagan AR, Hunter Z, Santos D, Tournhilac O, et al. (2004) Paradoxical increases in serum IgM and viscosity levels following rituximab in Waldenstrom's macroglobulinemia. Ann Oncol 15(10): 1481-1483.






























