Dendritic Cells and Regulatory T Cells Changes During ECP for Chronic GvHD in Pediatric Patients
Castiglia Sara, Rustichelli Deborah, Adamini Aloe, Albiani Roberto, Ferrero Ivana, Fagioli Franca and Berger Massimo*
Department of Paediatric Onco-Hematology, Regina Margherita Children’s Hospital, Italy
Submission: September 22, 2018; Published: October 03, 2018
*Corresponding author: Berger Massimo, Paediatric Onco-Hematology, Regina Margherita Children’s Hospital, City of Health and Science, Turin, Italy.
How to cite this article: Castiglia S, Rustichelli D, Adamini A, Albiani R, Ferrero I, Fagioli F, Berger M. Dendritic Cells and Regulatory T Cells Changes During ECP for Chronic GvHD in Pediatric Patients. Open Acc Blood Res Trans J. 2018; 2(5): 555597. DOI: 10.19080/OABTJ.2018.02.555597
Keywords: Dendritic cells; Chronic GvHD; Pediatric patients; Sequences of immunological events; Corticosteroids; UVA treatment; Regulatory T cells
Abbrevations: mDC: Myeloid Dendritic Cells; pDC: Plasmocytoid Dendritic Cells; Treg: Regulatory T Cells; GvHD: Graft-versus-host disease; APCs: Antigen-Presenting Cells; ECP: Extracorporeal Photopheresis; HSCT: Hematopoietic Stem Cell Transplantation; DCs: Dendritic Cells; PB: Peripheral Blood; PA: Photoactivated Apheresis; WBC: White Blood Cell
Mini Review
Graft-versus-host disease (GvHD) is a leading cause of post HSCT morbidity and mortality. It is mediated by alloreactive mature donor T lymphocytes, resulting in a harmful inflammatory response and tissue injury [1]. The pathophysiology of GvHD is constituted by precise sequences of immunological events such as the activation of antigen-presenting cells (APCs), activation, differentiation and migration of T cells, and finally the development of their full effector functions [2-4]. Dendritic cells (DCs) constitute the most professional APCs, promoting alloreactivity or clonal antigen-specific T cell responses. Moreover, tolerogenic DCs may play a pivotal role in GvHD exerting an immunomodulatory or even immunosuppressive effect on T cells [5]. DCs can be divided into two major subsets, plasmacytoid DCs (pDCs) and myeloid DCs (mDCs) which have distinct functions. pDCs play a pivotal role in peripheral tolerance through the generation of regulatory T (Treg). In addition to pDCs, mDCs also promote Th2 and Th0/Tr1 responses, depending on the activation signal types [6,7].
Although corticosteroids, with potent immunosuppressive and anti-inflammatory effects, are the first-line treatment for GvHD, only 25-50% of patients respond [1,8]. Extracorporeal photopheresis (ECP) is an alternative therapeutic strategy in patients who are resistant/refractory to steroids. ECP appears to act in an immunomodulatory fashion, inducing immunotolerance in GvHD by regulatory T lymphocytes, dendritic cells, in concert with the normalization of a T lymphocyte subset.
This pilot study focuses on these two cell populations, as well as on the patients’ whole immunological pattern [9-11]. Data from eleven patients affected by chronic GVHD were included and analyzed. The median age was 9 years (range 2-18), 6 out of 11 patients were females and suffered of myeloid malignancies (4 patients), acute lymphoblastic leukemia (2 patients), neuroblastoma (1 patient), Hemophagocytic Lymphohistiocytosis (1 patient), Ewing sarcoma (1 patient), Blackfan-Diamond Anemia (1 patient).
Seven patients received an unrelated Hematopoietic Stem Cell Transplantation HSCT (3 bone marrow, 3 peripheral blood, 1 cord blood), three patients had a sibling HSCT (1 patient with 1 Ag mismatch graft, all patients had bone marrow as the stem cell source) and one patient had an phenotypic identical-HSCT). All patients suffered moderate to severe cGvHD which was resistant/refractory to steroids. All patients received ECP from January 2016 to December 2016 in our center according to previously published techniques [12]. The aim of this study was to: i) monitor peripheral blood changes after 30 days of ECP and ii) describe the immunological changes in aphaeresis samples after UVA treatment.
A sample of peripheral blood (PB), a sample of apheresis pre-UVA photoactivation (pre-PA) and a sample of photoactivated apheresis (PA) were collected at the first day of ECP and every week for the first month of treatment. Informed consent was obtained from all patients. PB, pre-PA and PA samples were characterized at day 0, 8, 15, 21, 30 of ECP treatment. The percentage obtained by cytofluorimetric analysis was used to calculate the absolute number of cells/μl based on the white blood cell (WBC) number counted using a standard hemacytometer (DASIT).
Statistical analysis was performed using NCSS for Windows. Descriptive statistics are reported as medians, continuous variable differences between groups were calculated with the Student T test. The fold change was calculated by the ratio between the difference (day 30 – day 0) /day 0. The P value below 0.05 was considered as statistically significant.
As shown in Table 1, at day 30 we observed a 0.48 change of CD3+. After 30 days of treatment there was an improvement of the CD4+/CD8+ ratio from 0.49 to 0.86 (0.75 times), that resulted in a change of CD3+CD4+ (from 234 to 384 x109/L) compared to CD3+CD8+ (from 475 to 448 x109/L). Moreover, in the same period of study, we had a change of 0.75 times of CD3-CD56+ from 272 to 478 x109/L 62 and a smaller rise of CD19+ from 214 to 256 x109/L. The naïve CD4+ and CD8+ lymphocytes rose from 4 to 39x109/L (8.75-fold change, P=0.02) and from 27 to 58x109/L (1.14-fold change) over a month while their memory counterpart fell by 0.62 and 0.55-fold. When we calculated the CD4+ and CD8+ naïve/memory ratio we found a 35- and 4-fold change (P=0.01 and 0.02). Comparing the day 0 and day 30 peripheral blood samples, we observed a high rise of 0.66 and 1.14 times for mDCs (mDC from 9 to 15 x109/L [P=0.03] and pDC from 7 to 15 x109/L [P=0.05]) respectively together with a change of Tregs (from 2.9 to 9.1 x109/L, 3.5-fold change [P=0.04]). Finally, when we analyzed the changes between pre- and post-UVA photoactivation a significant change of Treg was observed (0.35-fold change, P=0.05), while a decrease in cell number was observed for CD8+ (P=0.05) (Table 2).

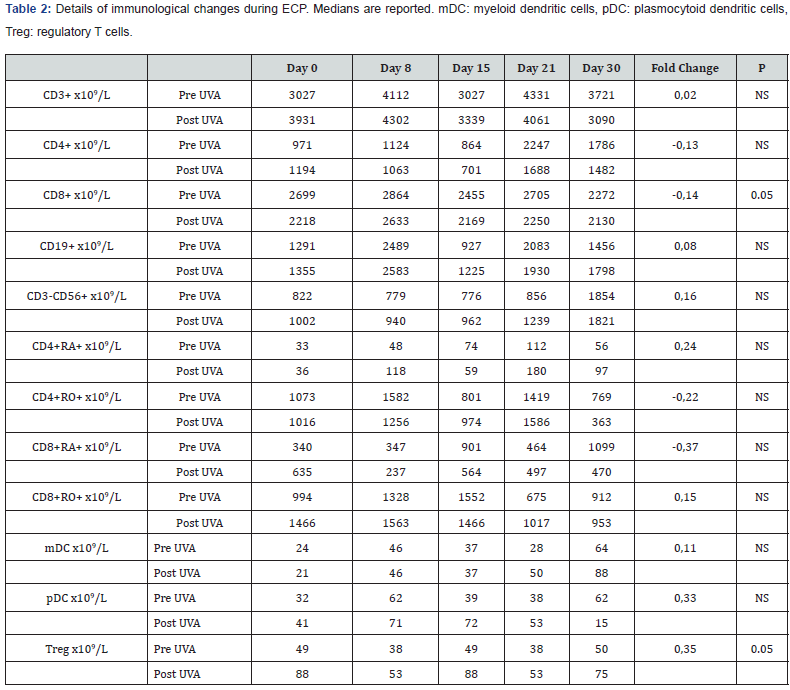
Considering the low number of patients enrolled in this study no firm conclusions can be drawn from a clinical point of view, while a biological effect was certainly highlighted. Our findings are in line with previous publications, however a larger cohort of patients is needed to establish a direct correlation between biological changes and clinical responses.
References
- Flinn AM, Gennery AR (2016) Extracoporeal photopheresis treatment of acute graft-versus-host disease following allogeneic haematopoietic stem cell transplantation.
- Reddy P, Ferrara JLM (2003) Immunobiology of acute graft-versushost disease. Blood Rev 17(4): 187-94.
- Jaramillo A, Fernández FG, Kuo EY, Trulock EP, Patterson GA (2005) Immune mechanisms in the pathogenesis of bronchiolitis obliterans syndrome after lung transplantation. Pediatr Transplant 9(1): 84-93.
- Heeger PS (2003) T-cell allorecognition and transplant rejection: a summary and update. Am J Transplant 3(5): 525-533.
- D’Asaro M, Dieli F, Caccamo N, Musso M, Porretto F (2006) Increase of CCR7- CD45RA+ CD8 T cells (T(EMRA)) in chronic graft-versus-host disease. Leukemia. marzo 20(3): 545-547.
- Schettini J, Mukherjee P (2008) Physiological role of plasmacytoid dendritic cells and their potential use in cancer immunity. Clin Dev Immunol 2008:106321.
- Fricke I, Gabrilovich DI (2006) Dendritic cells and tumor microenvironment: a dangerous liaison. Immunol Invest 35(3-4): 459- 483.
- Van Lint MT, Uderzo C, Locasciulli A, Majolino I, Scimé R, et al. (1998) Early treatment of acute graft-versus-host disease with high- or lowdose 6-methylprednisolone: a multicenter randomized trial from the Italian Group for Bone Marrow Transplantation. Blood. 1 ottobre 92(7): 2288-2293.
- Gorgun G, Miller KB, Foss FM (2002) Immunologic mechanisms of extracorporeal photochemotherapy in chronic graft-versus-host disease. Blood 100(3): 941-947.
- Di Renzo M, Rubegni P, Pasqui AL, Pompella G, De Aloe G, et al. (2005) Extracorporeal photopheresis affects interleukin (IL)-10 and IL-12 production by monocytes in patients with chronic graft-versus-host disease. Br J Dermatol 153(1): 59-65.
- Lorenz K, Rommel K, Mani J, Jin N, Hilgendorf I, et al. (2015) Modulation of lymphocyte subpopulations by extracorporeal photopheresis in patients with acute graft-versus-host disease or graft rejection. Leuk Lymphoma 56(3): 671-675.
- Berger M, Albiani R, Sini B, Fagioli F (2015) Extracorporeal photopheresis for graft-versus-host disease: the role of patient, transplant, and classification criteria and hematologic values on outcome-results from a large single-center study. Transfusion 55(4): 736-747.






























