Study of Bone Marrow Niche Cells in Aplastic Anemia and Itsí Change to Acute Leukemia Possibly
Ahmad Reza Rahnemoon*
Department of Hematology, Iran University of Medical Sciences, Tehran, Iran
Submission: February 28, 2018; Published: March 19, 2018
*Corresponding author: Ahmad Reza Rahnemoon, Department of Hematology, Iran University of Medical Sciences, Tehran, Iran, Email: ar.rahnemoon@gmail.com
How to cite this article: Ahmad Reza Rahnemoon. Study of Bone Marrow Niche Cells in Aplastic Anemia and Its' Change to Acute Leukemia Possibly. Blood Res Transfus J. 2018; 2(2) : 555581. DOI: 10.19080/OABTJ.2018.01.555581.
Abstract
In aplastic anemia bone marrow failure results from severe damage to the hematopoietic cell compartment; In other words, defects in hematopoietic stem cells(HSCs) and the stromal implicate in the disease but cause of this disease is still obscure. Actually imbalance between adipogenic and osteogenic differentiation of mesenchymal stem cell (MSC) can influence hematopoiesis importantly. Cells bearing the CD34 antigen , a marker of early hematopoietic cells, are greatly diminished and in functional studies committed stem cells like CFU-G, CFU-M, CFU-Mk and primitive progenitor cells like CFU-Mix are virtually reduced or absent. These findings suggest that reduced hematopoietic stem cells are the primary defect in abnormal hematopoiesis. Anyhow, we can say aplastic anemia is a stem cell disease firstly and second cytokine deficiency is not the etiologic problem in many cases of aplastic anemia because of some studies have shown relatively normal growth factor production and in some cases of aplastic anemia due to stromal cell defects possibility as well. Thus, here I try to discuss the HSCs and their microenvironment in aplastic anemia which how can support from normal hematopoiesis.
Introduction
Aplastic anemia (AA) is a disorder which have hypoplasia and severe pancytopenia. The disease has been associated with exposure to ionizing irradiation, also may be with drug, chemicals or infectious agents but frequently associated without any agents or idiopathic situation. Theoretically, some mechanisms may lead to aplastic anemia including; first, absent or defective hematopoietic stem cell; second, a defect intrinsic to the hematopoietic microenvironment or suppression of hematopoiesis by other host systems [1-4]. In the past, the disease attributed with congenital or acquired stem cell defects. Dameshek raised a provocative question: What do AA, paroxysmal nocturnal hemaglobinuria (PNH) and acute leukemia have in common? According to this knowledge, first, PNH can change to AA, second, overlap between AA and PNH may be occur. He thought, PNH was ecologically advantageous and also AA and PNH with each other different response to bone marrow in front of some agents like chemicals, viruses and ionizing radiation that resulting in a defective hematopoietic microenvironmental particularly in animal models primarily and then in some patients with AA and congenital hypoplastic anemia as well [5,8-10].
Discussion
The study has stated, AA patients, having a limited number of HSCs are more susceptible to damage of HSCs especially against higher doses of radiation but permanent aplasia occurs some months later which fails to support hematopoiesis. In addition, Knospe suggested that low dosage of irradiation might damage only stem cells whereas high dosage can damage the supportive stromal microenvironment as well. In animal models, irradiated marrow culture adherent cells, unable to support hematopoiesis. Also, after delivery of very high dosage irradiation, marrow environment with hypoplasia can’t support cell growth importantly [7,11-14]. Moreover, mice treated with cyclophosphamide or busulfan described a few transient HSCs with more prolong effect on hematopoietic microenvironment; and chloramphenicol can de-arranged hematopoietic microenvironment and prevent bone marrow regeneration which lastly irreversible AA occur. In CMV, the marrow cells can be infected that following physical changes in the structure of bone marrow niche with secondary aplasia in the end [12,13].
Abnormal stromal cell function and hematopoietic failure appeared in AA (Figure 1). The stromal cells with decrease in production includes regulators of hematopoiesis, extracellular matrix or in both possibly. After discontinuation with alkylating agent therapy, patients may develop aplasia and then into hypoplastic myelodysplastic syndrome (HMDS) as well [15-18]. In fact, in AA the alterations of physiological status in stromal microenvironment reported which revealed impair proliferation and differentiation. Also, the defective of hematopoiesis can be resulted from failure of the stromal microenvironment which produce essential growth factors or absence of proper growth receptors as well. Furthermore, the defective production of fibroblast showed decrease CFU-fibroblast (CFU-F) due to defective microenvironmental stromal fibroblast function which results of additional mitosis due to stromal precursor cell activated to support mesenchymal cells under position of stress marrow. On the other hand, MSCs are multipotent cells capable of multipotent differentiation with immunosuppressive virtues after the treatment of bone marrow transplantation (BMT). The role of MSCs after its transplantation, can be modified the recipient BM microenvironment by paracrine effect or cell-cell mediated reparative function. Thus, this change seems that the success of HSC transplantation (HSCT) base on the essential role of osteoblastic cells in the regulation of HSC [19-21]. Some researchers stated after MSC co-injection to establish of HSC in the niche and after the recovery as well, donor MSCs cleared possibly by the immune system and concerning in their animal experiments after transplantation and they suggested the necessary role of MSC transplantation to be only transient. Thus the role of BM microenvironment in BM failure syndromes explain that its supportive components as an additional tool for hematopoiesis improvement and support in the treatment diseases like MDS as well. In fact, the data support from this concept includes defects in BM microenvironment can be important of initiating steps in BM failure syndromes pathogenesis. In other words, microenvironmental dysfunction likely mediated at least in part by autoimmunity which lead to HSC depletion as a targetable component in initiated of the diseases process [22-25]. Chronic hematopoietic growth factors therapy resulting not only in reduced replicative function of stem cells, but also can effect in genetic instability too. Moreover, BM adipocytes are anti-hematopoietic growth factor, as a potent source, so increased in adipocyte cell of MSC may be the BM affected and contribute to the defective hematopoiesis. In AA, these growth factors can suppress apoptosis that help to eradication of damaged cells and so, contribute to the development of clonal disorders like MDS and acute myeloid leukemia (AML). In other words, in the disease programmed cell death (PCD) can be important in the patients and mechanisms controlling apoptosis may play an essential role with abnormal cell death and the relationship between death and advancement of AA may reversible which definitely increase of cellular apoptosis leads to involved in the disease and its progression. Thus, these finding suggest that, in most cases, growth factor deficiency is not the etiologic problem in the disease.
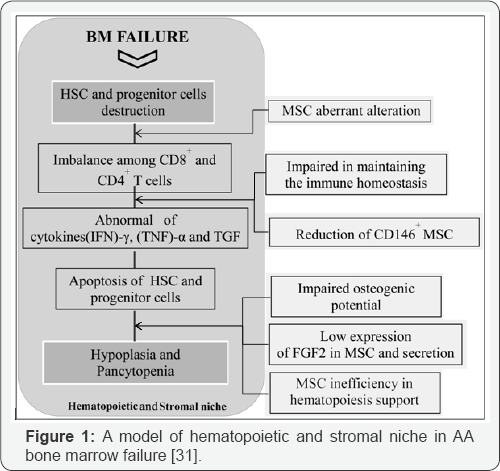
Genetic abnormalities includes ASXL1, DNMT3A and BCOR/ BCORL1 are found in half AA patients which represented in acute myeloid leukemia (AML). But PIGA mutations and uniparental disomy (UPD) in 6P (6PUPD) found in the patients as well. Also we can say DNMT3A and ASXL1 mutations are seen normally like in aged bone marrow individuals. In other words, age related clonal hematopoiesis (CH) by genetic analysis identified in 1-3% of patients with non hematologic cancers as well as normal persons that showed an important correlation with age and the high risk persons toward subsequent hematologic malignancy which suggested a common mechanism in relation of genomic aging between healthy elderly and bone marrow failure syndromes especially in AA. Also TET2 and JAK2 mutations infrequently presented among AA patients that suggested distinct mechanisms to manage in the clonal selection of bone marrow niche cells in AA (Figure 2 & 3). In our memory, the clonal selection should be observed much higher with BCOR, BCORL1 and PIGA mutations as well. Furthermore, DNMT3A and ASXL1 mutations may be over express in autoimmune BM environment of AA [26-28].
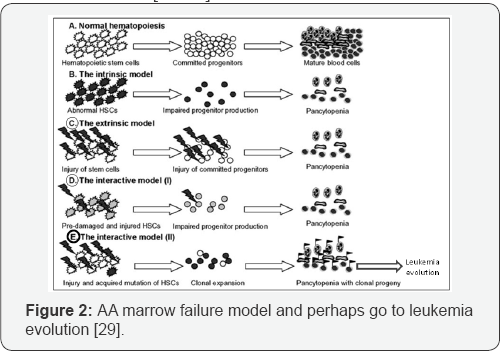
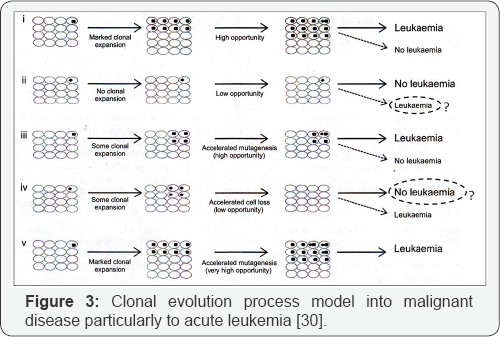
In cytogenetic abnormalities, the AA interpretation is complex possibly (Figure 2 & 3); because of monosomy 7 and multiple unfavorable mutations predicted perhaps with suitable blood count and prolonged survival after treatment. Moreover, according to WHO classification, MDS-U patients with mild BM failure, no dysplasia and monosomy 7 or del (7q) have the same criteria with some AA patients [28-31].
Conclusion
AA alterations in bone marrow niche cells and their defects including first, in stromal niche, second stem cell population particularly HSCs and/or other progenitor cells as well. In addition, CFU-F formation severely affected with decreased in AA. Thus, These alterations and other inhibitory effects are the feedbacks of proliferative defects on hematopoietic cells proliferation and their microenvironment as well. Here, I have some questions in AA disease as follows: first, we know AA clonal hematopoiesis is complex and highly variable, so what about the clonal hematopoiesis and its evolution? Second, what is the role of specific driver mutations which acquired in early, end and/or between them in the process of clonal hematopoiesis and its evolution to malignant disease possibly? In other words, we poorly know concerning how change AA to acute leukemia exactly or, in AA what is the exact relation between clonal hematopoiesis and acute leukemia possibly? Third, in some cases what is the borderline between AA and refractory anemia without ring sideroblast? In this case, the refractory anemia has dysplasia in erythroid cells only, no increase any blast in blood and BM and cytogenetic normally as well that like with some of AA patients. Finally, we must do a better understanding about these questions and also the mechanism of AA in the process of changing to possible acute leukemia.
References
- Young NS (2000) Introduction: acquired aplastic anemia. Semin Hematol 37: 2.
- Kagan WA, Ascensao JL, Fialk MA, Coleman M, Valera EB, et al. (1979) Studies on pathogenesis of aplastic anemia. Am J Med 66(3): 444-449.
- Kagan WA, Ascensao JA, Pahwa RN, Hansen JA, Goldstein G, et al. (1976) Aplastic anemia: presence in human bone marrow of cells that suppress myelopoiesis. Proc Natl Acad Sci 73: 2890-2894.
- Kagan WA (1977) Aplastic anemia: three different diseases. Clin Res 25: 476A.
- Young NS (1992) The problem of clonality in aplastic anemia: Dr. Dameshek's Riddle, restarted. Blood 79: 1385.
- Williams DM (1999) Pancytopenia, aplastic anemia, and pure red cell aplasia. In: Lee GR, et al. Wintrobe’s Clinical Hematology (volume 1), Maryland, Williams and Wilkins, pp. 1449.
- Alter BP, Young NS (1993) The bone marrow failure syndromes. In: Nathan DG, et al. Hematology and Infancy Childhood (volume 1), WB Saunders Company, Philadelphia, USA, pp. 216.
- Fried W, Kedo A, Barone J (1977) Effects of cyclophosphamide and busulfan on spleen colony forming units and on hematopoietic stromal. Cancer Res 37(4): 1205-1209.
- Knopse WH (1976) Bone marrow scanning with (Fe)59. Regeneration extension of marrow after ablative doses of radiotherapy. Cancer 37: 1432.
- Knopse WH, Crosby WH (1971) Aplastic anemia: a disorder of the bone marrow sinusoidal microcirculation rather than stem cell failure? Lancet 1: 20.
- Keung YK, Cobos E, Morgan D, Tonk V (1996) Development of minimally differentiated acute myeloblastic leukemia with novel isochromosome 18p and antecedent aplastic anemia. Cancer Genet Cytogenet 92: 1.
- Nara N, Bessho M, Hirashima K, Momo H (1982) Effects of chloramphenicol on hematopoietic inductive microenvironment. Exp Hematol 10(1): 20-25.
- Apperley JF, Dowding C, Hibbin J, Buiter J, Matutes E, et al. (1989) The effect of cytomegalovirus on hemopoiesis: in vitro evidence for selective infection of marrow stromal cell. Exp Hematol 17(1): 38-45.
- Quesenberry PJ (1990) The concept of hematopoietic stem cell. In: Williams WJ, et al. Hematology (volume 1), MC Grew Hill Company, New York, USA, pp. 129.
- Marsh JCW (2000) Hematopoietic growth factors in the pathogenesis and for the treatment of aplastic anemia. Seminars in Hematology 37(1): 81-90.
- Martin SJ, Green DR (1995) Apoptosis and cancer: the failure of controls on cell deathand cell survival. Crit Opin Hematol 18(2): 137153.
- Shadduck RK (2001) Aplastic anemia. In: Butler E, et al. Williams Hematology, sixth edition MC Grew Hill company, New York, USA, pp. 325.
- Hotta T, Kato T, Maeda H, Yamao H, Yamada H, et al. (1985) Functional changes in marrow stromal cells in aplastic anemia. Acta Hematol 74(2): 65-69.
- Kojima S, Matsuyama T, Kodera Y (1992) Hematopoietic growth factors releases by marrow stromal cells from patients by aplastic anemia. Blood 79(9): 2256-2261.
- Balderman SR, Calvi LM (2014) Biology of BM failure syndromes: role of microenvironment and niches. Hematology 2014(1): 71-76.
- Zhou Y, He Y, Xing W, Zhang P, Shi H, et al. (2017) An abnormal bone marrow microenvironment contributes to hematopoietic dysfunction in Fanconi anemia. Hematologica 102(6): 1017-1027.
- Ogawa S (2016) Clonal hematopoiesis in acquired aplastic anemia. Blood 128: 337-347.
- Jaganathan BG, Tisato V, Vulliamy T, Dokal I, Marsh J, et al. (2010) Effects of MSC co- injection on the reconstitution of aplastic anemia patient following hematopoietic transplantation. Leukemia 24(10): 1791-1795.
- Tripathy NK, et al. (2014) Enhanced adipogenicity of bone marrow mesenchymal stem cells in aplastic anemia. Stem Cells Int p. 1-6.
- Chatterjee S, et al. (2010) Alteration in marrow stromal microenvironment and apoptosis mechanisms involved in aplastic anemia: An animal model to study the possible disease pathology. Stem Cells Int p. 1-12.
- Yoshizato T, Dumitriu B, Hosokawa K, Makishima H, Yoshida K, et al. (2015) Somatic mutations and clonal hematopoiesis in aplastic anemia. The New England Journal of Medicine 373: 35-47.
- Nakao S, gale PR (2016) Are mild/moderate acquired idiopathic aplastic anemia and low risk myelodysplastic syndrome one or two diseases or both and how should it/they be treated? Leukemia 30(11): 2127-2130.
- Tesi B, Davidsson J, Voss M, Rahikkala E, Holmes TD, et al. (2017) Gain of function SAMD9L mutations cause a syndrome of cytopenia, immunodeficiency, MDS, and neurological symptoms. Blood 129(16): 2266-2279.
- Risitano AM, Maciejewski JP, Selleri C, Rotoli B (2007) Function and malfunction of hematopoietic stem cells in primary bone marrow failure syndromes. Cancer Stem Cell Research and Therapy 2(1): 3952.
- Grove CS, Vassiliou GS (2014) Acute myeloid leukemia: a paradigm for the clonal evolution of cancer? Disease Models and Mechanisms 7(8): 941-951.
- Gonzaga VF, Wenceslau CV, Lisboa GS, Frare EO, Kerkis I (2017) Mesenchymal stem cells benefits observed in bone marrow failure and acquired aplastic anemia. Stem Cells Int.






























