Glomerular Dysfunction in Adult Patients with β-Thalassemia Major
Sabry A Shoeib1, Mohamed A Abd El Hafez1, Alaa E Abd El Hamid1, Seham A Khodair2, Hesham G Amer2, Essam Aly Abd Elmohsen 3 and Heba Y Elkholy1*
1Departments of Internal Medicine, Menoufia University, Egypt
2Clinical Pathology, Faculty of Medicine, Menoufia University, Menoufia, Egypt
3Elmaadi Armed Forces institute, Egyp
Submission: June 17, 2017; Published: August 04, 2017
*Corresponding author: Heba Y Elkholy, Menoufia University, Egypt, Email: dr_hebaelkholy@hahoo.com
How to cite this article: Sabry A S, Mohamed A A E H, Alaa E A E H, Seham A K, Hesham G A, Essam A A E, Heba Y E. Glomerular Dysfunction in Adult Patients with β-Thalassemia Major . Open Acc Blood Res Transfus J. 2017; 1(3): 555564.
Abstract
Objective: The aim of the present study is to investigate the presence of glomerular dysfunctions in adults with β thalassemia major (β- TM), using early biomarkers of glomerular dysfunctions.
Background: β thalassemia major associated nephropathy has recently been a growing matter of concern affecting most aging thalassemia major patients. Serum creatinine is alone an unreliable indicator of renal functions.
Patients and methods: 50 patients with β-TM were subjected to history taking and clinical examination. Urinary albumin/creatinine ratio (ACR), Serum cystatin-C (SCys-C) levels were measured and CKD-EPI-CysC equation for estimated glomerular filtration rate (eGFR) were calculated. Presence of ACR>300mg/g and/or decreased eGFR cys were considered as glomerular dysfunction.
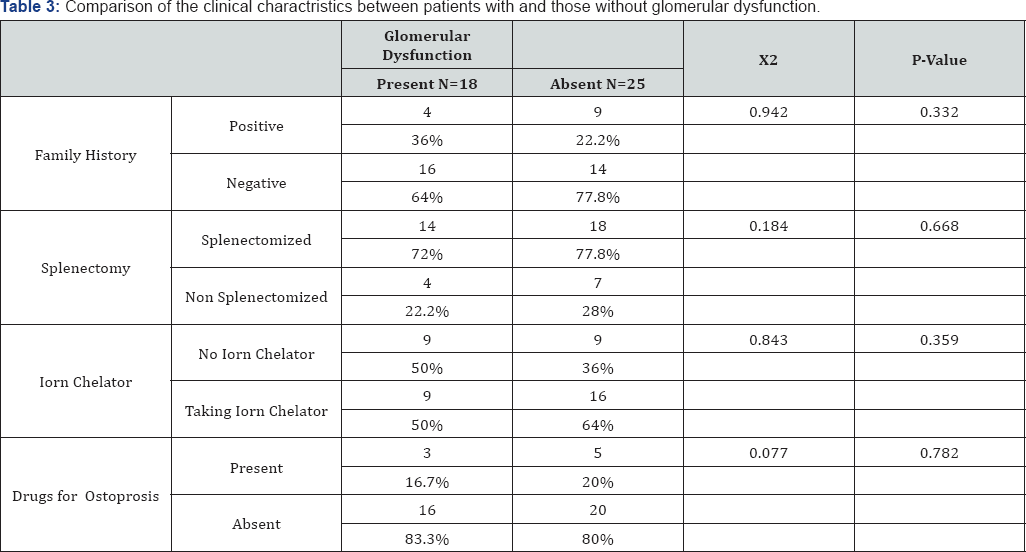
Results: Cases with HCV infection or diabetes were excluded from our analysis for possible risk factors for glomerular dysfunction, In the 43 patients with β-thalassemia major there were 18 patients with glomerular dysfunction (41.9%). There were significant differences between patients with and patients without glomerular dysfunction as regards age, urine albumin to creatinine ratio, serum Cyst-C, Serum creatinine, estimated GFRcys, hemoglobin and insignificant differences as regads sex, weight, height, BMI, splenectomy, using iron chelators or drugs for osteoporosis, blood requirement (ml/Kg/year) or ferritin level more than 1000.
Conclusion: our study revealed presence of glomerular dysfunctions in about (41.9%) of adult β-TM patients, age and pretransfusion hemoglobin level are predictors for glomerular dysfunctions. CKD-EPI-CysC equation for eGFR can detect early renal damage in these patients.
Keywords: β-thalassemia major; CKD-EPI-CysC; Glomerular dysfunctions; SCys-C: Serum Cystatin-C
Abbreviations: ACR: Albumin/Creatinine Ratio; eGFR: estimated Glomerular Filtration Rate; Ccr: Creatinine Clearance
Introduction
β-thalassemia syndromes are the most common inherited hemoglobinopathies in the world. Which is caused by an autosomal recessive genetic deficiency in the β-globin chain that leads to accumulation of unpaired a-globin chains [1].
β-thalassemia is a common hematologic disorder in the Mediterranean basin, parts of africa, the middle east, the Indian subcontinent, the Southern far east, and Southeast Asia; these areas make up the so-called thalassemia belt [2].
In Egypt It has been estimated that 1000 children out of 1.5 million live births are born annually with thalassemia major, the carrier rate in Egypt has been reported to be in the range of 9%-10% [3]. Aggregated a-chains damage the cell membrane and the membranes of intracellular organelles also it trigger the formation of reactive oxygen species, which further damage the protein and lipid constituents of cell membranes [4]. 3 main factors are accused in the pathophysiology of β-thalassemia patients: ineffective erythropoiesis, chronic anemia and hemolysis, and primary iron overload [5].
Ineffective erythropoiesis is the primary cause of anemia and is responsible for excessive intestinal iron absorption (primary iron overload) as well as the formation of extra medullary hematopoietic pseudotumors and osteoporosis [6].
Chronic anemia may be responsible for congestive cardiomyopathy, hepatosplenomegaly and growth and development retardation, hemolysis may lead to pulmonary hypertension and thromboembolic disease [7].
Transfusional iron overload leads to hepatic, endocrine and cardiac failure, and premature death [8]. Little attention has been paid to the possible kidney damage in patients with β-thalassemia, but recent studies outlined the presence of different tubular and glomerular dysfunction [5].
The aim of the present study was to investigate the presence of glomerular dysfunctions in adults with β-TM, using the early biomarkers for detection of glomerular dysfunctions.
Subjects and Methods
This is across-sectional study, it was carried out on 50 patients with β-TM; they were randomly selected from in and outpatients of the hematology unit, internal medicine department, faculty of medicine, menofia university from the period of August 2016 till February 2017. This study was conducted in agreement with the guidelines of the declaration of Helsinki, 1975 and its subsequent amendments (1983) also it was approved by the local ethics committee.
The studied patients with β-TM had in their history the following criteria at the time of initial diagnosis (age at first presentation was less than 2 years old with a mean hemoglobin level of 6-7g/dl, HbF >50%, and HbA 2<4% [9]. Patients with other hemolytic anemia, patients with other hemoglobinopathies, patients with systemic illness (heart failure, hepatic diseases, or diabetes mellitus) and patients with clinical or laboratory evidence of other causes of renal diseases were excluded from this study. No urinary tract infection or obstructive uropathy was noted at time of urine sampling. All patients were on regular blood transfusion.
Renal glomerular damage was considered if there is albuminuria. Albumin to creatinine ratio in early morning single spot urine sample greater than 300mg/g creatinine was considered albuminuria [10]. The estimated glomerular filtration rate (eGFR) was calculated using the 2012 CKD- EPI cystatin C equation: 133_min (SCysC/0.8, 1)_0.499_max (SCysC/0.8, 1)_1.328_0.996 Age[_0.932 if female], where SCysC is serum cystatin C (in mg/l), min indicates the minimum of SCysC/0.8 or 1 and max indicates the maximum of SCysC/0.8 or 1 [11].
Renal affection in this study was considered if eGFR is less than 60ml/min/1.73m2 and/or if the urinary ACR was greater than 300mg/g Cr. An eGFR creat of ≥;150mL/min/1.73m2 and ≥120mL/min/1.73m2 were considered as hyper filtration for <30 years old and older people, respectively [12]. Presence of ACR>300 mg/g creatinine and/or decreased eGFRcys were considered as glomerular dysfunction.
Patients included in our study were subjected to full medical history taking and complete clinical examination including age, sex, family history of β-thalassemia, weight, height, disease duration, first time of blood transfusion, number of blood transfusions/year, history of splenectomy, post-splenectomy duration and type, duration of chelation therapy, other comorbidities and drug history were obtained.
Patients were advised to fast overnight and to abstain from taking any medications (including chelation) in the previous 24h. The blood samples from patients were collected immediately before blood transfusion, about 10ml of venous blood after fasting and fresh second-morning midstream urine samples were collected from all the participants.
Blood samples were collected on EDTA (1.2mg/ml) for complete blood count using SysmexXT-1800i (Sysmex, Hyogo, Japan). And serum ferritin level was done using Cobas Integra 800 (Roche Diagnostics, Mannheim, Germany). Then serum samples were immediately divided into aliquots and frozen at -70 °C till serum cystatin-C levels were measured using the quantikine human cystatin-C immuno sorbent assay (ELISA) kit (Cat. No.: RD191009100; R&D Systems Inc., Minneapol is, Minnesota, USA), then the samples were incubated in microtitrate plate wells which was precoated with polyclonal anti-human cystatin-C antibody, and polyclonal anti-human cystatin-C antibody, conjugated with horseradish peroxidase is added to the wells after 30min of incubation and washing and incubated for 30min with the captured cystatin-C. The second washing step was also done, then the remaining horseradish peroxidase conjugate is left to react with the substrate solution (TMB).
Stopping of the reaction was done by addition of an acidic solution and absorbance of a resulting yellow product is measured .The absorbance is proportional to the concentration of cystatin-C. Construction of a standard curve is done by plotting absorbance values against concentrations of cystatin-C standards and concentrations of unknown samples are determined using this standard curve.
Urine was collected for the immediate assessment of urinary creatinine (creatinine-Jaff e enzymatic assay) and urinary albumin colorimetrically (Egypt Company for Biotechnology, Cairo, Egypt). Our collected data were tabulated and analyzed using SPSS version 17 software (S PSS Inc., Chicago, Illinois, USA). The categorical data were presented as numbers and percentages but quantitative data were expressed as means and SDs. Comparison of the continuous data between two groups was made by using the unpaired t-test for parametric data and the Mann-Whitney test for non-parametric data. Fisher's exact was used for comparison between categorical data. The Spearman test was used for correlations between different nonparametric parameters. The accepted level of significance in this work was stated at 0.05 (P<0.05 was considered significant).
Results
Our study included (50) patients with P-thalassemia major (26 females and 24 males) their age range from 18 to 50 years, the mean weight of patients was 57.5kg (range 35.0-80.0) and 2 patients one male and a female patient were below the third percentile for normal weight, the mean height is 158.8cm (range 140-170.0) and 4 male patients were below the third percentile for normal height, the mean BMI is 23.8 (range 20.2-27.1). 15 patients (30.0%) have family history of thalassemia, 38 patients (76.0%) had done splenectomy, 31 patients (62.0%) receive iron chelators of them 21 patients (42.0%) are on deferoxamine, 7 patients (14.0%) are on deferasirox and 3 patients (6.0%) are on deferiprone, there were 3 patients (6.0%) have diabetes melitus,9 patients (18.0%) have osteoprosis, 5 patients (10.0%) have HCV, 1 (2.0%) patient has cardiomyopathy, 1 (2.0%) patient has pulmonary embolism, 1 (2.0%) patient had underwent total hib replacement, the mean volume of transfused blood is 76.760ml per Kg per year. The mean serum Cyst-C (mg/dL) of patients was 0.91±0.34 range (0.23-2.25), the mean eGFR- cystatin was 0.91±0.34 range (30-210), the mean hemoglobin (mg/dL) was 7.4±8.5 and 43 patients (86.0%) have sereum ferritine level more than 1000.
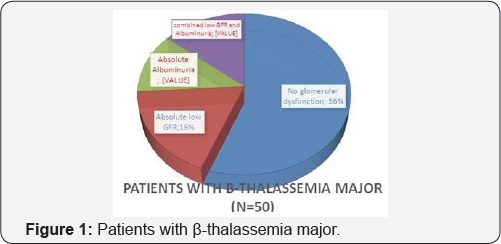
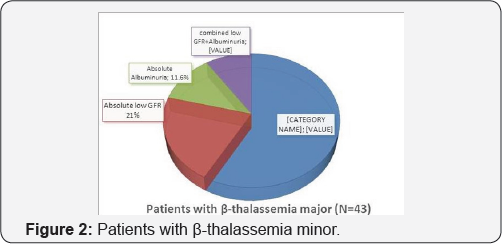
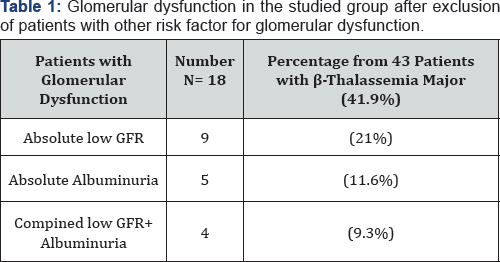
In the 50 Patients with β-thalassemia major there were 22 patients with glomerular dysfunction (44%) of them 9 patients (18%) had low eGFR cyst, 6 patients (12%) had albuminuria and 7 patients (14%) had combined low GFR and albuminuria. But, the number of cases further reduced to 43 cases as cases with HCV infection or diabetes were excluded from our analysis for possible risk factors for glomerular dysfunction. In the 43 Patients with β-thalassemia major there were 18 patients with glomerular dysfunction (41.9%) of them 9 patients (21%) had low eGFR cyst, 5 patients (11.6%) had albuminuria and 4 patients (9.3%) had combined low GFR and albuminuria (Figure 1 & 2) (Table 1).
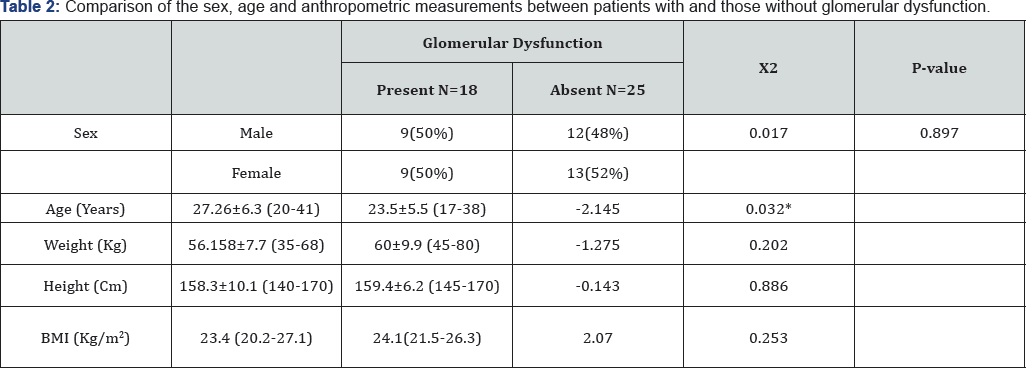
Our results showed significant differences between patients with and patients without glomerular dysfunction as regards age and insignificant differences as regards sex, weight, height, BMI, splenectomy, using iron chelators or drugs for osteoporosis (Table 2).
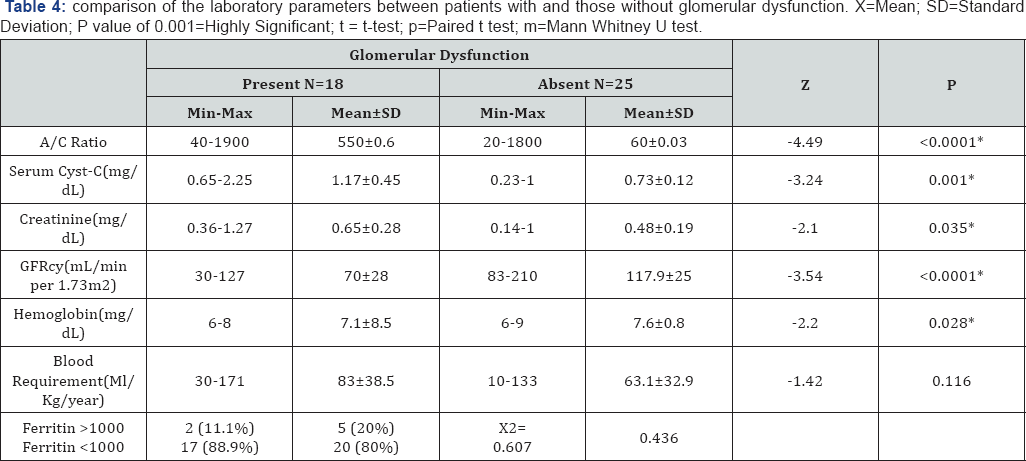
Our results showed significant differences between patients with and patients without glomerular dysfunction as regards urine albumin to creatinine ratio, serum Cyst-C, Serum creatinine, estimated GFRcys, hemoglobin and insignificant differences as regards blood requirement (ml/Kg/year) or ferritin level more than 1000 (Table 3 & 4).
There were significant negative correlation between eGFR cys and patients age, albuminuria and blood requirement, There were significant positive correlation between eGFR cys and patients weight and hemoglobin level. There were no significant correlation between eGFR cys and patients height or BMI.
Discussion
TM-associated nephropathy has recently been a growing matter of concern because renal failure starts to affect most aging TM patients. Renal injury once begins, the pattern of deterioration is insidious but progressive at the same time. Early awareness and intervention would probably decrease the morbidity and mortality [13].
The pathophysiology of renal injury in TM patients seems to be multi-factorial, attributed to various factors such as chronic anemia, oxidative stress due to ongoing hemolysis, excessive free iron, renal iron deposition and chelating agents [5].
Chronic anemia is thought to cause a decrease in systemic vascular resistance and subsequent increase in renal plasma flow which might play a role in glomerular hyper filtration, triggering the renal injury [14]. Regarding pathophysiology, glomerular hyper filtration, if untreated, can lead to stretching of the glomerular capillary wall due to increase in glomerular pressure, resulting in endothelial and epithelial injury together with transudation of macromolecules into mesangium [13].
Renin-angiotensin-aldosteron system seems to contribute to the intra renal pressure and this might preceed glomerulosclerosis and progressive tubulo-interstitial injury [15]. It is well-known that serum creatinine is alone an unreliable indicator of renal functions because muscle mass, protein intake and hepatic disease affects its serum levels. Cys-C is an endogenous, nonglycosylated basic protease inhibitor which has been an ideal marker of renal functions. Its production is not affected by age, gender or body mass index and correlates better than creatinine with measured GFR [13].
Besides among patients with hyper filtration, 31 (64.5%) had elevated levels of Cys-C. That's why elevated Cys-C levels should be thought as an early sign of renal injury in TM patients like in patients with diabetes mellitus type 1 [16].
CKD-EPI-CysC equation for eGFR detects more TM patients with renal impairment compared to equations that include serum creatinine. CKD-EPI-CysC equation for eGFR should be used in thalassemic patients as this equation can detect earlier renal damage [17].
The results of our study showed that 22 (44%) patients had glomerular dysfunction (eGFR <60ml/min/1.73m2 and/ or the urinary ACR was >300mg/g Cr), after exclusion of cases with HCV infection or diabetes from our analysis for possible risk factors for glomerular dysfunction there were 18 patients with Glomerular dysfunction (41.9%) of them 9 patients (21%) had low eGFR cyst, 5 patients (11.6%) had albuminuria and 4 patients (9.3%) had combined low GFR and albuminuria.
Our results contrast with the findings of studies that used eGFR and reported hyper filtration as Ponticelli et al. [18], Sumboonnanonda et al. [19], Koliakos et al. [20] Quinn et al. [21]. Our findings suggest that a larger population of patients with TM may ultimately develop CKD following their prolonged longevity. And this is in accordance with Milo et al. [22]. Which determine GFR by using insulin clearance and compare it with measured creatinine clearance (Ccr) and eGFR and cocluded that the estimating methods for determination of GFR are not clinically reliable; thus, the use of a more accurate method to assess GFR is warranted. Such a method may enable early detection of GFR decline and prevention or attenuation of its deterioration to CKD and the need for RRT. An important practical issue relates to the treatment of patients with TM with iron chelation drugs, antibiotics, cytotoxic drugs and other medications that need adjustment according to GFR because the use of estimated methods for GFR may cause erroneous dosing.
In accordance with our results, Hamed et al. [23] who found that impaired renal functions (eGFR <90ml/min/1.73m2) was reported in 47.83%. Also, Mohkam et al. [24], Hamed et al. [23] and Dimitriadou et al. [25], found proteinuria or abnormal ACR in 89.3, 82.61, and 68% of β-TM patients respectively. Elbedewy et al. found that 12 (30%) patients had renal affection (eGFR <90ml/min/1.73m2 and/or the urinary ACR was >30mg/gCr) [1].
Albuminuria was attributed mainly to the destruction of the glomerular filtration membrane which could be due to massive iron deposition in the tissues, resulting in an increase of free radical production, leading to apoptosis [26]. In addition, albuminuria could result from prolonged hyper filtration, prostaglandin secretion and chronic anemia [27]. Elbedewy et al. showed significantly higher levels of the urinary ACR in thalassemic patients when compared with the control group [1]. These results are in agreement with Aldudak et al. [28], Hamed et al. [23] and Ali and Mahmoud [29], who found statistically significant higher levels of the urinary ACR in the thalassemic group when compared with the control group.
Our results show that mean Serum Cyst-C (mg/dL) of patients was 0.91±0.34 range (0.23-2.25) and this is in accordance with Elbedewy et al. [1], that found mean Serum Cyst-C (mg/dL) of thalassemia patients was 0.99±0.2435 and mean Serum Cyst-C (mg/dL) of control group was 0.68±0.07618. Cystatin-C is a cysteine protease inhibitor that is synthesized from all human cells and secreted into the blood. The advantage of cystatin-C measurement in comparison with creatinine clearance is that it is not affected by height, sex, diet and muscle mass [30].
Serum cystatin-C is believed to be a more robust endogenous marker of GFR than creatinine as it is thought to be produced at a constant rate by all nucleated cells, freely filtered by the glomeruli, minimally bound to proteins, and totally reabsorbed and metabolized in the proximal tubule [31].
The cystatin-C molecule is more than 100 times larger than creatinine. In theory, narrowing of the glomerular filter could impair filtration of cystatin-C but still allow free passage of creatinine. This has led researchers to investigate whether an increase in plasma cystatin-C precedes the conventional creatinine [32].
Our results showed that age and pre transfusion hemoglobin level are independent predictors of glomerular dysfunction. The effect of age on glomerular function was studied by Eliana Lai et al. [33], this retrospective study evaluated the changes in estimated glomerular filtration rate (eGFR) in 81 adult patients with TM followed for 10 years. Only patients who had an eGFR of >90mL/min/1.73m2 at presentation were admitted to the study. All patients were regularly followed for at least 10 years. At 10 years, 15 patients (18.5%), eGFR decreased to <90mL/ min (from 98.1 to 78.2mL/min/1.73m2; P=0.004), patients with elevated phosphaturia, elevated uricuria and/or abnormal levels of calcaemia show a significant decline in eGFR over time, suggesting that tubular damage acquired in childhood caused by either TM or its treatment may eventually result in abnormal eGFR.
Eliana Lai et al. [33], concluded that large variations in haemoglobin levels were also associated with an increased risk of decline in eGFR, showing the importance of maintaining pre- transfusional levels of haemoglobin between 9.5 and 10.5g/ dL. Iron overload and anaemia are likely to be the main factors responsible for these tubular abnormalities.
Conclusion
Our study revealed presence of glomerular dysfunctions in about (41.9%) of adult β-TM patients, age and pre-transfusion hemoglobin level are predictors for glomerular dysfunctions. CKD-EPI-CysC equation for eGFR can detect early renal damage in these patients.
References
- Elbedewy T, Gawaly A, Abd El-Naby A (2015) Serum cystatin-C and urinary N-acetyl-p-D-glucosaminidase as biomarkers for early renal dysfunction in adult Egyptian patients with β-thalassemia major. Tanta Medical Journal 43(1): 31-38.
- Elmezayen A, Kotb S, Sadek N, Abdalla E (2015) p-Globin Mutations in Egyptian Patients With β-thalassemia. Lab Med 46(1): 4-13.
- El-Shanshory M, Hagag A, Shebl S , Badria I, Abd Elhameed A, et al. (2014) Spectrum of Beta globin gene mutations in Egyptian children with β-thalassemia. Mediterr J Hematol Infect Dis 6(1): e2014071.
- Nienhuis A, Nathan D (2012) Pathophysiology and Clinical Manifestations of the p-halassemias. Cold Spring Harb Perspect Med 2: a011726.
- Mallat N, Mallat S, Musallam K, Taher A (2013) Potential mechanisms for renal damage in beta-thalassemia. JNEPHROL 26(5): 821-828.
- Cappellini MD, Cohen A, Eleftheriou A, Piga A, Porter J, et al. (2008) Guidelines for the clinical management of thalassaemia. Thalassaemia International Federation Conference.
- Musallam KM, Taher AT (2011) Thrombosis in thalassemia: why are we so concerned? Hemoglobin 35(5-6): 503-510.
- Brittenham GM (2011) Iron-chelating therapy for transfusional iron overload. N Engl J Med 364(2): 146-156.
- Hoffman R, Benz EJ, Shattil SJ, Furie B, Silberstein LE, et al. (2008) Basic principles and practice. Churchill Livingstone, Philadephia, pp. 535-564.
- Alvarez O, Montane B, Lopez G, Wilkinson J, Miller T (2006) Early blood transfusions protect against microalbuminuria in children with sickle cell disease. Pediatr Blood Cancer 47(1): 71-76.
- Inker LA, Schmid CH, Tighiouart H, et al. (2012) Estimating glomerular filtration rate from serum creatinine and cystatin C. N Engl J Med 367: 20-29.
- Palatini P (2013) Glomerular hyperfiltration: a marker of early renal damage in pre-diabetes and pre-hypertension. Nephrol Dial Transplant 27(5): 1708-1714.
- Gokce M, Kup H, Tugcu D, Salcioglu Z, Aydogan G, et al. (2014) Insidious Renal Damage in Patients with Thalassemia Major: Is it More Serious than Appreciated? J Hematol Thrombo Dis 2: 173.
- Hostetter TH (2003) Hyperfiltration and glomerulosclerosis. Semin Nephrol 23(2): 194-199.
- Davis LE, Hohimer AR (1991) Hemodynamics and organ blood flow in fetal sheep subjected to chronic anemia. Am J Physiol 261(6 Pt 2): R1542-1548.
- Papassotiriou I, Margeli A, Hantzi E, Delaporta P, Sergounioti A, et al. (2010) Cystatin C levels in patients with beta-thalassemia during deferasirox treatment. Blood Cells Mol Dis 44: 152-155.
- Voskaridou E, Christoulas D, Papatheodorou A, Oikonomopoulos P, Komninaka V, Repa K, et al. (2013) The CKD-EPI-Cystatin c equation for the estimation of glomerular filtration rate detects higher number of patients with thalassemia major and renal impairment, before and after treatment with deferasirox; is it time to change from MDRD To CKD-EPI-Cystatin C equation in thalassemia? Blood 122: 3451.
- Ponticelli C, Musallam KM, Cianciulli P, Cappellini MD (2010) Renal complications in transfusion-dependent beta thalassaemia. Blood Rev 24(6): 239-244.
- Sumboonnanonda A, Malasit P, Tanphaichitr VS, Ong-ajyooth S, PetraratS, et al. (2003) Renal tubular dysfunction in alpha thalassemia. Pediatr Nephrol 18(3): 257-260.
- Koliakos G, Papachristou F, Koussi A, Perifanis V, Tsatra I, et al. (2003) Urine biochemical markers of early renal dysfunction are associated with iron overload in beta-thalassaemia. Clin Lab Haematol 25(2): 105-109.
- Quinn CT, Johnson VL, Kim HY, Trachtenberg F, Vogiatzi MG, et al.(2011) Thalassemia Clinical Research Network: Renal dysfunction in patients with thalassaemia. Br J Haematol 153(1): 111-117.
- Milo G, Feige R, Pazgal I, Gafter A, Shpilberg O, et al. (2015) GFR in Patients with β-thalassemia Major. Clin J Am Soc Nephrol 10(8): 13501356.
- Hamed EA, ElMelegy NT (2010) Renal functions in pediatric patients with beta-thalassemia major: relation to chelation therapy: original prospective study. Ital J Pediatr 36: 39-48.
- Mohkam M, Shamsian BS, Gharib A, Nariman S, Arzanian MT (2008) Early markers of renal dysfunction in patients with beta-thalassemia major. Pediatr Nephrol 23(6): 971-976.
- Dimitriadou M, Christoforidis A, Economou M, Teli A, Printza N, et al. (2011) Fok-I polymorphism of vitamin D receptor gene and the presence of renal dysfunction in patients with β-thalassemia major. Pediatr Hematol Oncol 28(6): 509-516.
- Link G, Athias P, Grynberg A, Pinson A, Hershko C (1989) Effect of iron loading on transmembrane potential, contraction, and automaticity of rat ventricular muscle cells in culture. J Lab Clin Med 113(1): 103-111.
- Koren G, Kochavi-Atiya Y, Bentur Y, Olivieri NF (1991) The effects of subcutaneous deferoxamine administration on renal function in thalassemia major. Int J Hematol 54(5): 371-375.
- Aldudak B, Karabay Bayazit A, Noyan A, Ozel A, Anarat A, et al. (2000) Renal function in pediatric patients with beta-thalassemia major. Pediatr Nephrol 15(1-2): 109-112.
- Ali BA, Mahmoud AM (2014) Frequency of glomerular dysfunction in children with beta thalassaemia major. Sultan Qaboos Univ Med J 14(1): e88-94.
- Randers E, Erlandsen EJ (1999) Serum cystatin C as an endogenous marker of the renal function - a review. Clin Chem Lab Med 37(4): 389395.
- Tenstad O, Roald AB, Grubb A, Aukland K (1996) Renal handling of radiolabelled human cystatin C in the rat. Scand J Clin Lab Invest 56(5): 409-414.
- Herget-Rosenthal S, Marggraf G, Husing J, Goring F, Pietruck F, et al. (2004) Early detection of acute renal failure by serum cystatin C. Kidney Int 66(3): 1115-1122.
- Eliana Lai M, Spiga A, Vacquer S, Paola Carta M, Corrias C, Ponticelli C (2012) Renal function in patients with p-thalassaemia major: a longterm follow-up study. Nephrol Dial Transplant 27(9): 3547-355.






























