Efficacy and Challenges of Bortezomib for Adult Refractory TTP-A Case Report
Ryan Low1* and Tina Dutt 2
1Department Postgraduate, Central Manchester University Hospitals NHS Foundation Trust, UK
2Roald Dahl Centre for Thrombosis and Haemostasis, Royal Liverpool and Broadgreen University Hospitals, UK
Submission: June 26, 2017; Published: July 31, 2017
*Corresponding author: Ryan Low, Department Postgraduate, Central Manchester University Hospitals NHS Foundation Trust, Oxford Road, Manchester, UK, Tel: 07398164687, Email: ryan.low@doctors.org.uk
How to cite this article: Ryan L, Tina D. Efficacy and Challenges of Bortezomib for Adult Refractory TTP-A Case Report. Open Acc Blood Res Transfus J. 2017; 1(3) : 555563.
Abstract
Thrombotic Thrombocytopenic Purpura (TTP) is a rare but life threatening condition. Therapeutic plasma exchange (PLEX) remains the mainstay treatment - adjuvant immunosuppressant therapy with rituximab is also required in some patients despite this some patient remain refractory. Bortezomib has been suggested through case reports as an adjuvant therapy for refractory TTP. However, challenges exist regarding its use.
We report the case of an acute acquired TTP that did not respond to PLEX or rituximab however appeared to rapidly respond to the proteasome inhibitor bortezomib. The case is of a 59 year old gentleman with acute TTP who was commenced on once daily PLEX with an inadequate response. Twice daily PLEX and prescribed four doses of rituximab were initiated. Again only a partial response was observed, a course of bortezomib was trialed alongside the twice daily PLEX.A relatively quick recovery in platelet count was observed, and although difficult to interpret, the ADAMTS13 inhibitor titre and ADAMTS13 activity normalized.
This case supports the use of bortezomib as an adjuvant therapy in the management of acute TTP where PLEX and rituximab have been ineffective. However it also highlights the limitations of monitoring disease activity and response using ADAMTS 13 assays during twice daily PEX, and this should be considered when interpreting response to bortezomib. Thrombotic thrombocytopenia pupura (TTP) is a rare but life threatening condition. The majority of cases are due to inhibitory auto antibodies against ADAMTS 13, an enzyme required for the cleavage of ultra-large VWF multimers. Therapeutic daily plasma exchange (PEX) has remained the mainstay treatment for TTP since the 1920s reducing the overall mortality from around 90% to less than 20% [1].
A small proportion of patients however do not respond to daily plasma exchange alone. Refractory disease has been defined as the progression of clinical symptoms or persistent thrombocytopenia despite PEX (reference-BCSH). Intensification of PEX frequency or volume with the addition of corticosteroids and/or adjuvant immunosuppressant therapy may provide benefit [2,3]. Rituximab the anti-CD20 monoclonal antibody has shown to improve time to remission and is currently an agent of choice for refractory disease [4]. However, there remain a small number of patients who do not respond, and more recently Bortezomib, a proteasome inhibitor more widely used in the treatment for myeloma, has been used in a small number of refractory cases of TTP. The drug appears to be a safe and effective treatment for a subgroup of refractory patients [5].
A clinical dilemma remains regarding at which stage to initiate such therapies. Furthermore, the most representative parameter for monitoring response for patients receiving multiple therapies in addition to intensive plasma exchange remains unclear and often presents a challenge when assessing clinical response. Here we present a case of acute refractory TTP that responded to Bortezomib, but highlights the challenges in monitoring to assess disease remission.
Keywords: Thrombotic thrombocytopenic pupura; Bortezomib; Plasma exchange; Rituximab; ADAMTS 13
Abbreviations: PEX: Plasma Exchange; TTP: Thrombotic Thrombocytopenic Purpura
Case Report
A 59 year old Caucasian gentleman presented to his General Practitioner with a one week history of vomiting, dizziness and lethargy. He was initially diagnosed with labyrinthitis, however his symptoms worsened and he attended the local Emergency Department the following day. On initial examination he was found to be afebrile and jaundiced with wide-spread bruising. Admission bloods showed Hb 137g/L, MCV 93.4fl, Platelets 6.0 x 109/L, Urea 13.5mmol/L, Creatinine 118mmol/L, normal coagulation screen and LDH 4000U/L. The blood film showed multiple schistocytes, polychromasia and confirmed a true thrombocytopenia.
The patient developed confusion overnight and TTP was suspected. The patient was transferred urgently to the regional TTP Specialist Centre where he required restraint and intubation due to severe agitation. Plasma exchanges 1.5 volumes once daily was commenced with 1g methyl prednisolone once daily for three days. ADAMTS 13 was confirmed to be less than 5% with an inhibitor level of 71U/ml confirming a diagnosis of acute immune mediated TTP.
Overnight the patient suffered two cardiac arrests and demonstrated progressive renal impairment despite plasma exchange. On day 2 plasma exchange was escalated to twice daily and on day 7 Rituximab was commenced 375mg/m2 every fourth day. Multiple attempts to extubate were complicated by ongoing severe confusion and agitation. Following an initial rise in platelet count to 151 x 109/L this subsequently fell to 22 x 109/L.
On day 25 a bone marrow biopsy was performed to exclude other causes for the thrombocytopenia. This was found to be morphologically normal. Flow cytometry confirmed B-cell depletion (4%) with the presence of plasma cells (1.2%).
Bortezomib was initiated at a dose of 1mg/m2 given subcutaneously every 3 days (day 22, 26 and 29). The patient received a total of 3 doses with a prompt response in the platelet count after the first treatment followed by a progressive gradual rise to normal range by day 52. Twice daily plasma exchange was continued until day 44. Pre-exchange samples of ADAMTS 13 activity and antibody were measured along with platelet count, LDH and Hb (Figure 1).
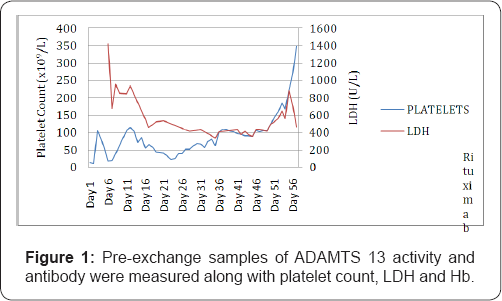
The patient was extubated on day 36, steroid and plasma exchange was discontinued on day 47. He was discharged on day 57 with a normal full blood count and ADAMTS 13 activity. Following discharge the patient was registered partially blind and with a high frequency hearing loss as a consequence of multiple infarcts sustained during the acute illness, confirmed by MRI imaging.
Discussion
This case report supports the potential role of Bortezomib in achieving clinical remission in patients with acute refractory TTP. The use of Bortezomib for TTP was first reported by Shortt et al in 2013 for a 53 year old lady, who appeared refractory to bi-daily plasma exchange, corticosteroids, cyclophosphamide, Rituximab and N-acetyl cysteine [6]. In the same year the drug was used in an adolescent with acquired refractory TTP demonstrating clinical deterioration despite Rituximab and daily plasma exchange. In both of these cases Bortezomib was commenced after 2 out of the 4 total doses of Rituximab due to no observed improvement in hematological indices or clinical status [7]. A further two case reports have been published since reporting it use in acute TTP [8,9].
The effectiveness of Bortezomib is thought to lie in the mode of action. Bortezomib is a proteasome inhibitor that works via the ubiquitin-proteasomal pathway and results in apoptosis of malignant cells [10]. It has also been found to cause apoptosis of plasma cells and both naive and memory B cells leading to a substantial reduction in autoantibody formation [11]. Through the destruction of auto-reactive B cells and plasma cells there may also be a reduction in ADAMTS 13 auto antibodies. Alternative mechanisms of action have been suggested to explain the role of Bortezomib in TTP. Sorvillo et al. [12] demonstrated that ADAMTS 13 is endocytosed by immature dendritic cells and presented to CD4+ T cells on major histocompatibility complex class II molecules. Alongside evidence that Bortezomib induces apoptotic cell death substantially more in immature dendritic cells compared with mature, this led to the conclusion that the drug may prevent endocytosis of ADAMTS 13 into dendritic cells, hence reducing the production of auto antibodies [12-14].
The exclusive value of Bortezomib has been questioned in patients receiving Rituximab and other concomitant therapies due to the possibility of a delayed/ cumulative effective of the other drugs. Mazepa et al. [8] reported the case of a patient treated without concomitant Rituximab where Bortezomib was found to be beneficial in achieving a plasma exchange independent remission versus achieving a normal platelet count [8]. A similar case has been described by Yates et al [9].
Our patient had received all of the 4 prescribed doses of Rituximab prior to receiving Bortezomib. Possible reasons for the presumed inefficacy for Rituximab in some patients may include increased drug clearance due to intensified plasma exchange regimens and a therefore greater time requirement to achieve therapeutic drug concentrations. McDaonald et al. [15] reported that in patients on once daily PEX the plasma Rituximab levels are undetectable prior to their subsequent PEX [15].
Flow cytometry has been used to study the effective B cell depletion which in both this and the case reported suggested by Shortt et al suggested effective depletion post Rituximab. There is a lack of data around the clearance and drug concentration levels of Bortezomib during plasma exchange however as the drug is given subcutaneously one could postulate that the degree of clearance maybe less.
The exclusive contribution of an intensified PEX regimen has been difficult to quantify as other treatments are often initiated or intensified simultaneously [2]. In particular the reliability of using ADAMTS 13 to monitor response in this setting is questionable. It is intuitive that as drug clearance is likely to be enhanced, interpretation of ADAMTS 13 and autoantibody due to increased cycling is also likely to be affected. The case reported by Shortt et al, also receiving Bortezomib in conjunction with bi-daily PEX, demonstrated decline of the autoantibody and an ADAMTS 13 coinciding with recovery whilst on the exchange programme. In our case, however, monitoring was found to be unhelpful and an unreliable representation of disease activity whilst on bi-daily PEX due to the effect of administered plasma on blood samples taken.
In addition to a limited number of reports, this case supports the evidence regarding the role of Bortezomib as an effective adjuvant therapy in the management of refractory TTP. For complex, refractory TTP, the limitations of monitoring disease activity and response using ADAMTS 13 assays during twice daily PEX should be taken in to consideration when assessing clinical recovery. Further understanding of the potential efficacy of this drug through prospective trials may offer a critical choice for patients faced with this challenging condition.
References
- Lian ECY (2005) Pathogenesis of thrombotic thrombocytopenic purpura: ADAMTS13 deficiency and beyond. Semin Thromb Hemost 31(6): 625-632.
- Nguyen TC, Han YY (2011) Plasma exchange therapy for thrombotic microangiopathies. Organogenesis 7(1): 28-31.
- Bandarenko N, Brecher ME (1998) United states thrombotic thrombocytopenic purpura apheresis study group (US TTP ASG): Multicenter survey and retrospective analysis of current efficacy of therapeutic plasma exchange. J Clin Apher 13(3): 133-141.
- Scully M, Cohen H, Cavenagh J, Benjamin S, Starke R (2007) Remission in acute refractory and relapsing thrombotic thrombocytopenic purpura following rituximab is associated with a reduction in IgG antibodies to ADAMTS-13. Br J Haematol 136(3): 451-461.
- Patriquin CJ, Thomas MR, Dutt T, McGuckin S, Blombery PA (2016) Bortezomib in the treatment of refractory thrombotic thrombocytopenic purpura. Br J Haematol 173(5): 779-785.
- Shortt J, Oh DH, Opat SS (2013) ADAMTS13 Antibody Depletion by Bortezomib in Thrombotic Thrombocytopenic Purpura. New England Journal of Medicine 368(1): 90-92.
- van Balen T, Schreuder MF, de Jong H, van de Kar NC (2014) Refractory thrombotic thrombocytopenic purpura in a 16-year-old girl: Successful treatment with bortezomib. Eur J Haematol 92(1): 80-82.
- Mazepa MA, Raval JS, Stephan M, Alice M, Park YA (2014) Bortezomib induces clinical remission and reduction of ADAMTS13 inhibitory antibodies in relapsed refractory idiopathic thrombotic thrombocytopenic purpura. British Journal of Haematology 164(6): 900-902.
- Yates S, Matevosyan K, Rutherford C, Shen YM, Sarode R (2014). Bortezomib for chronic relapsing thrombotic thrombocytopenic purpura: a case report. Transfusion 54(8): 2064-2067.
- Kouroukis TC, Baldassarre FG, Haynes AE, Imrie K, Reece DE, et al. (2014) Bortezomib in multiple myeloma: systematic review and clinical considerations. Curr Oncol 21(4): e573-e603.
- Mulder A, Heidt S, Vergunst M, Roelen DL, Claas FH (2013) Proteasome inhibition profoundly affects activated human B cells. Transplantation 95(11): 1331-1337.
- Sorvillo N, Pos W, van den Berg LM, Fijnheer R, Martinez-Pomares L (2012) The macrophage mannose receptor promotes uptake of ADAMTS13 by dendritic cells. Blood 119(16): 3828-3835.
- Subklewe M, Sebelin-Wulf K, Beier C, Lietz A, Mathas S, et al. (2007) Dendritic Cell Maturation Stage Determines Susceptibility to the Proteasome Inhibitor Bortezomib. Hum Immunol 68(3): 147-155.
- Park SJ, Cheong HIl, Shin JIl (2013) Antibody depletion by bortezomib through blocking of antigen presentation. New England Journal of Medicine, 368(14): 1364-1365.
- McDonald V, Manns K, Mackie IJ, Machin SJ, Scully MA (2010) Rituximab pharmacokinetics during the management of acute idiopathic thrombotic thrombocytopenic purpura. J Thromb Haemost8(6): 1201-1208.






























