- Proceeding
- Introduction
- Pathophysiology of Thrombosis
- Overview of the Coagulation Cascade
- Predictors of Thromboembolic Manifestations
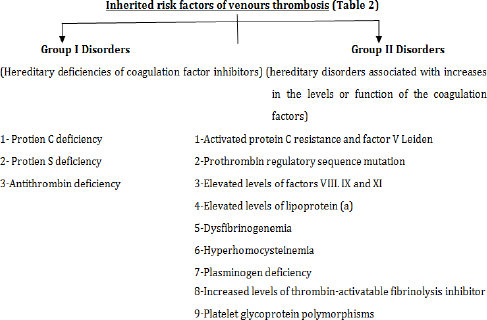
- Inherited Risk Factors Of Venous Thrombosis
- Hereditary Deficiencies of Coagulation Factor Inhibitors
- Hereditary Disorders Associated with Increases in the Levels or Function Of the Coagulation Factors
- The Role of the Platelets
- Laboratory Investigation of Thrombosis (Figure 14)
- Laboratory Investigations of Thombophelic Parameters
- Anti-coagulation Therapy
Diagnostic Approach of Thrombophilia in Critical Situations
Eman Abd El-Hay Mashhour*
Tanta University, Egypt
Submission: June 24, 2017; Published: July 28, 2017
*Corresponding author: Eman Abd El-Hay Mashhour, Tanta University, Egypt, Email: hodaaboufreikha@aucegypt.edu
How to cite this article: Eman A E-H M. Diagnostic Approach of Thrombophilia in Critical Situations. Open Acc Blood Res Transfus J. 2017; 1(3) : 555562.
- Proceeding
- Introduction
- Pathophysiology of Thrombosis
- Overview of the Coagulation Cascade
- Predictors of Thromboembolic Manifestations
- Inherited Risk Factors Of Venous Thrombosis
- Hereditary Deficiencies of Coagulation Factor Inhibitors
- Hereditary Disorders Associated with Increases in the Levels or Function Of the Coagulation Factors
- The Role of the Platelets
- Laboratory Investigation of Thrombosis (Figure 14)
- Laboratory Investigations of Thombophelic Parameters
- Anti-coagulation Therapy
Introduction
Thrombosis of the veins and arteries, together with complicating embolic phenomena, is perhaps the most important cause of sickness and death in the developed countries of the world at the present time. It is of far greater overall clinical importance than all of the hemorrhagic disorders combined. Thrombosis thus involves the interplay of vascular, cellular and humoral factors within a flowing stream of blood. It is a dynamic process to be distinguished from the static phenomenon of blood coagulation.
- Proceeding
- Introduction
- Pathophysiology of Thrombosis
- Overview of the Coagulation Cascade
- Predictors of Thromboembolic Manifestations
- Inherited Risk Factors Of Venous Thrombosis
- Hereditary Deficiencies of Coagulation Factor Inhibitors
- Hereditary Disorders Associated with Increases in the Levels or Function Of the Coagulation Factors
- The Role of the Platelets
- Laboratory Investigation of Thrombosis (Figure 14)
- Laboratory Investigations of Thombophelic Parameters
- Anti-coagulation Therapy
Pathophysiology of Thrombosis
The hemostatic apparatus is intrinsically self-propagating and has the potential to occlude the vascular system as an extension of its physiologic actions. Regulatory processes act to counterbalance haemostatic phenomena, and thrombosis may develop whenever the dynamic balance between prothrombotic and anti-thrombotic processes becomes altered. Hemostatic mechanisms and their inhibitory counterparts are illustrated. Thrombosis may result when an imbalance of these phenomena occurs. The intereactions indicated mainly occur in the microvasculature, which because of the large endothelial surface in proportion to the small volume of flow may act to clear circulation of prothrombotic debris (Figure 1).
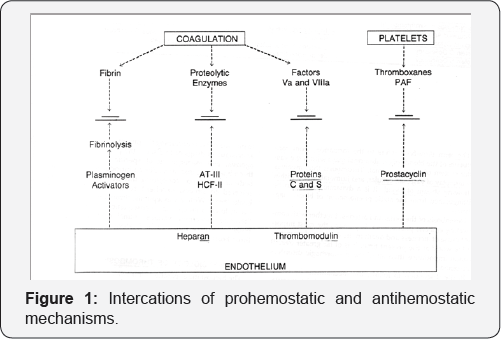
That abnormalities of the vessel wall, alterations of blood flow, and changes in the composition of the blood are the major factors in the pathophysiology of thrombosis was well recognized in the nineteenth century. The hematological terms of these three factors, usually known as the triad of Virchow.
- Proceeding
- Introduction
- Pathophysiology of Thrombosis
- Overview of the Coagulation Cascade
- Predictors of Thromboembolic Manifestations
- Inherited Risk Factors Of Venous Thrombosis
- Hereditary Deficiencies of Coagulation Factor Inhibitors
- Hereditary Disorders Associated with Increases in the Levels or Function Of the Coagulation Factors
- The Role of the Platelets
- Laboratory Investigation of Thrombosis (Figure 14)
- Laboratory Investigations of Thombophelic Parameters
- Anti-coagulation Therapy
Overview of the Coagulation Cascade

The coagulation cascade consists of a series of highly regulated proteins that circulate in their inactive, or zymogen, form. Coagulation is initiated when factor VIIa (the only coagulation factor that circulates in substantial quant ities in its activated form) binds to tissue factor on the surface of endothelial cells and monocytes at sites of vascular injury (Figure 2). The tissue factor-factor Vila complex activates factor X to factor Xa and factor IX to factor IXa. Factor Xa, in the presence of factor Va, proteolytically activates prothrombin into thrombin, which is the pivotal enzyme of coagulation. Thrombin cleaves soluble fibrinogen, forming insoluble fibrin that constitutes the scaffolding of the hemostatic plug.
- Proceeding
- Introduction
- Pathophysiology of Thrombosis
- Overview of the Coagulation Cascade
- Predictors of Thromboembolic Manifestations
- Inherited Risk Factors Of Venous Thrombosis
- Hereditary Deficiencies of Coagulation Factor Inhibitors
- Hereditary Disorders Associated with Increases in the Levels or Function Of the Coagulation Factors
- The Role of the Platelets
- Laboratory Investigation of Thrombosis (Figure 14)
- Laboratory Investigations of Thombophelic Parameters
- Anti-coagulation Therapy
Predictors of Thromboembolic Manifestations
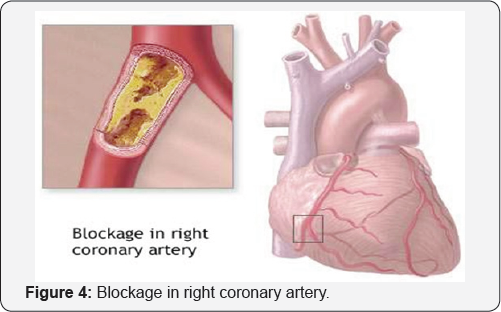
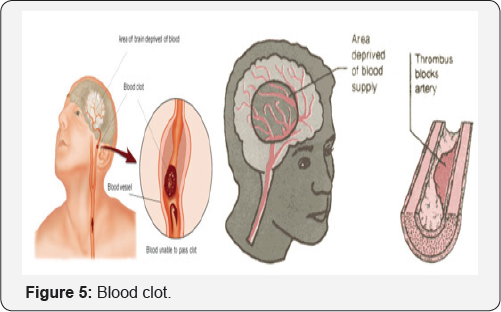
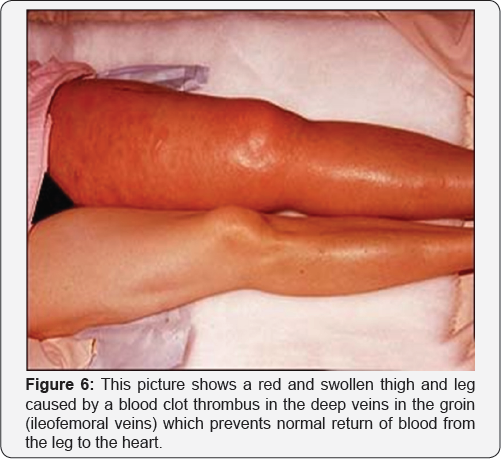
Classic acquired risk factors for venous thrombosis
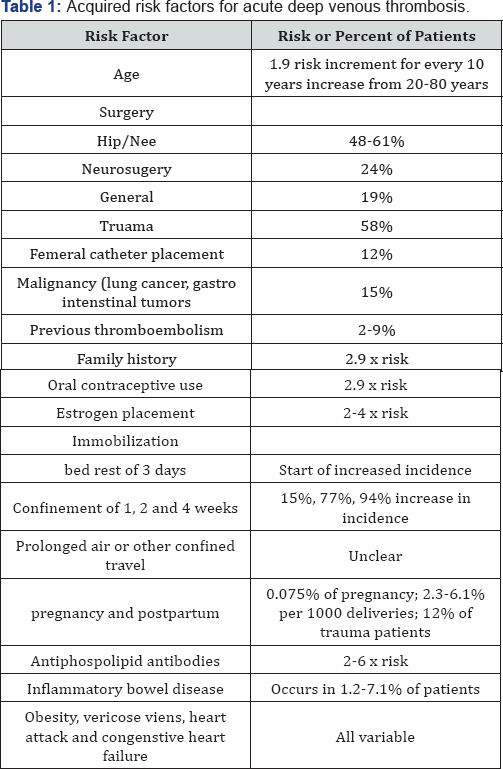
These factors include trauma, immobilization, pregnancy, surgery, malignancy and infection. These are all factors that may cause damage, stasis of the blood or changes in blood composition (Table 1).
Long air travel
Recent reports have linked air travel with venous thromboembolism (VTE) Research has shown that correctly fitting anti-thrombosis stockings increase blood flow, thus lowering the risk of DVT in those at risk. Advice related to stocking socks should apply to all forms of travel when a passenger is sitting still for a long period of time and taking off the shoses accompanied with circle movement of the ankle every now and then. If you are in a very high-risk category you should seek advice from your doctor and consider postponing your travel plans (Figure 7).

- Proceeding
- Introduction
- Pathophysiology of Thrombosis
- Overview of the Coagulation Cascade
- Predictors of Thromboembolic Manifestations
- Inherited Risk Factors Of Venous Thrombosis
- Hereditary Deficiencies of Coagulation Factor Inhibitors
- Hereditary Disorders Associated with Increases in the Levels or Function Of the Coagulation Factors
- The Role of the Platelets
- Laboratory Investigation of Thrombosis (Figure 14)
- Laboratory Investigations of Thombophelic Parameters
- Anti-coagulation Therapy
Inherited Risk Factors Of Venous Thrombosis
In inherited risk factors for venous thrombosis, most of which concern defects in the procoagulant and anticoagulant pathways, account for a substaintial proportion of all thrombotic events. Table 2 summarizes prevalences and relative risks of established gentic risk factors.
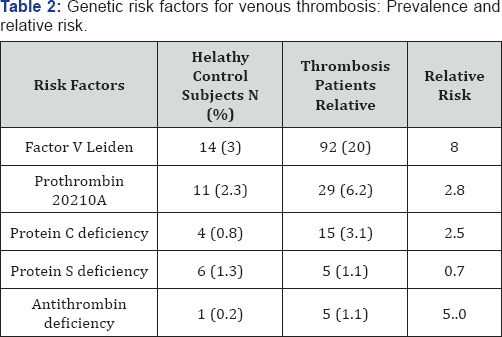
Data are from the leiden thrombophilia study which involved 474 consecutive patiens with a first deep vein thrombosis and 474 healthy control subjests. Protein S deficiency was not associated with increased thrombotic risk in this case-control study, which contrasts with previous findings in family studies.
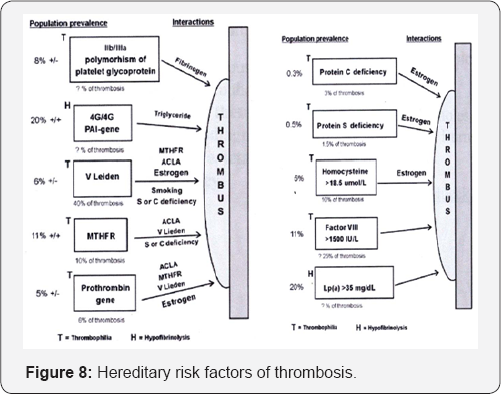
Hereditary risk factors of thrombosis
(Figure 8)
- Proceeding
- Introduction
- Pathophysiology of Thrombosis
- Overview of the Coagulation Cascade
- Predictors of Thromboembolic Manifestations
- Inherited Risk Factors Of Venous Thrombosis
- Hereditary Deficiencies of Coagulation Factor Inhibitors
- Hereditary Disorders Associated with Increases in the Levels or Function Of the Coagulation Factors
- The Role of the Platelets
- Laboratory Investigation of Thrombosis (Figure 14)
- Laboratory Investigations of Thombophelic Parameters
- Anti-coagulation Therapy
Hereditary Deficiencies of Coagulation Factor Inhibitors
Protein C deficiency
Protein C deficiency associated with venous thrombosis was first described in 1981.Physiologically, protein C is activated by thrombin complexed to thrombomodulin. Activated protein C is a potent anticoagulant that in-activates factors Va and VIIIa, a reaction requiring its co-factor, protein S. Protein C deficiency is found in approximately 0.2% of the normal population and in 2.5% to 6% of patients with venous thrombosis. Most patients with venous thrombosis and protein C deficiency will have functional protein C levels that are about 50% of normal, and many patients will have an episode of venous thrombosis by age 60 years.
Protein S deficiency (Figure 9)
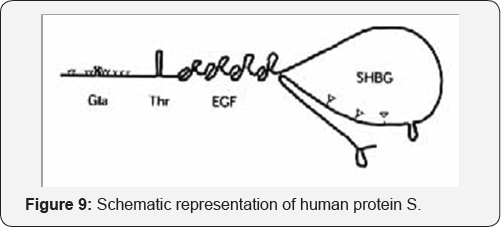
Protein S is a polyoeptide protein that normally present in the plasma and in the a-granules of platelets. It functions as a cofactor for the action of protein C. Deficiency of protein S is inherited as an autosomal dominant trait and is associated with a lifelong thromboembolic diathesis.
Protein S exists in the plasma in a free form that is in equilibrium with a nonfunctional form bound to the C4b, component of complement. Thrombosis is associated with reductions in free or functional protein S and assays for this form are thus required for diagnosis. Measurements of C4b, total protein S antigen, and C4b-protein S complexes provide additional information.
The clinical manifestations of protein S deficiency are identical to those of protein C deficiency.
Anti-thrombin III deficiency
Patients with anti-thrombin III deficiency are at higher risk for thrombosis than patients with any other congenital thrombophilic state; approximately 60% of patients with antithrombin deficiency will have an episode of venous thrombosis.
There are three types of hereditary AT deficiency:
a. Type 1: Classic -There is reduced synthesis of the AT molecule. Agene deletion or frame shift mutation results in a truncated protein which is unstable.
b. Type 2: The level of AT produced is normal, but the protein is dysfunctional.
c. Type 3: The quantity and quality of AT is normal but it lacks the receptor for heparin; therefore, it cannot be accelerated.
- Proceeding
- Introduction
- Pathophysiology of Thrombosis
- Overview of the Coagulation Cascade
- Predictors of Thromboembolic Manifestations
- Inherited Risk Factors Of Venous Thrombosis
- Hereditary Deficiencies of Coagulation Factor Inhibitors
- Hereditary Disorders Associated with Increases in the Levels or Function Of the Coagulation Factors
- The Role of the Platelets
- Laboratory Investigation of Thrombosis (Figure 14)
- Laboratory Investigations of Thombophelic Parameters
- Anti-coagulation Therapy
Hereditary Disorders Associated with Increases in the Levels or Function Of the Coagulation Factors
Factor V leiden and activated protein C resistance (APCR)
Schematic models of factor V. Factor V contains three A-modules, one B-module and two C-modules. Thrombin cleaves three peptide bonds, as indicated by the arrows. In factor Va. the Al-AZ containing heavy chain and the A3-C1, -C2 composed light chain form a calcium-dependent complex. Factor Va is a substrate for APC, which cleaves the peptide bond at Arg 306, Arg 506 and Arg 679. The cleavage at 506 is preferred and this is also the site that is mutated in APC-resistance. a mutation which predicts replacement of the Arg with a Gln (Figure 9).
In the 1993 Dahlback et al. described the most frequent genetic abnormality of the coagulation system: the APC resistance (APCR). In more than 90% of cases, APCR is due to a unique mutation of the FV gene, the G1691A transition in exon 10, responsible for the substitution of arginine by glutamine at position 506 of FV (Figure 10).

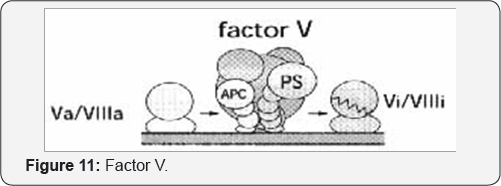
Speculative molecular model of APC-mediated degradation of factor Va and factor VIlla. A complex formed between protein S and factor V on the membrane surface may constitute a binding site for APC. APC efficiently inhibits blood coagulation through degradation of factors VIIIa and Va (Figure 11).
Prothrombin Gene Mutation (Fll Leiden)
A new genetic risk factor was recently associated with VTE.'2. This factor is like FV Leiden a unique mutation that affects the 3' untranslated part of the prothrombin (FI1) gene (G20210A). This mutation is also called FII Leiden. In contrast to the previously described "loss of function" genetic abnormalities, the prothrombin gene mutation upregulates prothrombin levels, thus being a "gain of function" mutation.
Elevated Levels of factors VIII, IX and XI
When compared with healthy controls, patients with a history of venous thrombosis are more likely to have persistent elevation of the levels of coagulation factors VIII, IX, and XI. In addition, siblings of patients with both venous thrombosis and elevated factor VIII levels also have persistently elevated factor VIII levels, suggesting a hereditary predisposition.
Elevated levels of lipoprotein (a) & Hyperhomocysteinemia
Recently, elevated levels oflipoprotein (a) have been suggested to be an additional risk factor for venous thromboembolism. This theory is not widely accepted at present; if elevations of this factor are a risk for venous thrombosis, however, their ability to interfere with normal fibrinolytic function is the likely mechanism.
High levels of homocysteine are associated with both venous and arterial thrombosis. The potential mechanisms include endothial-activation, proliferation of smooth-muscle cells, changes in endothelial nitric oxide production, or changes in endothelial sterol metabolism. Congenital hyper homocysteinemia is most commonly due to mutations affecting the cystathione-b-synthase (CBS) gene or the methylene tetrahydrafolate reductase (MTHFR) gene.
Elevated Level of von willebrand Factor
Von Willebrand factor is a large multimeric protein synthesized in endothelial cells and megakaryocytes that serves 2 important functions in hemostasis: carrier protein for factor VIII and the ligand that initiates adhesion of platelets to the damaged vessel, von Willebrand factor binds to the glycoprotein lb receptor on platelets and to collagen in the vessel wall.
Von Willebrand factor levels vary with blood type. Individuals with type O blood have the lowest levels, those with type AB have the highest levels, and those with types A and B have intermediate levels.
Elevated Fibrinogen & dysfibrinogenaemia
Fibrinogen is a 340-kd soluble glycoprotein, the plasma component of which is synthesized exclusively in the liver. Fibrinogen is modified by thrombin to produce fibrin monomers that are the primary constituent of the fibrirn clot. Decreased levels of fibrinogen are associated with an increased risk of bleeding. Increased levels of fibrinogen have been proposed as potentially contributing to thrombophilia, and fibrinogen polymorphisms resulting in increased fibrinogen levels have been described. Increased fibrinogen levels are postulated to enhance thrombus formation by altering the kinetics of the coagulation cascade, resulting in increased fibrin formation, augmenting platelet interaction by increased binding to the glycoprotein IIb/IIIa receptor, and increasing plasma viscosity (Figure 12).
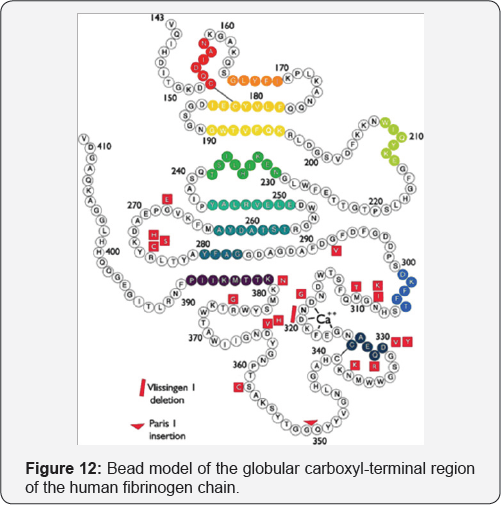
Bead model of the globular carboxyl-terminal region of the human fibrinogen chain, from Val143 to Val411. Mutations responsible for -dysfibrinogenemias are presented in red. Colored beads indicate stretches of helix or strand structure.
- Proceeding
- Introduction
- Pathophysiology of Thrombosis
- Overview of the Coagulation Cascade
- Predictors of Thromboembolic Manifestations
- Inherited Risk Factors Of Venous Thrombosis
- Hereditary Deficiencies of Coagulation Factor Inhibitors
- Hereditary Disorders Associated with Increases in the Levels or Function Of the Coagulation Factors
- The Role of the Platelets
- Laboratory Investigation of Thrombosis (Figure 14)
- Laboratory Investigations of Thombophelic Parameters
- Anti-coagulation Therapy
The Role of the Platelets

Overall, here is what happens. Vascular injury leads to the margination of platelets to the site of vessel injury. The platelets adhere to the damaged endothelial cells via von Willebrand Factor. (Small amounts of fibrinogen assist in creating this platelet plug.) The platelets are then stimulated to degranulate and express receptors for Factors V and VllI as well as leukocytes. At this same time, blood is exposed to tissue factor and the clotting pathways are initiated. The necessary cofactors (V and VIII) bind to receptors on platelet surfaces and allow for efficient propagation of the cascade. This eventually leads to the formation of fibrin. Since fibrin strands are formed in the vicinity of platelets, the two can easily come together and form a clot (Figure 13).
There are many instances when the clotting system becomes over productive and leads to the formation of pathologic clots Like increased platelet numbers, hyperactive platelet and secreting thrombogenic platelet constituents or presence of Platelet glycoprotein polymorphisms.
- Proceeding
- Introduction
- Pathophysiology of Thrombosis
- Overview of the Coagulation Cascade
- Predictors of Thromboembolic Manifestations
- Inherited Risk Factors Of Venous Thrombosis
- Hereditary Deficiencies of Coagulation Factor Inhibitors
- Hereditary Disorders Associated with Increases in the Levels or Function Of the Coagulation Factors
- The Role of the Platelets
- Laboratory Investigation of Thrombosis (Figure 14)
- Laboratory Investigations of Thombophelic Parameters
- Anti-coagulation Therapy
Laboratory Investigation of Thrombosis (Figure 14)
When Is It Appropriate to Perform Laboratory Evaluation?
Laboratory investigation should be performed 3 to 6 months after thrombosis and in the absence of oral anti-coagulation. Testing for PC and PS is not reliable during oral anticoagulation, and it is preferable that the patient has been off this kind of therapy for at least 10 days and if possible for 1 month.
- Proceeding
- Introduction
- Pathophysiology of Thrombosis
- Overview of the Coagulation Cascade
- Predictors of Thromboembolic Manifestations
- Inherited Risk Factors Of Venous Thrombosis
- Hereditary Deficiencies of Coagulation Factor Inhibitors
- Hereditary Disorders Associated with Increases in the Levels or Function Of the Coagulation Factors
- The Role of the Platelets
- Laboratory Investigation of Thrombosis (Figure 14)
- Laboratory Investigations of Thombophelic Parameters
- Anti-coagulation Therapy
Laboratory Investigations of Thombophelic Parameters
Laboratory Findings that may be associated with the Presence of Thromboembolism
Routine Laboratory tests:
Activated Prothrombin Time (aPT)
Activated Partial Thromboplastic Time (aPTT)
Thrombin Time
Bleeding & Time
Fibrinogen Concentrations
a. Specific testes
Assays for Lupus Anticoagulants
Factor VIII Inhibitor Assay
von Willebrand Disease Assays
Platelet specific Tests
Platelet specific "marker" proteins (PF4, B-thromboglobulin)
Increased platelet turnover
Increased platelet procoagulant activity
Another Laboratory tests (Figure 15,16)
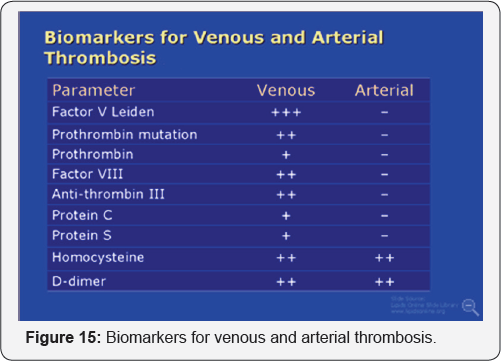
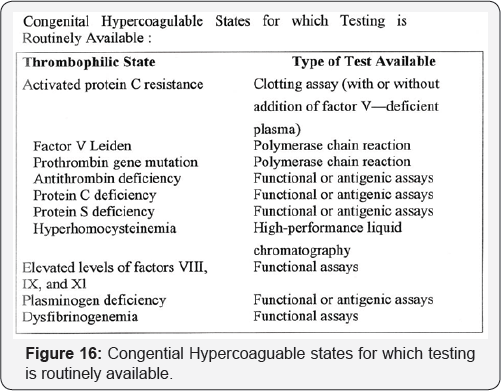
Reduced fibrinolytic activity
Circulating fibrin monomer complexes
Prothrombin fragment 1.2
Abnormal levels of fibrinopeptide A
Thrombin-antithrobin III and plasmin antiplasmin complexes
Abnormal levels of fibrin degradation products
- Proceeding
- Introduction
- Pathophysiology of Thrombosis
- Overview of the Coagulation Cascade
- Predictors of Thromboembolic Manifestations
- Inherited Risk Factors Of Venous Thrombosis
- Hereditary Deficiencies of Coagulation Factor Inhibitors
- Hereditary Disorders Associated with Increases in the Levels or Function Of the Coagulation Factors
- The Role of the Platelets
- Laboratory Investigation of Thrombosis (Figure 14)
- Laboratory Investigations of Thombophelic Parameters
- Anti-coagulation Therapy
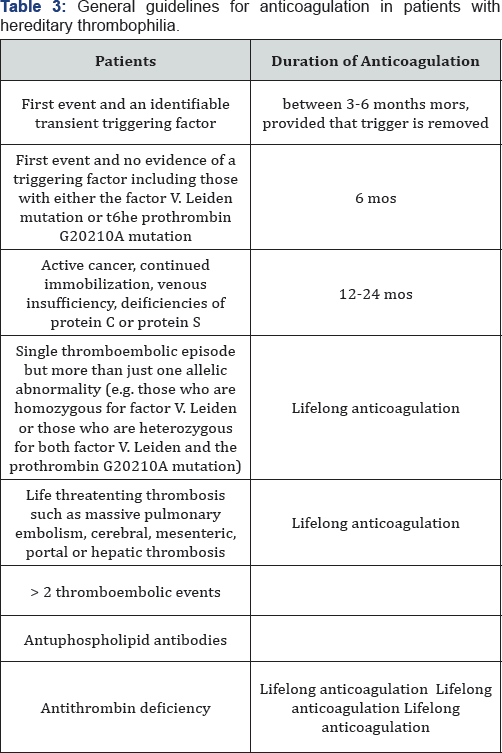
Anti-coagulation Therapy
Heparin
Mechanisn of Action: The anticoagulant action of heparin is the result of its interaction with a physiologic inhibitor-ATIII It acts directly in the peripheral blood and does not affect the hepatic biosynthesis or the plasma levels of any coagulation factor (Figure 17).
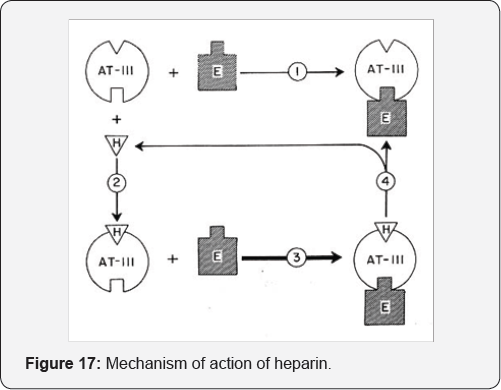
Heparin may bind directly to sites on thrombin and may displace factor xa from phospholipid bindings sites by a mechanism that independent of ATIII. Most of the enzymes involved in blood coagulation are serine-active proteases. As a consequence heparin may inhibit every step in coagulation (Table 3).