The Use of Blood-Derived Products as an Effective Element in the Repair of Cartilage Lesions
Mahdieh Sadat Ghiasi1*, Sahar Farzaneh2 and Seyed Khalil Pestehei3
1Neuroscience Institute, Tehran University of Medical Sciences, Tehran, Iran
2Department of development Biology, Islamic Azad University of Parand, Tehran, Iran
3Department of Anesthesiology, School of Medicine Imam Khomeini Hospital Complex Tehran University of Medical Science, Tehran, Iran
Submission: April 11, 2023; Published: May 22, 2023
*Corresponding author: Mahdieh Sadat Ghiasi, Neuroscience Institute, Tehran University of Medical Sciences, Tehran, Iran Email: mahdieh.ghiasi@yahoo.com
How to cite this article: Mahdieh Sadat G, Sahar F. The Use of Blood-Derived Products as an Effective Element in the Repair of Cartilage Lesions. Nov Tech Arthritis Bone Res. 2023; 4(1): 555628. DOI 10.19080/NTAB.2023.04.555628
Abstract
Treatment of cartilage lesions due to the absence of neurons and blood vessels is one of the most important problems in the field of orthopedics and maxillofacial. Its problems can lead to chronic pain and functional limitations in patients. Cartilage damage caused by trauma, tumors, or ageing. Today, with the help of tissue engineering, various structures such as natural and artificial scaffolds were created to cover and regenerate cartilage tissue. Blood is one of the rich sources of regenerative cells, growth factors, cytokines, and suitable scaffolds in the production of biomaterials and obtaining progenitor cells for cartilage repair. In this study, a collection of research and review articles were collected until 2023 from reliable websites, to explain the use of various products obtained from blood in the repair of cartilage lesions and entered the study. The purpose of this study is to review the clinical and research evidence related to the effective elements derived from blood tissue in the treatment of cartilage tissue damage. While the most of recent research were emphasized on the use of artificial materials from natural autologous materials such as stem cells and growth factors, in this study it will point to other structures that are more efficient and derived from platelets. It seems that by using tissue engineering strategies and doing more research on the blood tissue, it can be used as a biomaterial in the repair of cartilage tissue lesions.
Keywords:Repair; Cartilage Tissue; PRP: Platelet-Rich Plasma; Tissue Engineering; Blood Biomaterial
Keywords:OA: Osteoarthritis; MSC: Mesenchymal Stem Cell; BMS: Bone Marrow Stimulation; CFCs: Colony Forming Cells; PRGF: Plasma-Rich in Growth Factors; PRF: Plat Let-Rich Fibrin; MSC: Mesenchymal Stem Cells; MMPs: Matrix Metalloproteinases; HA: Hyaluronic Acid
Introduction
Cartilage is an essential elastic connective tissue in the body’s skeletal system, which supports the various organs such as the respiratory tract, ear, pharynx, nose, bone joints and reduces the mechanical pressure in joints. Some degenerative diseases such as osteoarthritis (OA), aging, exercise and abrasion of joints, tumors, continuous stress, inflammation, and strikes may lead to cartilage damage [1]. Cartilage tissue has a very limited regeneration and self-repairing potential due to absence of blood vessels, lymph, nerves and a small number of progenitor cells [2], and its repair is often in the form of mesenchymal stem cells infiltrating the damaged area and differentiating it into chondrocytes; But the new fibrocartilage tissue, has a much lower mechanical stress tolerance than the origin cartilage tissue, and it degrades during the time [3,4]. Therefore, the treatment of cartilage lesions is currently an important research topic in traumatology [5], orthopedics and dentistry. Many efforts have been made to find the best treatment for repair of cartilage lesions, such as cell therapy and the use of tissue engineering in order to design suitable scaffolds for tissue. In cell therapy with methods such as Bone Marrow Stimulation (BMS) [6], autograft, allograft and cartilage cell transplantation [7,8], repair based on Mesenchymal Stem Cell (MSC) [ 9,10] and grafting of tissue engineered cartilages [11-14] stimulate the repair, regeneration and creation of cartilage tissue in the damaged areas. With the progress of biology and development of new sciences such as tissue engineering, alternative methods have been created as an effective solution to improve the recovery of diseases. Tissue engineering is an interdisciplinary science that uses engineering and molecular cell biology to develop biological substitutes by creating three-dimensional structures.
Three necessary factors in tissue engineering include: selection of suitable cells for the target tissue, suitable scaffold, and growth factors. The cell used on the surface of the scaffold to regenerate the damaged tissue must have the ability to multiply, prevent immune responses and maintain the properties of the scaffold. The source of cells in tissue engineering can be bone marrow, umbilical cord blood, fat tissue, fibroblast, embryonic stem, blood stem cells etc. [15]. Although embryonic stem cells are more potency to reproduction and differentiation than adult stem cells such as blood, obtaining, maintaining, and extracting them requires more time and expense than adult cells, and due to ethical issues, there are prohibitions in their use. Scaffolds used in tissue engineering are also designed to have biocompatibility and mechanical properties for cell processes such as migration, proliferation, and differentiation towards functional tissue. In various research, both natural and artificial (synthetic) materials have been used, including string structures, spongy-porous, woven, or non-woven webs, and hydrogels in order to create suitable scaffolds for cartilage tissue with different shapes. Also, the growth factors used in the tissue regeneration process must be molecularly bioactive and increase the rate of formation of the desired tissue. For example, cytokines and blood tissue growth factors have very important effects on cells during the cartilaginous process. Therefore, according to the limitations and mentioned points about the types of cells and scaffolds used in cartilage tissue engineering, in this study, review and research articles about blood-derived biomaterials until 2023 have been collected from reliable websites about clinical applications and reflection of them in the repair of cartilage tissue.
Blood Tissue and its Derived Products
PRP
Blood is a connective tissue of mesodermal origin, which is rich in various types of cells such as platelets, growth factors, cytokines, stem cells such as mesenchymal stem cells derived from bone marrow and has the potency of division, differentiation into other types of cells such as mature blood cells or cartilage tissue (chondrogenic). The main nature of blood stem cells is called colony forming cells (CFCs), which remain pluripotent after entering the circulation system or become one of several single cell types like platelets [16]. Different formulations have been created by researchers to extract various types of blood cells for more than several years for different uses. These materials and their formulations have drawn a lot of attention in tissue regeneration studies with the aim of improving soft and hard tissues. Also, blood products are one of the most suitable options for tissue engineering because of signaling molecules, growth factors, cytokines, chemokines, and all types of leukocytes (immune cells).
Molecules and chemical compounds released by these cells in the blood tissue are one of the most suitable options for tissue engineering because they act as natural antibiotics and anti-inflammatory in case of possible contamination in these products [17]. Many blood derivatives products such as plasma concentrate, Plat Let-Rich Fibrin (PRF), Platelet-Rich Plasma (PRP), Plasma-Rich in Growth Factors (PRGF), hydrogels, fibrin glue and. are extracted from blood tissue. Since the whole blood of an adult human consists of two compounds, cells, and plasma, after separating its components by centrifugation with determined cycles or due to the presence or absence of chemicals and anticoagulants materials, various therapeutic products are obtained. One of these products is Platelet Rich Plasma (PRP), which is usually prepared by using two stages of centrifugation of blood containing the heparin anticoagulant, which platelets are four to seven times thickens than their normal amount in Blood [18].
Many in vivo and in vitro research have shown the remarkable effects of using PRP in repairing all kinds of joint, bone and gum tissue lesions. For example, Beige et al in 2018 after the simultaneous induction of 3D alginate scaffolds and PRP for 16 weeks in a rabbit model observed that the matrix of the defective area expressed high levels of chondrocyte genes Sox9, aggrecan and collagen [19]. Or in the treatment of degree II knee osteoarthritis lesions and acute tendonitis in athletes with lesions, by injecting RPR at standard time, it has high effective on proliferation of neotocites and improving the pain in patients [20]. Also, after the induction of mesenchymal stem cells of human (MScs) by PRP, the expression of Runx2 gene, which plays a significant role in the differentiation of chondrogenesis, were significantly increased [21]. Platelets are produced by megakaryocytes and contain a variety of growth factors, adhesion molecules, cytokines, chemokines and integrins, which after stimulating the proliferation activation of chondrocytes and pluripotent mesenchymal stem cells (MSC), cause to synthesis of chondrocytes by strengthening collagen II and aggrecan genes [22]. Also, due to the presence of anti-inflammatory factors in blood, they reduce inflammation by preventing the catabolic effects of cytokines, such as IL-1β and matrix metalloproteinases (MMPs) in arthritis areas, reducing pain and protecting cartilage tissue [22]. Chen and et al have investigated the effects of PRP on the proliferation and induction expression of cartilage-specific genes in the two-dimensional culture of human intervertebral disc nucleus cells [23]. The purpose of this study was to determine the accumulation of proteoglycan in intervertebral disc nucleus cells treated with PRP and their anti-apoptotic effects. They announced that by using PRP they were able to increase the proliferation of intervertebral disc nucleus cells and the accumulation of proteoglycans seven to eleven times more than the control [23]. In the clinical trial in 2020 Ghazi et al evaluated the repairment of knee in a 53-year-old patient with osteoarthritis, they found that combining platelet-rich plasma (PRP) with stem cells derived from autologous adipose tissue and injecting that causes the fasting regeneration of cartilage in the area after 18 months. They followed the MRI scan images and examined chondrogenic markers and found that the expression of genes related to cartilage formation increased significantly after the injection [24]. Also, in extensive clinical studies on 48 patients conducted in 2010 by Giannini S and et al, after using PRP as a scaffold in the repair of osteochondral lesions with MRI and pathological evaluations for 2 years, it showed that the amount of Cartilage tissue recovery improved up to 91% and high degrees of tissue regeneration were showed in patients. In (Table 1) summarizes some applications of PRP on cartilage tissue.
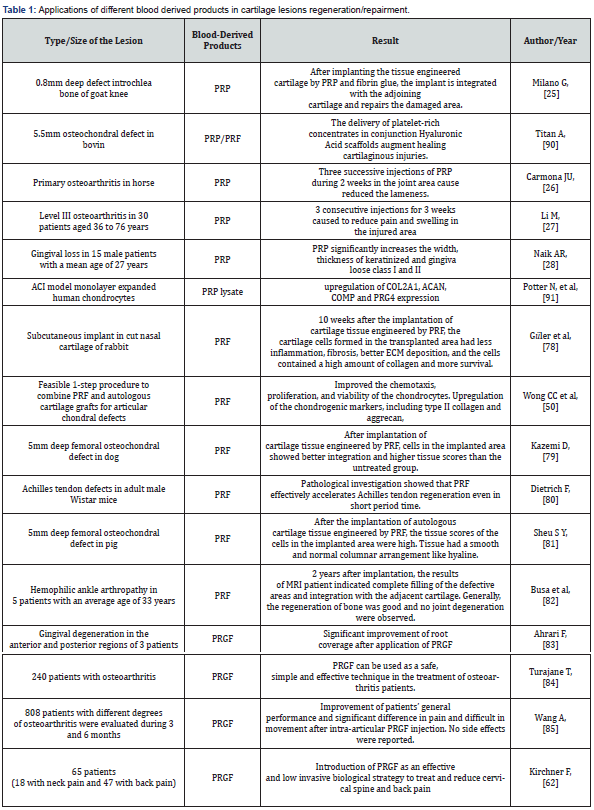
Many studies have shown that blood products can be used as a potential alternative source in the repair and regeneration of different tissues, especially cartilage. For example, research on PRP indicates the ability of this biological material to improvement lesions directly on the processes of proliferation, differentiation and angiogenesis, or cells recruitment through the process of chemotaxis and lateral environment control of the inflammation. In fact, according to the reports of studies, PRP has the power to attract mesenchymal stem cells through the process of chemotaxis [25]. Bahman pour et al compared fibrin-rich plasma, growth factors derived from stromal cells, and PRP in transplantation of rabbit knee defects. They found that the transplanted group with fibrin-rich plasma along with growth factors derived from stromal cells had the greatest ability to repair knee cartilage defects in rabbits [26]. In addition, during experiments, it was shown that PRP can absorb the peripheral blood monocytes by using a dosedependent method and ultimately lead to a change in the release profiles of pro-inflammatory cytokines [27]. Several research reported the ability of PRP in the field of inflammatory migration. Activated PRP has shown high levels of growth factors such as TNF-α [28].
In another study, Haleem et al. reported that transplantation of bone marrow-derived mesenchymal stem cells along with plasma rich in platelets/ fibrin glue can heal and restore the knee area in rabbits [29]. In other osteoarthritis models which used osteoarthritis cartilage and synovium of patients, PRP (with or without leukocytes) showed similar anti-inflammatory effects [30]. Chakroun’ et al revealed that filling a tooth socket by PRF can enhance the healing process without any complication [31]. Furthermore, PRP has shown the potential to protect cell viability and survival during cell cryopreservation [32]. Therefore, PRP promises to increase and amplification the proliferation of stem cells with minimal loss of their stemness. Altogether, a series of reports mentioned the role of PRP in the release of growth factors and its importance on the process of chemotaxis and inflammation. It is not strange that PRP is considered as the management of inflammation and pain of arthritis [33]. Shao et al showed that stem cells-derived bone marrow loaded in fibrin glue can create relatively favorable cartilage tissue in the osteochondral defect area of the rabbit knee [34].
In addition to improving axon growth, PRP has also shown neuroprotective properties. In the brain of a mice model suffering from brain stroke, PRP extract, which was used locally or systemically, produced neuroprotective effects [35]. However, local intra-arterial injection of PRP extract with the aim of reducing the volume and level of cerebral infarcts showed better results than its systemic injection. In addition, PRP also minimized neurological impairment in mice models. Also, PRP showed antimicrobial activity by inhibiting the growth of bacteria. According to the results of various experiments, PRP inhibits the growth of various bacteria such as Pseudomonas aeruginosa, Staphylococcus aureus, Streptococcus faecalis, Prohormones gingival is and Aggregatibacter actinomycetemcomitans [36,37]. This inhibition is done by the release of antibacterial chemokine ligands 1, 3 and 5. Wu et al used the combination of plasma-rich platelet scaffold and autologous chondroblasts to repair the cartilage area of the rabbit knee and confirmed the formation of cartilage tissue [38]. During a study, Lee et al can repair the osteochondral defect in the rabbit knee by transplanting platelet gel and stem cells derived from the synovial membrane [39].
PRF
Another blood product is Platelet Rich Fibrin (PRF), which does not require chemical additives and is produced A-PRF (Active PRF) immediately after blood collection before coagulation of the sample with a one-time centrifugation [40]. According to the results, A-PRF contains more platelets, which are mostly found in the end layers of the PRF membrane [41]. This product increases the potential of angiogenesis through the expression of matrix metalloproteinase 9 enzyme. [42]. PRF can release a high amount of several growth factors including TGF-β1, PDGF and VEGF [43]. The main difference between PRF and PRP is the constituent elements of the fibrin structure in them. In PRF, this network is gently formed during the centrifugation process in the absence of anticoagulant agents, leading to a compact fibrin structure. This structure in PRF acts as a network that traps platelets and leukocytes during the centrifugation process. This reserve property of the fibrin network increases the gradual release of growth factors and other mediators, as a result, helps in long-term preservation and stimulation of stem cells by PRF [44]. In fact, the patterns of growth factor diffusion such as TGF-β and PDGF are between PRP and PRF. In the PRP method, the release of TGF-β and PDGF is clearly reduced after the first day, while PRF shows a significant release of TGF-β and PDGF growth factors up to two weeks [45]. Ehrenfest et al. confirmed the difference in leukocyte-derived VEGF release profiles in PRP compared to PRF [46].
Overall, these studies suggest that PRF membranes may be able to release a higher amount of growth factors for a longer period of time [47]. In vitro research on the various effects of using PRF in the construction of different types of cartilage tissue indicates an increase in the expression of chondrogenic mRNAs such as GAGs, proteoglycans [48] in human cell lines, increase in the expression of ACAN mRNA and markers related to osteoarthritis such as ADAMTS4, PTGS2 and MMP13 [49] and improving chemotaxis, proliferation, and survival of chondrocytes [50] in rabbit chondrocytes. In many studies, PRF has been used to repair cartilage tissue in various human and animal lesions. Even in other cases such as skin lesions, products derived from blood tissue can be used to speed up the treatment process. For example, in the treatment of nail fungal infections [51], the infection can be quickly removed by PRF, or in androgenic hair loss [52], PRP and its related growth factors affect the cellular and molecular cycles of the hair follicle and used as a useful clinical method to treat hair loss, especially in the early stages. In (Table1). summarizes some applications of Platelet Rich Fibrin on cartilage tissue.
PRP and PRF have many clinical applications in the field of regenerative medicine. Several in vitro and animal studies have been reported in this field. The cultured Stem cells on scaffolds or in combination with scaffolds have been specially evaluated in orthopedic, maxillofacial surgery, lesions and burn injuries treatment. The use of PRP has been reported with the aim of speeding up the repairing of chronic skin lesions and improving the results of fat grafts using. For example, patients with ulcers of the lower limbs have been treated by PRP combined with primary adipose tissue and a control treatment patient consisting of collagen combined with hyaluronic acid. After 7 and 10 weeks, respectively, PRP-treated lesions were completely epithelialized in 61.1 and 88.9% compared to the control group (40 and 60%). A similar study was conducted to investigate the effect of PRP on the treatment of various mandible and facial lesions. The patients examined in these studies were treated with PRP and fat grafting or just fat grafting. One year after surgery, the survival and threedimensional tissue volume in the group treated by PRP was 70%; While these tissue characteristics were reported only 31% in the group which treated by just fat grafting [53].
In orthopedic fields, PRP has been used as an effective bioactive substance in the treatment of diseases. For example, in a human clinical trial aimed to the treatment of malformation, 21 patients underwent high bone osteotomy as well as treatment with mesenchymal stem cells and PRP injections. In this clinical trial, 23 patients were only subjected by osteotomy of the tibia bone and treated with PRP injection as the control. This study showed that the addition of mesenchymal stem cells causes a significant reduction in the pain and significant increase in the formation of cartilage tissue [54]. In the field of dentistry and maxillofacial surgery, PRP was used to treat a disease called Black Triangle, which the distance between the teeth is greater than normal, and it is caused by the receding height of the papilla. In this study, 10 patients participated, each of whom performed between 1 and 5 PRP injections, and the treatment follow-up time in this study was considered more than 69 months. All patients reported that they did not report important side effects and their satisfaction level with the treatment was very high. Patients showed varying degrees of repairment and defect filling, and two patients also showed complete regeneration [55]. However, the involvement of progenitor cells in regeneration largely remains unappreciated. In summary, preliminary clinical results showed that platelet-based blood derivatives such as PRP and possibly PRF are promising supplements for stem cell-based therapies. Specifically, they are always prepared, accessible and safe sources of human growth factors.
PRGF
Plasma rich in growth factors (PRGF) is another blood product of concentrated platelets that has a high potential in the process of tissue regeneration [56]. In fact, PRGF is a cocktail of proteins and growth factors that stimulates the proliferation, migration, and chemotaxis of cells by increasing the expression of autocrine and proangiogenic factors such as vascular endothelial growth factor and growth factors [57]. Research on PRGF has been limited but satisfy results have been obtained in the field of tendon and cartilage repair [58,59]. In a case report on the treatment of femoral condyle in 2003 by Mikel Sanchez and et al, was found that the use of autologous PRGF causes the formation of cartilage without extra bone in the damaged area. MRI scan images showed that after 18 months of following the recovery process, the cartilage was completely formed and reached the standard thickness in patients [58]. In a randomized study on dog models of with severe osteoarthritis, scientists showed that intra-articular transplantation of ASCs in the presence of PRGF improves physical performance and reduces pain [60,61]. Several reports have been done on the reparative effects of PRGF on hard bone tissue, but less on soft tissue. Plasma rich in growth factors (PRGF) is plasma rich in platelets without leukocytes and can reduce pain and improve the injured area in various conditions such as chronic degenerative [62]. Also, the combined use of PRGF with other products such as hyaluronic acid (HA) is more effectively reduce pain in knee osteoarthritis patients by suppressing the catabolic environment of inflammatory cells [63]. Platelet-derived growth factors help cartilage to proliferate and maintain its pseudo-hyaline phenotype [64,65]. In (Table1), some use of the different blood derived products (PRP/PRF/PRGF) in the repair and regeneration of cartilage tissue are collected.
Cell Therapy of Cartilage Tissue
As mentioned before, other types of stem cells derived from blood such as umbilical cord blood can be used in the treatment of cartilage lesions. Cord blood extracted from placental blood in the umbilical cord connected to the fetus. After the baby is born, the remaining blood in the umbilical cord and placenta, which is rich in stem cells, is thrown away as biological trash. While this blood contains stem cells that can be used in the treatment of many disorders and diseases. The most important advantages of umbilical cord are immortality and pluripotency. Also, these cells do not get old due to successive proliferation, so by injecting or replacing them in tissues that have serious damage, it can help to improve and form new cells and tissue. These cells are very easy to obtain. Bone marrow stem cells are the most useful sources of the body’s immune system and hematopoiesis. Bone marrow has the highest percentage of hematopoietic stem cells (1-3%) and cord blood has 0. 6 to 1% progenitor and hematopoietic stem cells. In the peripheral blood, less than 0. 2% of hematopoietic stem cells have been identified. The advantages of using umbilical cord blood compared to bone marrow are the rate of graft rejection reactions against the host GVHD / Graft versus host disease of umbilical cord blood recipients is lower than that of bone marrow blood. GVHD is a serious and sometimes mortal reaction following a bone marrow transplantation, in which the immune cells of the transplant donor attack the host’s body and cause damage to parts of the host’s body. The presence of primary and immature cells of the immune system in the umbilical cord blood reduces the immunological rejection of the transplant to the bone marrow. So severe transplant rejection has been seen in about 60% of cord blood transplants [66].
By using umbilical cord blood, it is possible to perform a successful transplant with less HLA similarity between donor and recipient than bone marrow blood, so it will include a large number of recipients. Cord blood donation is not dangerous for the donor (fetus and mother). Collecting cord blood is simpler and easier than bone marrow. In this way, after the birth of the newborn, the umbilical cord and placenta are separated, and the blood is collected by a bag or syringe. Mother and baby are not harmed during this process. In addition, it is completely painless. This is despite the fact that bone marrow blood collection requires surgical procedures and is usually done under anesthesia and can be painful. The rate of viral infections of the donor, including cytomegalovirus, is lower in umbilical cord blood than in bone marrow blood. Umbilical cord blood stem cells and progenitors have more proliferative potency than bone marrow. Because that a large number of babies are born daily, many blood units are available, which makes their use easy. Studies have shown that umbilical cord stem cells produce more blood cells than bone marrow.
Umbilical cord blood cells produce ten times more cells and therefore a successful transplant can be done even with a small number of cells. Nowadays, there is umbilical cord blood bank in most of the developed countries, which stores the baby’s umbilical cord blood instead of throwing it away and uses it for him/herself or other patients in the future. Parents register in this bank before the baby is born. Immediately after childbearing, the umbilical cord is separated from the baby and the blood from the umbilical cord and placenta is collected by a needle and transferred to the blood bags. Then, the baby’s umbilical cord blood is frozen and stored in the bank to use for treatment if the baby becomes infected with diseases such as leukemia. Although the use of stem cells in the treatment of diseases is not very common still, these cells are used in the treatment of type 1 diabetes (insulin-dependent), cardiovascular diseases, lupus, neurological diseases such as stroke, Parkinson’s and Alzheimer’s, anemia and immunodeficiency, liver diseases [67,68]. In the systemic studies conducted by Dond Hwan Lee et al in 2022 on the therapeutic applications of stem cells derived umbilical cord blood on cartilage tissue regeneration, by following the process of cartilage repair inpatients who underwent surgery using mesenchymal stem cells derived from human umbilical cord blood, cartilage repair was significantly higher in patients who were treated with these cells compared to group who were treated with other types of stem cells such as bone marrow, [69].
Fibrin glue is another product derived from blood which has not been studied much. In the studies conducted by Ghiasi and et al on the regeneration of cartilage cells in 2019, they found that the use of hydrogel scaffold made by fibrin, causes significant differentiation of cells in the culture medium into chondrogenic cells [70]. They used natural polymers with high adhesion and biocompatibility based on the matrix in creating hydrogel-shaped engineered scaffolds and showed that this scaffold compared to other materials such as alginate were increase the survival and differentiation of cells and increase the expression of chondrogenic genes such as collagen II and Aggrecan which indicates the greater and better efficiency of this type of scaffold [70]. Or, in other studies that were conducted on the ear cartilage of Canin dogs, it was shown that the use of hydrogel scaffolds obtained from platelet-rich plasma with other stem cells significantly creates normal cartilage in the damaged areas [71]. Researchers have found that the use of blood products can increase the migration of stem cells and growth factors in the damaged area of the cartilage, thus accelerating its regeneration [72].
Conclusion
Although there are many reports of the advantage use of blood production in the field of orthopedics, skin, hair, and vascular applications, there are still many challenges to investigate them. Blood-derived compounds show high variability between patients [73]. In addition, the variety of the blood derivatives is related to some factors such as age, gender, and diseases in patients. Moreover, how these changes affect the behavior of stem cells in vitro, is still largely unstudied. These challenges can be systematically searched and investigated by starting powerful operational analysis. In addition, proper transfer and delivery of blood-derived compounds can have an important effect on tissue regeneration. Specially, the biological characteristics of these materials, such as hardness, degradability, porosity, and bioactivity of compounds derived from blood, are important in their effects on cartilage tissue regeneration processes. Currently, the delivery of these bioactive substances is possible based on their mass release with poor controllability. As a result, long time treatments require other multiple treatments such as a large number of injections. These results are strongly fluctuating and changing according to the concentration of growth factors, which causes disturbances in the prediction of clinical treatments. Biological materials can act as means of controlling the release process, which provides the possibility of stability and need to transfer these multiple compounds of growth factors [74-91]. In addition, it is possible that biological materials can covalently form a special link with growth factors, which will maintain a high level of these molecules locally. Therefore, in general, according to the mentioned materials and the high ability and capability of blood-derived products in repairing and renewing cartilage tissue, and the possibility of easy access and low risk of its use, it can be a potential and inexpensive source for treatment. Cartilaginous lesions are used.
References
- Rackwitz L, Djouad F, Janjanin S, Nöth U, Tuan RS (2014) Functional Cartilage Repair Capacity of De-differentiated, Chondrocyte-and Mesenchymal Stem Cell-Laden Hydrogels In Vitro. Osteoarthr Cartil 22(8): 1148-1157.
- Frisch J, Orth P, Venkatesan JK, Rey RA, Schmitt G, et al. (2017) Genetic Modification of Human Peripheral Blood Aspirates Using Recombinant Adeno-Associated Viral Vectors for Articular Cartilage Repair with a Focus on Chondrogenic Transforming Growth Factor-β Gene Delivery. Stem cells Transl Med 6(1): 249-260.
- Hunziker EB (2002) Articular Cartilage Repair: Basic Science and Clinical Progress A Review of the Current Status and Prospects Osteoarthritic Cartel 10(6): 432-463.
- Orth P, Rey RA, Venkatesan JK, Madry H, Cucchiarini M (2014) Current Perspectives in Stem Cell Research for Knee Cartilage Repair. Stem Cells Cloning 7: 1-17.
- Zhu Y, Fu W (2022) Peripheral Blood-Derived Stem Cells for the Treatment of Cartilage Injuries: A Systematic Review. Front Bioeng Biotechnol 22(10): 956614.
- Jin LH, Choi BH, Kim YJ, Park SR, Jin CZ, et al. (2011) Implantation of bone marrow-derived buffy coat can supplement bone marrow stimulation for articular cartilage repair. Osteoarthritis Cartilage 19(12): 1440-1448.
- Makris EA, Gomoll AH, Malizos KN, Hu JC, Athanasiou KA (2015) Repair and tissue engineering techniques for articular cartilage. Nat Rev Rheumatol 11(1): 21-34.
- Riboh JC, Cvetanovich GL, Cole BJ, Yanke AB (2017) Comparative efficacy of cartilage repair procedures in the knee: a network meta-analysis. Knee Surg Sports Traumatol Arthrosc 25(12): 3786-3799.
- Fu WL, Ao Y, Ke X, Zheng Z, Gong X, et al. (2014) Repair of large full-thickness cartilage defect by activating endogenous peripheral blood stem cells and autologous periosteum flap transplantation combined with patellofemoral realignment. Knee 21(1): 609-612.
- Li X, Ding J, Zhang Z, Yang M, Yu J, et al. (2016) Kartogenin-Incorporated Thermogel Supports Stem Cells for Significant Cartilage Regeneration. ACS Appl Mater Interfaces 2(8): 5148-5159.
- Zhao D, Li Y, Zhou X, Yang Z (2018) Peripheral Blood Mesenchymal Stem Cells Combined with Modified Demineralized Bone Matrix Promote Pig Cartilage Defect Repair. Cells Tissues Organs 206(1-2): 26-34.
- Ding J, Zhang J, Li J, Li D, Xiao C, et al. (2019) Electrospun polymer biomaterials. Prog Polym Sci p. 1-34.
- Wang C, Feng N, Chang F, Wang J, Yuan B, et al. (2019) Injectable Cholesterol-Enhanced Stereocomplex Polylactide Thermogel Loading Chondrocytes for Optimized Cartilage Regeneration. Adv Healthc Mater 8: e1900312.
- Zhang Y, Yu J, Ren K, Zuo J, Ding J, et al. (2019) Thermosensitive Hydrogels as Scaffolds for Cartilage Tissue Engineering. Biomacromolecules 20(4): 1478-1492.
- Alonso SR, Merchan ML, Ciriza J, Burgo LSD, Pedraz JL (2021) Tendon tissue engineering: Cells growth factors scaffolds and production techniques. J Control Release 10(333): 448-486.
- Masoudi E, Ribas J, Kaushik G, Leijten J, Khademhosseini A (2016) Platelet-Rich Blood Derivatives for Stem Cell-Based Tissue Engineering and Regeneration. Curr Stem Cell Rep 2(1): 33-42.
- Ding ZY, Tan Y, Peng Q, Zuo J, Li N (2021) Novel applications of platelet concentrates in tissue regeneration (Review). Exp Ther Med Mar 21(3): 226.
- Dhurat R, Sukesh M (2014) Principles and Methods of Preparation of Platelet-Rich Plasma: A Review and Author's Perspective. J Cutan Aesthet Surg 7(4): 189-197.
- Beigi MH, Atefi A, Ghanaei HR, Labbaf S, Ejeian F, et al. (2018) Activated platelet-rich plasma improves cartilage regeneration using adipose stem cells encapsulated in a 3D alginate scaffold. J Tissue Eng Regen Med 12(6): 1327-1338.
- Tischer T, Bode G, Buhs M, Marquass B, Nehrer S (2020) Platelet-rich plasma (PRP) as therapy for cartilage, tendon and muscle damage - German working group position statement. J Exp Orthop 7(1): 64.
- Giannini S, Buda R, Vannini F, Cavallo M, Grigolo B (2009) One-step bone marrow-derived cell transplantation in talar osteochondral lesions. Clin Orthop Relat Res 467(12): 3307-3320.
- Xie, X, Zhang C, Tuan RS (2014) Biology of platelet-rich plasma and its clinical application in cartilage repair. Arthritis Res Ther 16(1): 204.
- Chen WH, Lo WC, Lee JJ, Su CH, Lin CT, et al. (2006) Tissue-engineered intervertebral disc and chondrogenesis using human nucleus pulposus regulated through TGF-beta1 in platelet-rich plasma. J Cell Physiol 209(3): 744-754.
- Ghiasi M, Farzaneh S, Bigdelo M (2020) Assessment of human cartilage regeneration in a patient with knee osteoarthritis using autologous adipose-tissue-derived stem cells and Platelet-rich plasma: a case study. J Surg Trauma 8(2): 73-78.
- Moreira TLS, Leijten JC, Wennink JW, Chatterjea AG, Feijen J, et al. (2012) The effect of platelet lysate supplementation of a dextran-based hydrogel on cartilage formation. Biomaterials 33(14): 3651-3661.
- Bahmanpour S, Ghasemi M, Sadeghi NM, Ragerdi KI (2016) Effects of Platelet-Rich Plasma & Platelet-Rich Fibrin with and without Stromal Cell-Derived Factor-1 on Repairing Full-Thickness Cartilage Defects in Knees of Rabbits. Iran J Med Sci 41(6): 507-517.
- El Sharkawy H, Kantarci A, Deady J, Hasturk H, Liu H, et al. (2007) Platelet-rich plasma: growth factors and pro- and anti-inflammatory properties. J Periodontol 78(4): 661-669.
- Bendinelli P, Matteucci E, Dogliotti G, Corsi MM, Banfi G, et al. (2010) Molecular basis of anti-inflammatory action of platelet-rich plasma on human chondrocytes: mechanisms of NF-kappaB inhibition via HGF. J Cell Physiol 225(3): 757-766.
- Haleem AM, Singergy AA, Sabry D, Atta HM, Rashed LA, et al. (2010) The Clinical Use of Human Culture-Expanded Autologous Bone Marrow Mesenchymal Stem Cells Transplanted on Platelet-Rich Fibrin Glue in the Treatment of Articular Cartilage Defects: A Pilot Study and Preliminary Results. Cartilage 1(4): 253-261.
- Osterman C, McCarthy MB, Cote MP, Beitzel K, Bradley J, et al. (2015) Platelet-Rich Plasma Increases Anti-inflammatory Markers in a Human Coculture Model for Osteoarthritis. Am J Sports Med 43(6): 1474-1484.
- Choukroun J, Diss A, Simonpieri A, Girard MO, Schoeffler C, et al. (2006) Platelet-rich fibrin (PRF): a second-generation platelet concentrate. Part IV: clinical effects on tissue healing. Oral Surg Oral Med Oral Pathol Oral Radiol Endod 101(3): 56-60.
- Murphy MB, Blashki D, Buchanan RM, Yazdi IK, Ferrari M, et al. (2012) Adult and umbilical cord blood-derived platelet-rich plasma for mesenchymal stem cell proliferation, chemotaxis, and cryo-preservation. Biomaterials 33(21): 5308-5316.
- Andia I, Maffulli N (2013) Platelet-rich plasma for managing pain and inflammation in osteoarthritis. Nat Rev Rheumatol 9(12): 721-730.
- Shao XX, Hutmacher DW, Ho ST, Goh JC, Lee EH (2006) Evaluation of a hybrid scaffold/cell construct in repair of highload- bearing osteochondral defects in rabbits. Biomaterials 27(7): 1071-1080.
- Zhang Y, Ying G, Ren C, Jizhang Y, Brogan D, et al. (2015) Administration of human plateletrich plasma reduces infarction volume and improves motor function in adult rats with focal ischemic stroke. Brain Res 1594: 267-273.
- Mariani E, Filardo G, Canella V, Berlingeri A, Bielli A, et al. (2014) Platelet-rich plasma affects bacterial growth in vitro. Cytotherapy 16: 1294-1304.
- Badade PS, Mahale SA, Panjwani AA, Vaidya PD, Warang AD (2016) Antimicrobial effect of platelet-rich plasma and platelet-rich fibrin. Indian J Dent Res 27(3): 300-304.
- Wu W, Chen F, Liu Y, Ma Q, Mao T (2007) Autologous injectable tissue-engineered cartilage by using platelet-rich plasma: experimental study in a rabbit model. J Oral Maxillofac Surg 65(10): 1951-1957.
- Lee JC, Min HJ, Park HJ, Lee S, Seong SC (2013) Synovial membrane-derived mesenchymal stem cells supported by platelet-rich plasma can repair osteochondral defects in a rabbit model. Arthroscopy 29(6): 1034-1046.
- Anilkumar K, Geetha A, Umasudhakar, Ramakrishnan T, Vijayalakshmi R (2009) Platelet-rich-fibrin: a novel root coverage approach. J Indian Soc Periodontol 13(1): 50-54.
- Ghanaati S, Booms P, Orlowska A, Kubesch A, Lorenz J, et al. (2014) Advanced platelet-rich fibrin: a new concept for engineering by means of inflammatory cells. J Oral Implantol. cell-based tissue 40(6):679-689.
- Christoffersson G, Vågesjö E, Vandooren J, Lidén M, Massena S, et al. (2012) VEGF-A recruits a proangiogenic MMP 9-delivering neutrophil subset that induces angiogenesis in transplanted hypoxic tissue. Blood 120(23): 4653-4662.
- Dohan EDM, Rasmusson L, Albrektsson T (2009) Classification of platelet concentrates: from pure platelet-rich plasma (P-PRP) to leucocyte- and platelet-rich fibrin (L-PRF). Trends Biotechnol 27(3): 158-167.
- Wang M, Li J, Liu J, Lin X, Xu W (2012) The comparison of platelet-rich fibrin and platelet-rich plasma in releasing of growth factors and their effects on the proliferation and differentiation of adipose tissue derived stem cells in vitro. 30(6): 641-649.
- He L, Lin Y, Hu X, Zhang Y, Wu HA (2009) comparative study of platelet-rich fibrin (PRF) and platelet-rich plasma (PRP) on the effect of proliferation and differentiation of rat osteoblasts in vitro. Oral Surg, Oral Med, Oral Pathol, Oral Radiol Endodontol 108(5): 707-713.
- Dohan EDM, De Peppo GM, Doglioli P, Sammartino G (2009) Slow release of growth factors and thrombospondin-1 in Choukroun's platelet-rich fibrin (PRF): a gold standard to achieve for all surgical platelet concentrates technologies. Growth Factors 27(1): 63-69.
- Su CY, Kuo YP, Tseng YH, Su CH, Burnouf T (2009) In vitro release of growth factors from platelet-rich fibrin (PRF): a proposal to optimize the clinical applications of PRF. Oral Surg, Oral Med, Oral Pathol, Oral radiol Endodontics 108(1): 56-61.
- Chien CS, Ho HO, Liang YC, Ko PH, Sheu MT, et al. (2012) Incorporation of exudates of human platelet-rich fibrin gel in biodegradable fibrin scaffolds for tissue engineering of cartilage. J Biomed Mater Res Part B 100(4): 948-955.
- Raouf MA, Wang X, Miusi S, Chai J, Mohamed AAB, et al. (2017) Injectable-platelet rich fibrin using the low speed centrifugation concept improves cartilage regeneration when compared to platelet-rich plasma. Platelets 30(2):213-221.
- Wong CC, Chen CH, Chan WP, Chiu LH, Ho WP, et al. (2017) Single-Stage Cartilage Repair Using Platelet-Rich Fibrin Scaffolds with Autologous Cartilaginous Grafts. Am J Sports Med 45(13): 3128-3142.
- Ghiasi M, Bigdello M, Hamidieh A (2021) The extraordinary efficacy of platelet rich fibrin membrane on fungal infections of nails in a human model. Journal of Basic and Clinical Pathophysiology 9(2): 11-14.
- Sheykhhasan M, Bakhtiari PH, Ghiasi M (2016) Autologous platelet-rich plasma (PRP) for the treatment of pattern hair loss: A review. Jdc 7(3) :169-185.
- Cervelli V, Gentile P, Scioli MG, Grimaldi M, Casciani CU, et al. (2009) Application of platelet-rich plasma in plastic surgery: clinical and in vitro evaluation. Tissue Eng Part C Methods 15(4): 625-634.
- Koh YG, Kwon OR, Kim YS, Choi YJ (2014) Comparative outcomes of open-wedge high tibial osteotomy with platelet-rich plasma alone or in combination with mesenchymal stem cell treatment: a prospective study. Arthroscopy 30(11): 1453-1460.
- Yamada Y, Nakamura S, Ueda M, Ito K (2015) Papilla regeneration by injectable stem cell therapy with regenerative medicine: long-term clinical prognosis. J Tissue Eng Regen Med 993: 305-309.
- Ghiasi M, Mehdizadeh M, Khatibshad L (2022) Designing Nanofiber Multilayer Composite Scaffolds and Lyophilized Blood Growth Factors in the Process of Osteogenesis. J Mazandaran Univ Med Sci 32(210): 1-12.
- Anitua E, Tejero R, Zalduendo MM, Orive G (2013) Plasma rich in growth factors promotes bone tissue regeneration by stimulating proliferation migration, and autocrine secretion in primary human osteoblasts. J Periodontol 84(8): 1180-1190.
- Sánchez M, Azofra J, Anitua E, Andía I, Padilla S, et al. (2003) Plasma rich in growth factors to treat an articular cartilage avulsion: a case report. Med Sci Sports Exerc 35(10):1648-1652.
- Sánchez M, Anitua E, Azofra J, Andía I, Padilla S, et al. (2007) Comparison of surgically repaired Achilles tendon tears using platelet-rich fibrin matrices. Am J Sports Med 35(2): 245-251.
- Anitua E, Prado R, Troya M, Zalduendo M, Fuente M, et al. (2016) Implementation of a more physiological plasma rich in growth factor (PRGF) protocol: anticoagulant removal and reduction in activator concentration. Platelets 27(5): 459-466.
- Vilar JM, Morales M, Santana A (2013) Controlled, blinded force platform analysis of the effect of intraarticular injection of autologous adipose-derived mesenchymal stem cells associated to PRGF-Endoret in osteoarthritic dogs. BMC Veterinary Research 9: 131.
- Kirchner F, Milani I, Martinez A, Kirchner BN, Prado R, et al. (2021) Plasma Rich in Growth Factors (PRGF) in the Treatment of Cervical and Lumbar Back Pain: A Retrospective Observational Clinical Study. Pain Physician 24(5): 649-660.
- Raeissadat SA, Rayegani SM, Ahangar AG, Abadi PH, Mojgani P, et al. (2017) Efficacy of Intra-articular Injection of a Newly Developed Plasma Rich in Growth Factor (PRGF) Versus Hyaluronic Acid on Pain and Function of Patients with Knee Osteoarthritis: A Single-Blinded Randomized Clinical Trial. Clin Med Insights Arthritis Musculoskelet Disord 10: 1179544117733452.
- Ulrich VM, Maloney MD, Schwarz EM, Rosier R, O Keefe RJ (2003) Articular Cartilage Biology. J Am Acad Orthop Surg 11(6): 421-430.
- O Keefe RJ, Crabb ID, Edward PJ, Rosier RN (1994) Effects of transforming growth factor-beta 1 and fibroblast growth factor on DNA synthesis in growth plate chondrocytes are enhanced by insulin-like growth factor-I. J Orthop Res 12(3): 299-310.
- Urashima M, Hoshi Y, Shishikura A, Kamijo M, Kato Y, et al. (1994) Umbilical cord blood as a rich source of immature hematopoietic stem cells. Acta Paediatr Jpn 36(6): 649-655.
- Waller WR (2011) Umbilical cord blood: information for childbirth educators. J Perinat Educ 20(1): 54-60.
- Harris DT (2014) Stem Cell Banking for Regenerative and Personalized Medicine. Biomedicines 2(1): 50-79.
- Lee DH, Kim SA, Song JS, Shetty AA, Kim BH, et al. (2022) Cartilage Regeneration Using Human Umbilical Cord Blood Derived Mesenchymal Stem Cells: A Systematic Review and Meta-Analysis. Medicina (Kaunas) 58(12): 1801.
- Ghiasi M, Mehdizadeh M, Mohammadsharifi A, Nasr G, Bigdelo M, et al. (2019) Use of Mesenchymal Adult Stem Cell for Cartilage Regeneration by Hydrogel. IJML 6(3): 207-218.
- Ghiasi M (2021) The Effects of Allogeneic cADSCs on an Experimental Ear Auricular Defect to Evaluate Cartilage Regeneration in a Canine Model. J Clin Med Res 2(1): 1-11.
- Chen YR, Yan X, Yuan FZ, Ye J, Xu BB (2020) The Use of Peripheral Blood-Derived Stem Cells for Cartilage Repair and Regeneration In Vivo: A Review. Front Pharmacol 11: 404.
- Sarkar MR, Augat P, Shefelbine SJ, Schorlemmer S, Huber LM, et al. (2006) Bone formation in a long bone defect model using a platelet-rich plasma-loaded collagen scaffold. Biomaterials 27(9): 1817-1823.
- Milano G, Deriu L, Sanna PE, Masala G, Manunta A (2012) Repeated platelet concentrate injections enhance reparative response of microfractures in the treatment of chondral defects of the knee: an experimental study in an animal model. Arthroscopy 28(5): 688-701.
- Carmona JU, Arguelles D, Climent F, Prades M, Soler R, et al. (2005) Autologous platelet-rich plasma injected intraarticularly diminished synovial effusion and degree of lameness in horses affected with severe joint disease. Annual Scientific Meeting of the European College of Veterinary Surgeons. Lyon.
- Li M, Zhang C, Ai Z, Yuan T, Feng Y, et al. (2011) Therapeutic effectiveness of intra-knee-articular injection of platelet-rich plasma on knee articular cartilage degeneration 25(10): 1192-1196.
- Naik AR, Ramesh AV, Dwarkanath CD, Naik MS, Chinnappa AB (2013) Use of autologous platelet rich plasma to treat gingival recession in esthetic periodontal surgery. J Indian Soc Periodontol 17(3): 345-353.
- Güler İ, Billur D, Aydin S, Kocatürk S (2015) Efficacy of platelet-rich fibrin matrix on viability of diced cartilage grafts in a rabbit model. Laryngoscope 125(3): 104-111.
- Kazemi D, Shams Asenjan K, Dehdilani N, Parsa H (2017) Canine articular cartilage regeneration using mesenchymal stem cells seeded on platelet rich fibrin: Macroscopic and histological assessments. Bone Joint Res 6(2): 98-107.
- Dietrich F, Duré LG, Klein PC, Bampi FV, Padoin VA, et al. (2015) Platelet-Rich Fibrin Promotes an Accelerated Healing of Achilles Tendon When Compared to Platelet-Rich Plasma in Rat. World J Plast Surg 4(2): 101-109.
- Sheu SY, Wang CH, Pao YH, Fu YT, Liu CH, et al. (2017) The effect of platelet-rich fibrin on autologous osteochondral transplantation: An in vivo porcine model. Knee 24(6): 1392-1401.
- Buda R, Cavallo M, Castagnini F, Cenacchi A, Natali S (2015) Treatment of Hemophilic Ankle Arthropathy with One-Step Arthroscopic Bone Marrow-Derived Cells Transplantation. Cartilage 6(3): 150-155.
- Ahrari F, Keshavarzi F, Bijani A, Jenabian N (2020) Efficacy of Application of Plasma Rich in Growth Factors Along with the Tunnel Technique for Treatment of Gingival Recession: a Clinical Trial. J Dent (Shiraz) 21(4): 275-283.
- Turajane T, Sriratanavudhi C, Saengsirinavin P, Chaweewannakorn U, Lappaiwong W, et al. (2019) Safety and clinical efficacy of platelet rich growth factors (prgf) in managing knee osteoarthritis after failed conservative treatment: evidence from real practices. J Southeast Asian Med Res 3(1): 1.
- Wang SA, Cugat R, Ares O, Seijas R, Cuscó X (2011) Infiltration of plasma rich in growth factors for osteoarthritis of the knee short-term effects on function and quality of life. Arch Orthop Trauma Surg 131(3): 311-317.
- Frazer A, Bunning R, Thavarajah M, Seid J, Russell R (1994) Studies on type II collagen and aggrecan production in human articular chondrocytes in vitro and effects of transforming growth factor-β and interleukin-1β. Osteoarthr Cartil 2(4): 235-245.
- Ujol JP, Chadjichristos C, Legendre F, Bauge C, Beauchef G, et al. (2008) Interleukin-1 and transforming growth factor-beta 1 as crucial factors in osteoarthritic cartilage metabolism. Connect Tissue Res 49(3): 293-297.
- Schmidt MB, Chen EH, Lynch SE (2006) A review of the effects of insulin-like growth factor and platelet derived growth factor on in vivo cartilage healing and repair. Osteoarthr Cartil 14(5): 403-412.
- Martin JA, Buckwalter JA (2000) The role of chondrocyte-matrix interactions in maintaining and repairing articular cartilage. Biorheology 37(1-2): 129-140.
- Titan A, Schär M, Hutchinson I, Demange M, Chen T (2020) Growth Factor Delivery to a Cartilage-Cartilage Interface Using Platelet-Rich Concentrates on a Hyaluronic Acid Scaffold. Arthroscopy 36(5): 1431-1440.
- Pötter N, Westbrock F, Grad S, Alini M, Stoddart MJ, et al. (2021) Evaluation of the influence of platelet-rich plasma (PRP), platelet lysate (PL) and mechanical loading on chondrogenesis in vitro. Sci Rep 11 20188.






























