Multimodal Anti-Inflammatory Approach to Osteoarthritis Management - Review of T Cell Immunomodulation with Undenatured (Native) Collagen Type II, and LOX Inhibition with Boswellia
Rakesh Mahajan1, Niranjan R Rathod2 and Varsha Narayanan3*
1 Director of Orthopaedics, Institute for Bone, Joint Replacement, Orthopaedics Spine & Sports Medicine, BLK Super speciality Hospital, New Delhi
2Consultant Orthopaedic Surgeon, Rathod Orthopaedic Hospital, Borivali, Mumbai
3Chief - Medical Affairs, Integrace Health (Bone Health and Pain Management), Mumbai
Submission: February 08, 2019; Published: February 25, 2019
*Corresponding author: Varsha Narayanan, Integrace Health, Raheja Platinum, Marol, Mumbai 400059
How to cite this article: Rakesh M, Niranjan R R, Varsha N. Multimodal Anti-Inflammatory Approach to Osteoarthritis Management - Review of T Cell Immunomodulation with Undenatured (Native) Collagen Type II and LOX Inhibition with Boswellia. Nov Tech Arthritis Bone Res. 2019; 3(4): 555618. DOI: 10.19080/NTAB.2019.03.555618
Abstract
Osteoarthritis, known to be a degenerative disease, is now seeing increasing evidence of also having an inflammatory pathophysiology involving multiple pathways. T cell mediated immune response to joint cartilage collagen is now one of the recognized pathways causing cartilage destruction. T helper cell activation, involvement of pro-inflammatory cytokines and increased destruction of cartilage by Matrix Metalloproteinase enzymes, along with a relative suppression of anti-inflammatory cytokines and T regulatory cells appear to be important inflammatory mechanisms perpetuating continuous cartilage loss. Conversion of Phospholipids released from tissue injury to arachidonate and thereafter to prostaglandins and leukotrienes by the Cyclo-oxygenase 2 (COX2) and Lipo-oxygenase 5 (5LOX) enzymes respectively, is also well known.
While COX2 inhibition via Non-steroidal anti-inflammatory drugs is well established in Osteoarthritis, the role of Leukotrienes in increasing inflammatory activity and cartilage destruction requiring appropriate inhibition needs further emphasis. Therefore, controlling cartilage destruction in Osteoarthritis through addressing these multiple inflammatory pathways can be a rational therapeutic approach. This article aims to elaborate on the pharmacotherapeutic approaches involving modulating T cell immune response to cartilage and inhibiting the LOX pathways by Undenatured (Native) Collagen Type II and Boswellia extract respectively.
Keywords: Osteoarthritis; Cartilage; Undenatured (Native) Collagen Type II; Boswellia; T Cell; COX; LOX; Leukotrienes; Matrix Metalloproteinase; Cytokines
Introduction
Osteoarthritis (OA) has traditionally been a degenerative disease caused by long term and repeated wear and tear of the cartilage of weight bearing joints leading to loss of cartilage followed by exposure and damage to underling bone surfaces [1]. The joint hyaline cartilage is made up of Chondrocytes which synthesize and maintain the main components of cartilage: Type II Collagen and Extracellular Matrix (ECM) Proteoglycans containing Glycosaminoglycans (GAGs) like Chondroitin sulphate and Hyaluronic acid [2]. Chondrocytes also produce lubricating proteins like Lubricin in the synovial surfaces and fluid. Loss and destruction of cartilage leads to decreased number of chondrocytes and thereby also a decrease in synthesis of Collagen type II and GAGs. Therefore, in Osteoarthritis, an overall imbalance due to excess cartilage loss and deficient synthesis sets in [3].
Inflammatory Pathways in Osteoarthritis
Recent concepts in Osteoarthritis have shown that inflammation in the joint as a result of T cell mediated immune response plays an important role in ongoing cartilage destruction as well as symptoms of pain, swelling and loss of mobility. Studies have shown increased number of CD4 cells in the serum and synovial fluid of OA patients [4]. Response to autologous chondrocytes of peripheral T cells isolated from OA patients is greater than that of peripheral T cells isolated from controls, and this response is partially blocked by antibodies against human leukocyte antigen (HLA) classes I and II, CD4, and CD8 [5]. T cells in the synovial fluid of OA patients expressed class II HLA (an indicator of activated T cells) and the percentages of CD4+ and CD8+ cells in the syno vial fluid of OA patients were even found to be like those found in RA patients [6]. Synovial aggregates from OA patients express CD80, an inducible costimulatory ligand involved in T-cell activation suggesting that synovial aggregates in OA patients are areas of antigen recognition and T-cell activation [7].
T cell mediated immune response is postulated to be as a result of glycosylation alterations in joint cartilage collagen, and this T cell activation leads to increase in pro-inflammatory cytokines like IL1, IL6, and TNF alpha, which activate matrix metalloproteinase (MMP) and cause further destruction of cartilage [8-9]. Consequently, there is a decrease in T regulatory cell action and reduction in anti-inflammatory cytokines (IL10 and TGF beta) which induce T regulatory differentiation and suppress MMPs [10-11]. Cartilage destruction leads to release of phospholipids converted to arachidonic acid by phospholipases which are then converted by Cyclo-oxygenase (COX2) and Lipo-oxygenase (5LOX) to prostaglandins and leukotrienes respectively. The inflammatory actions of prostaglandins (PGE2) in causing pain, swelling and increase in temperature are well studied and therefore NSAIDs (COX inhibitors) are the most recognized drugs in OA management [12]. However, the LOX pathway also plays an important role in the inflammatory response in OA [13].
The 5LOX enzymes specifically act on arachidonate to convert them to Leukotrienes (LTs). Leukotrienes (like LTB4 and cysteinyl Leukotrienes) lead to increase in pro-inflammatory cytokines. LTB4 enhances the production of interleukin (IL)-1 and Tumour necrosis factor (TNF)-α which activate MMP mediated cartilage destruction. LTs also activate Interstitial Cell Adhesion Molecules (ICAMs) which act as potent chemotactic agents stimulating migration and activation of T cells and macrophages, leading to phagocytic activity and release of Reactive Oxygen Species (ROS), which further mediate cartilage destruction [14-16]. These properties of LTB4, similar to PG E2, also play a role in stimulating bone resorption in OA [17]. Therefore, cartilage destruction and loss in OA is a result of an interplay of multiple inflammatory pathways, and this should be taken into consideration during therapeutic choices for managing pain and inflammation in OA (Figure 1).
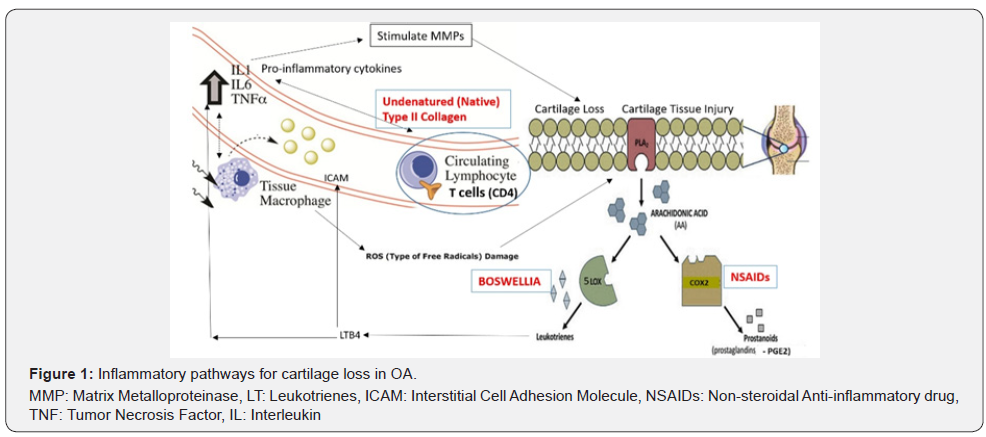
Therapeutic Approaches in OA
Current non-surgical treatment options in OA consist of Non-steroidal anti-inflammatory drugs (NSAIDs) which inhibit COX2 thereby reducing pain and inflammatory tissue injury caused by PGE2. NSAIDs though effective, have long term limitations of gastric side effects due to also inhibiting COX1, requiring co-use of proton pump inhibitors (PPIs) also associated with long term safety concerns. NSAIDs also do not address the aetiology of continued T cell immune mediated or LOX mediated cartilage destruction. Inhibiting only COX pathways with NSAIDs can increase Leukotriene synthesis through diverting arachidonate through the LOX5 pathway.18 Drugs like Corticosteroids act by Phospholipase inhibition which may address both prostaglandin and leukotriene synthesis however they are generalized immune-suppressants as against the requirement of specific immune-suppression of T cell response against cartilage collagen II in OA [19-20].
Corticosteroids are also associated with systemic side effects and patient tolerance concerns. Diacerein, an anti-inflammatory agent acts by suppressing one of the pro-inflammatory cytokines IL1[21]. Hydrolysed collagen supplements and SYSADOAs- Systemic slow acting drugs in Osteoarthritis (GAGs and their precursors like Chondroitin sulphate, Glucosamine sulphate and Hyaluronic acid) act by providing building blocks for regenerating cartilage. These agents do not present any patient tolerance or side effect issues but rely on the body’s ability to assimilate these supplements to regenerate lost cartilage [22]. This proves not only to be a slow approach but also far less effective as these agents do not act upon arresting the ongoing immune mediated and in flammatory cartilage destruction [23].Therefore, there is a need to address both symptom relief as well as control ongoing cartilage destruction in a multiprong manner in OA patients so that the body can then regenerate and rebuild cartilage more effectively.
Undenatured (Native) Collagen Type II
The joint cartilage is made up predominantly of type II collagen synthesized by chondrocytes. Collagen is a complex protein present only in animals and humans, with a triple helical structure. Our body cannot absorb intact collagen therefore collagen supplements are made up of heat denatured and acid hydrolysed collagen with the prospect of absorption by the body and further assimilation into the triple helical collagen type II by the joint cartilage chondrocytes [24]. However, the absorption and assimilation of hydrolysed collagen would be an uphill task against on-going immune mediated inflammatory destruction of cartilage.
Undenatured (Native) Collagen Type II -U(N)C II is non-hydrolysed collagen with intact structure, active epitopes and antigenicity while hydrolysed collagen loses its native structure and antigenicity [25-26]. Undenatured type II collagen is extracted from chicken sternum using little or no heat unlike the high heat used in hydrolysed collagen, and very limited processing just enough to concentrate the collagen and make it soluble [27]. The manufacturing process ensures that the collagen remains biologically active in its most native, triple helix form with antigenic sites (epitopes) intact for immunomodulation. ELISA studies with simulated human gastric fluid at 32 degrees C and pH 2 have shown maintenance of triple helical form of Undenatured Type II Collagen.
Therefore, the mechanism of action of Undenatured (Native) Collagen Type II is not related to its absorption or assimilation as it acts in the small intestine itself through a process called oral tolerance (Figure 2) to help slow down the T cell mediated inflammatory damage of type II collagen in the joint cartilage [25]. Payer’s patches (also called (GALT- Gut-associated lymphoid tissue) are GI lymph nodes rich in T cells. Dendritic cells (DCs) in GALT take up the Undenatured (Native) Collagen Type II in its glycosylated form, and repeated low dose administration of the same with its intact antigenic sites stimulates T regulatory cell and suppresses T helper cell stimulation [28].
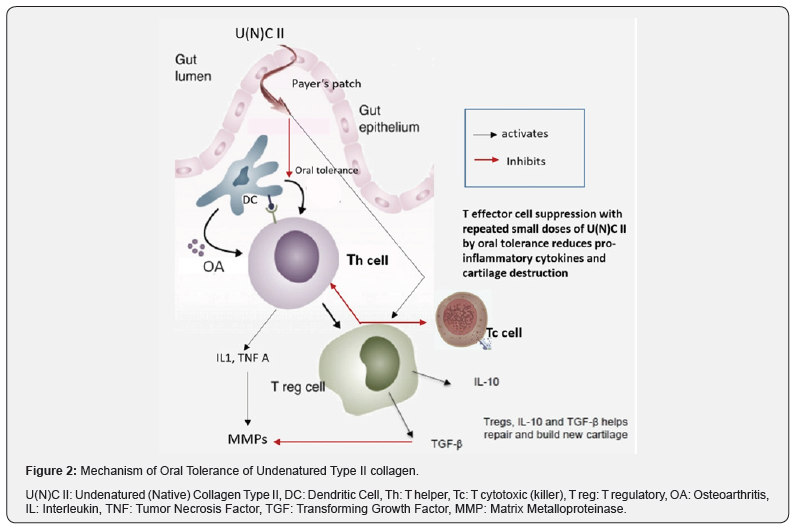
T helper cells (Th) develop ‘tolerance’ thereby attenuating the immune response against cartilage collagen. The immune balance thereby shifts towards T regulatory cells (Treg) and increased anti-inflammatory cytokines like IL 10 and TGF Beta which suppresses the cartilage collagen degrading MMP enzymes [29]. Therefore, endogenous collagen synthesis can be more effective in the light of collagen destruction and inflammatory response being suppressed. Understanding the difference between undenatured (native) and hydrolysed collagen supplements attains crucial importance as Undenatured (Native) Collagen Type II is required in very small quantities and acts by the process of oral tolerance to specifically reduce T cell mediated inflammatory immune response against cartilage collagen type II, while hydrolysed collagen needs to be supplemented in large doses (often along with GAGs and its precursors (like Chondroitin sulphate, Glucosamine sulphate etc) as it intends to supply building blocks for chondrocytes to assimilate and rebuild cartilage [24].
The distinction lies clearly in Undenatured (Native) Collagen Type II reducing cartilage destruction while hydrolysed collagen intending to help regenerate cartilage. However, our body is already constantly rebuilding cartilage but the efficiency of the same is limited and falls short in view of senescence and increased ongoing cartilage destruction.
Efficacy and Safety of Undenatured (Native) Collagen Type II in OA
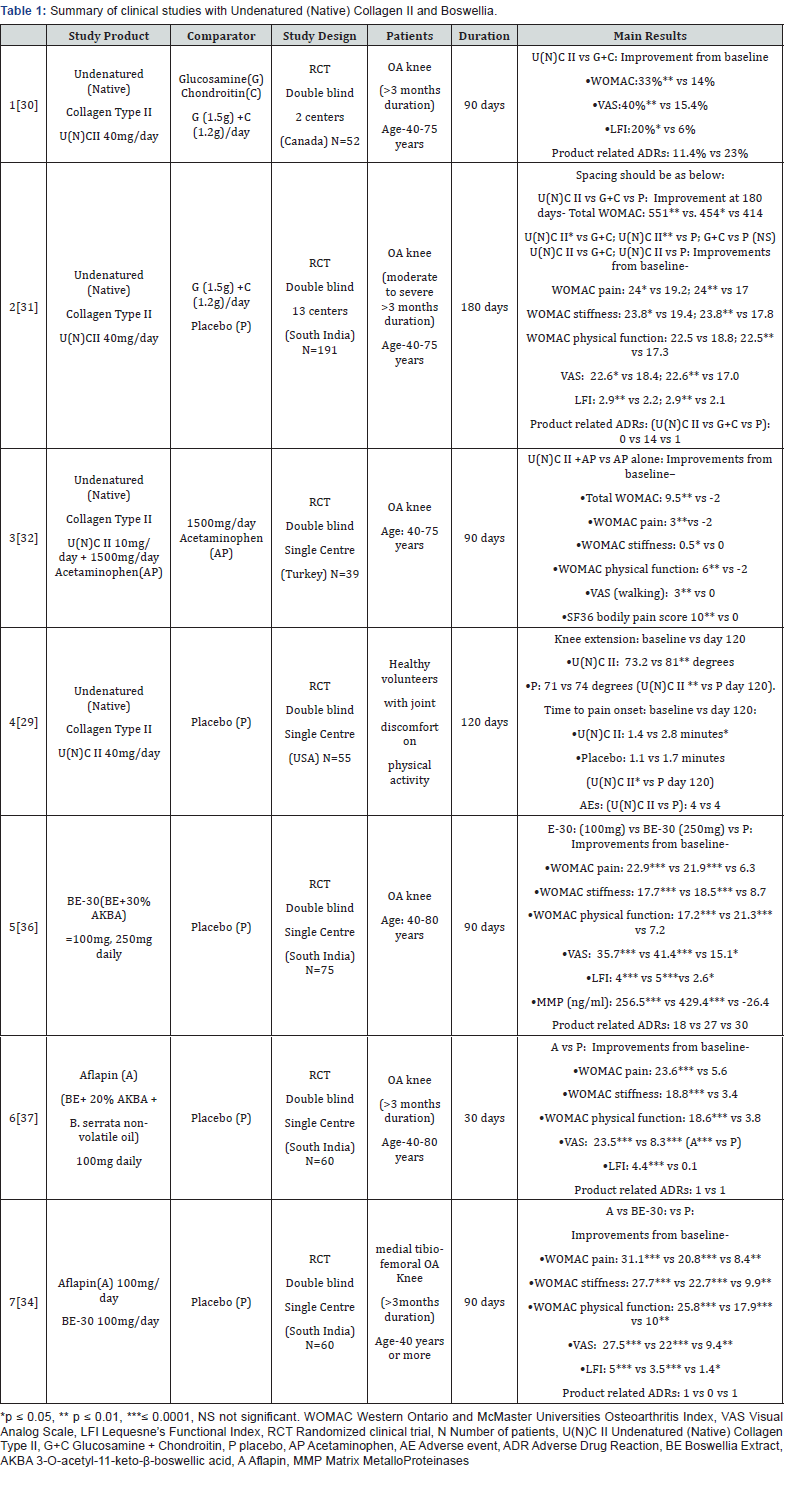
The efficacy and tolerance of Undenatured (Native) Collagen Type II - U(N)C II (40mg) in Osteoarthritis has been demonstrated in various clinical studies (Table 1). In the first study versus combination of Glucosamine and Chondroitin sulphate (G+C: 1500+1200 mg/day), U(N)C II treatment was more efficacious resulting in a significant reduction in all assessments from the baseline at 90 days which was not observed in G+C treatment group (N=26/group). Treatment with U(N)C II reduced the Western Ontario and McMaster Universities Osteoarthritis Index (WOMAC score), Visual Analog Scale (VAS) score and Lequesne’s Functional Index (LFI) score by 33%, 40%, 20% as compared to 14%, 15.4% and 6% in G+C treated group after 90 days suggesting a more significant reduction in pain and enhancement in daily activities and quality of life [30]. A higher number of subjects (23%) on G+C demonstrated adverse events possibly related to product as compared to 11.4% of subjects on U(N)C II (P<0.05). For U(N) C II the possible adverse events related to products were intermittent constipation and headaches while for G+C it was bloating, stomach pain, rash, swelling around the eyes and scars, hives and headache.
In the second study 191 volunteers were randomized into three groups receiving a daily dose of U(N)C II (40 mg), G+C (1500 mg G+1200 mg C), or placebo. At day 180, the U(N)C II group demonstrated a significant reduction in overall WOMAC score compared to placebo and G+C: U(N)C II compared to placebo ( -551 vs. -414; 95 % CI -232 to -42; p = 0.002) and compared to G+C ( -551 vs. -454; 95 % CI -190 to -3; p = 0.04). Supplementation with U(N)C II also resulted in significant changes for pain and stiffness WOMAC subscales versus G+C and placebo: pain (24 vs 19.2 vs 17 ; p = 0.016 vs. G+C; p = 0.0003 vs. placebo;); stiffness (23.8 vs 19.4 vs 17.8; p=0.044 vs. G+C ; p = 0.004 vs. placebo); and significant changes for physical function versus placebo (22.5 vs 17.3; p = 0.007 vs. placebo).
The U(N)C II supplemented group had a significant decrease in mean VAS score at day 180 versus both G+C and placebo (22.6 vs 18.4 vs 17.0; p = 0.025 vs G+C; p = 0.002vs placebo). A significant reduction was also observed in the LFI score for the U(N) C II group at day 180 versus G+C and placebo (2.9 vs 2.2 vs 2.1; p = 0.008 vs G+C; p = 0.009; vs placebo). No significant change was observed between the G+C and placebo for both VAS and LFI scores. Safety outcomes did not differ among the groups. U(N)C II improved knee joint symptoms in knee OA subjects and was well-tolerated [31]. 15 product related adverse events were seen, 14 of which belonged to the GC group (gastro-intestinal) and 1 to placebo while no AEs noted for the U(N)C II cohort were deemed to be product related.
Another study in 39 patients was performed to evaluate the effect of adding Undenatured (Native) Collagen Type II to Acetaminophen (Paracetamol) 1500mg/day [32]. After 3 months of treatment, the patients of the combination group showed significant improvements in VAS walking (p<0.001), WOMAC pain (p=0.003), WOMAC total (p=0.004), WOMAC physical functioning (p=0.016) scores and subscales of SF36 bodily pain score (p=0.016) while the same was not significant with the group receiving only Acetaminophen. Comparisons between the groups revealed a significant difference in VAS walking score in favour of the combination group (50% reduction) as compared to the Acetaminophen monotherapy group (p=0.002). A study done in non-arthritic healthy volunteers who had knee pain on physical activity showed significantly better response to Undenatured (Native) Collagen Type II versus placebo in increasing degree of knee extension and time to pain onset on physical activity [29].
Boswellia
Boswellia serrata is a tree from India also called Indian frankincense, Salai or Indian Olibanum. Boswellic Acids (BAs) with the characteristic pentacyclic triterpene ring, present in the gum resin of B. serrata are the main constituents having anti-inflammatory properties as they specifically inhibit 5LOX enzymes responsible for leukotriene production, without affecting other LOX and COX activities. 3-O-acetyl-11-keto-β-Boswellic acid (AKBA) possesses the most potent inhibitory activity on 5LOX [33]. Aflapin is a preparation that contains B. serrata extract selectively enriched with AKBA and B. serrata non-volatile oil and has shown superior efficacy as an anti-inflammatory, chondroprotective and anti- osteoarthritic agent, with better oral bioavailability compared to B. serrata extracts (BEs) commercially available in the market [34-35]. Inhibition of 5LOX and Leukotriene formation reduce the levels of pro-inflammatory mediators IL1, TNF alpha and ICAM, leading to decreased cartilage destruction by MMPs, increased synthesis of GAGs, decreased chemotaxis of inflammatory cells and overall tilting the balance in favour of cartilage regeneration over cartilage loss.
Efficacy and Safety of Boswellia extract in OA
The efficacy and safety of Boswellia have been shown in various clinical studies (Table 1). A double-blind, randomized, study in 75 OA patients with Boswellia (BE-30 containing 30% AKBA) 100mg, 250mg) and placebo once daily showed clinically significant improvements in pain scores (VAS score) and physical function scores (Lequesne Functional Index- LFI) as compared to placebo with both Boswellia dosage groups at 90 days, with the 250-mg dose showing improvements in pain score and functional ability in 7 days after the start of treatment [36]. Both doses of BE- 30 also showed a significant fall in MMP enzymes while the same showed a rise in placebo group.
Another double blind randomized study with Boswellia (50mg BID Aflapin : Encapsulated B. serrata extract selectively enriched with 20% AKBA and B. serrata specific non-volatile oil) in 60 patients with mild or moderate unilateral or bilateral OA of at least 3 months duration, showed clinically significant improvements with 37.6%, 32.0%, 40.1%, 41.3% and 38.8% reductions in VAS, LFI, WOMAC pain, WOMAC stiffness and WOMAC function scores respectively over the placebo group. Improvement with Alfapin was early as 5 days. No significant changes in blood, urine or laboratory parameters were seen [37].
A third study compared the efficacy of both the above Boswellia preparations (Aflapin and BE-30 both 100mg/day against placebo) in 60 subjects with OA. Both groups showed clinically significant improvements in pain scores and physical function scores versus placebo [34]. In comparison to placebo, the improvements in in VAS, LFI, WOMAC pain, WOMAC stiffness and WOMAC functional ability scores, were better in Aflapin group as compared to BE30 with Aflapin group showing improvements of 47.3% (P<0.0001), 35.8% (P=0.0004), 61.7% (P<0.0001), 60.1% (P=0.0001) and 49.4% (P=0.0001) as compared to 31.6% (P=0.006), 18.35% (P=0.060), 30.3% (P=0.009), 42.2% (p=0.006) and 21.25% (P=0.078) respectively.
Both groups showed significant improvement in pain score and functional ability as early as 7 days of treatment with better results for Aflapin over BE-30. After 7 days, the BE-30 treatment group exhibited 8.09% (P=0.002), 8.68% (P=0.031) and 8.35% (p=0.015) reductions in VAS, WOMAC pain and WOMAC function respectively over baseline scores while Aflapin treatment group exhibited 12.8% (P=0.0004), 9.17% (P=0.003), 11.78% (P=0.012), 18.48% (P=0.012) and 10.24% (p=0.005) reductions in VAS, LFI WOMAC pain, WOMAC stiffness and WOMAC function scores respectively, over baseline scores. Aflapin and BE-30 were seen to significantly reduce TNF-α level by 65.04% and 38.83% respectively and Aflapin reduced TNF alpha induced ICAM1 expression significantly and more than BE-30 [32-34]. Aflapin also inhibited MMP enzymes better than BE-30 by 41.36% [33].
Conclusion
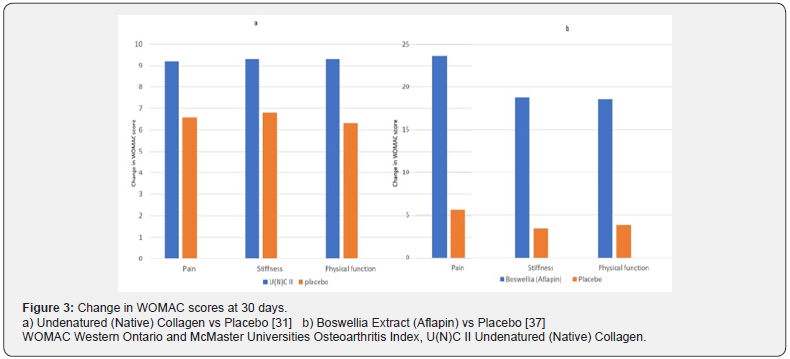
Cartilage destruction in Osteoarthritis involves multiple Inflammatory pathways like COX2 action to produce Prostaglandins, 5LOX action producing Leukotrienes and T cell activation in response to altered Collagen II glycosylation in hyaline cartilage. The resulting activation of inflammatory mediators like IL1, TNF alpha and ICAM, increases the action of MMP enzymes which degrade cartilage, and leads to recruiting more inflammatory T cells and macrophages leading to continuation of inflammatory damage to cartilage. Therefore, a multimodal therapeutic approach seems rational with agents which can inhibit inflammatory pathways at multiple levels. While NSAIDs act on the COX pathway, Undenatured (Native) Collagen Type II by its mechanism of oral tolerance attenuates inflammatory T cell response and activates T regulatory cells, which reduces cartilage damage. Boswellia acts by inhibiting the LOX pathway and thereby further reduces the action of inflammatory mediators. Therefore, using these agents together can be an effective way of reducing cartilage damage, and increasing cartilage regeneration. Initiating treatment with combination therapies like U(N)C II with BE for early symptomatic relief and rapid reduction in inflammation(Figure 3), and then sustaining prolonged immune tolerance with U(N)C II alone can also be a possible approach. More studies with combination of these agents will add further value to this therapeutic approach.
References
- Haq I, Murphy E, Dacre J (2003) Osteoarthritis- Review. Postgrad Med J 79(933): 377-383.
- Goldring MB (2012) Chondrogenesis, chondrocyte differentiation, and articular cartilage metabolism in health and osteoarthritis. Ther Adv Musculoskelet Dis 4(4): 269-285.
- Maldonado M, Nam J (2013) The Role of Changes in Extracellular Matrix of Cartilage in the Presence of Inflammation on the Pathology of Osteoarthritis. Biomed Res Int 2013: 284873.
- Li Ys, Luo W, Zhu Sa, Lei GH (2017) T Cells in Osteoarthritis: Alterations and Beyond. Front. Immunol 8: 356.
- Sakata M, Masuko Hongo K, Nakamura H, Onuma H, Tsuruha JI, et al. (2003) Osteoarthritic articular chondrocytes stimulate autologous T cell responses in vitro. Clin Exp Rheumatol 21(6): 704-710.
- Hussein MR, Fathi NA, El Din AM, Hassan HI, Abdullah F, et al. (2008) Alterations of the CD4(+), CD8 (+) T cell subsets, interleukins-1beta, IL-10, IL-17, tumor necrosis factor-alpha and soluble intercellular adhesion molecule-1 in rheumatoid arthritis and osteoarthritis: preliminary observations. Pathol Oncol Res 14(3): 321-328.
- Haynes MK, Hume EL, Smith JB (2002) Phenotypic characterization of inflammatory cells from osteoarthritic synovium and synovial fluids. Clin Immunol 105(3): 315-325.
- Zhang JM, An J (2007) "Cytokines, inflammation, and pain". International Anesthesiology Clinics. 45 (2): 27-37.
- Wassilew GI, Lehnigk U, Duda GN, Taylor WR, Matziolis G, et al. (2010) The Expression of Proinflammatory Cytokines and Matrix Metalloproteinases in the Synovial Membranes of Patients with Osteoarthritis Compared with Traumatic Knee Disorders. Arthroscopy: The Journal of Arthroscopic & Related Surgery 26(8): 1096-1104.
- Carter NA, Rosser EC, Mauri C (2012) Interleukin-10 produced by B cells is crucial for the suppression of Th17/Th1 responses, induction of T regulatory type 1 cells and reduction of collagen-induced arthritis. Arthritis Research & Therapy 14(1): R32.
- Wan YY, Flavell RA. TGF-Beta and Regulatory T Cells. Regulatory T Cells and Clinical Application pp 91-109.
- Ricciotti E, FitzGerald GA (2011) Prostaglandins and Inflammation. Arterioscler Thromb Vasc Biol. 31(5): 986-1000.
- Attur M, Dave M, Abramson SB, Amin A (2012) Activation of diverse eicosanoid pathways in osteoarthritic cartilage: a lipidomic and genomic analysis. Bull NYU Hosp Jt Dis 70(2): 99-108.
- Martel Pelletier J, Mineau F, Fahmi H, Laufer S, Reboul P, et al. (2004) Regulation of the expression of 5-lipoxygenase-activating protein/5-lipoxygenase and the synthesis of leukotriene B (4) in osteoarthritic chondrocytes: role of transforming growth factor beta and eicosanoids. Arthritis Rheum 50(12): 3925-3933.
- He W, Pelletier JP, Martel-Pelletier J, Laufer S, Di Battista JA (2002) Synthesis of interleukin 1beta, tumor necrosis factor-alpha, and interstitial collagenase (MMP-1) is eicosanoid dependent in human osteoarthritis synovial membrane explants: interactions with anti-inflammatory cytokines. J Rheumatol 29(3): 546-553.
- Kurokouchi K, Kambe F, Yasukawa K, Izumi R, Ishiguro N, et al. (1998) TNF-alpha increases expression of IL-6 and ICAM-1 genes through activation of NF-kappa B in osteoblast-like ROS17/2.8 cells. J Bone Miner Res. 13(8): 1290-1299.
- Garcia C, Boyce BF, Gilles J, Dallas M, Qiao M, et al. (1996) Leukotriene B4 stimulates osteoclastic bone resorption both in vitro and in vivo. J Bone Miner Res. 11(11): 1619-1627.
- Jackson SE, Holloway JW, Warner JA, Sampson AP (2012) Interleukin-13, but Not Indomethacin, Increases Cysteinyl-Leukotriene Synthesis in Human Lung Macrophages. J Allergy (Cairo) 2012: 348741.
- Kragballe K (1989) Topical Corticosteroids: Mechanism of Action. Acta Derm Venereol Suppl (Stockh). 151: 7-10; discussion 47-52.
- Auphan N, DiDonato JA, Rosette C, Helmberg A, Karin M, et al. (1995) Immunosuppression by glucocorticoids: inhibition of NF-kappa B activity through induction of I kappa B synthesis. Science 270(5234): 286-290.
- Medhi B, Singh PK, Prakash A, Sen R, Wadhwa S, et al. (2007) Diacerein: A New Disease Modulating Agent in Osteoarthritis. IJPMR 18(2): 48-55
- Ullal SD, Narendranath SD, Kamath RK, Pai MRSM, Kamath SU, et al. (2019) Prescribing Pattern for Osteoarthritis in A Tertiary Care Hospital. Journal of Clinical and Diagnostic Research 4(3): 2421-2426.
- Steinmeyer J (2000) Pharmacological Basis for the Therapy of Osteoarthritis. In: Grifka J, Ogilvie-Harris DJ (eds) Osteoarthritis. Springer Pp 54-65.
- Abraham (2017) Collagen for Joint Health. Supplements in Review.
- Bagchi D, Misner B, Bagchi M, Kothari SC, Downs BW, et al. (2002) Effects of Orally Administered Undenatured Type II Collagen against Arthritic Inflammatory Diseases: A mechanistic exploration. Int J Clin Pharm Res 22(3-4): 101-110.
- Trentham DE, Halpner AE, Trentham RA, Bagchi M, Kothari S, et al. (2001) Use of Undenatured Type II Collagen in the treatment of Rheumatoid Arthritis. Clinical Practice of Alternative Medicine 2(4): 254-259.
- James P Lugo, Zainulabedin M Saiyed, Francis C Lau, Jhanna Pamela L Molina, Michael N Pakdaman, et al. (2013) Undenatured type II collagen (UC-II®) for joint support: a randomized, double-blind, placebo-controlled study in healthy volunteers J Int Soc Sports Nutr 10(1): 48.
- Park KS, Park MJ, Cho, Kwok SK, Ju JH, et al. (2009) Type II collagen oral tolerance; mechanism and role in collagen-induced arthritis and rheumatoid arthritis Mod Rheumatol 19(6): 581-589.
- Lugo PJ, Saiyed ZM, Lau FC, Molina JPL, Pakdaman M, et al. (2013) Undenatured type II collagen (U(N)C II II®) for joint support: a randomized, double-blind, placebo-controlled study in healthy volunteers. J Int Soc Sports Nutr 10: 48.
- Crowley DC, Lau FC, Sharma P, Evans M, Guthrie N, et al. (2009) Safety and efficacy of undenatured type II collagen in the treatment of osteoarthritis of the knee: a clinical trial. International Journal of Medical Sciences 6(6): 312-321.
- Lugo JP, Saiyed ZM, Lane NE (2016) Efficacy and tolerability of an undenatured type II collagen supplement in modulating knee osteoarthritis symptoms: a multicenter randomized, double-blind, placebo-controlled study. Nutrition Journal 15:14.
- Bakilan F, Armagan O, Ozgen M, Tascioglu F, Bolluk O, et al. (2016) Effects of Native Type II Collagen Treatment on Knee. Osteoarthritis: A Randomized Controlled Trial. Eurasian J Med 48(2): 95-101.
- Suva MA, Kheni DB, Sureja VP (2018) Aflapin: A novel and selective 5-lipoxygenase inhibitor for arthritis management. Indian J Pain 32(1): 16-23.
- Sengupta K, Krishnaraju AV, Vishal AA, Mishra A, Trimurtulu G, et al. (2010) Comparative efficacy and tolerability of 5-Loxin and Aflapin against osteoarthritis of the knee: a double blind, randomized, placebo controlled clinical study. Int J Med Sci 7(6): 366-377.
- Roy S, Khanna S, Shah H, Rink C, Phillips C, Preuss H, et al. (2005) Human genome screen to identify the genetic basis of the anti-inflammatory effects of Boswellia in microvascular endothelial cells. DNA Cell Biol 24(4): 244-255.
- Sengupta K, Alluri KV, Satish AR, Mishra S, Golakoti T, et al. (2008) A double blind, randomized, placebo-controlled study of the efficacy and safety of BE-30 for treatment of osteoarthritis of the knee. Arthr Res Ther 10(4): R85.
- Vishal AA, Mishra A, Raychaudhuri SP (2011) A double blind, randomized, placebo controlled clinical study evaluates the early efficacy of Aflapin in subjects with osteoarthritis of knee. Int J Med Sci 8(7): 615-622.






























