The Osteoarthritis-Associated Catabolic Factor, Interleukin-1β, Down Regulates Chondrocyte Energy Metabolism by Modulating Adenosine Monophosphate-Activated Protein Kinase in OA
Naoko Yui1, Takanori Kumai1, Hajime Kobayashi1, Kamada Toshikazu2, Hiroto Fujiya1, Satoshi Kishiro3, Kenji Uehara3, Hisateru Niki3 and Kazuo Yudoh4*
1Department of Sports Medicine, St. Marianna University School of Medicine, Japan
2Department of Orthopedic Surgery and Rheumatology, Hara Orthopedic Hospital, Japan
3Department of Orthopaedic Surgery, St. Marianna University School of Medicine, Japan
4 Department of Frontier Medicine, Institute of Medical Science, St. Marianna University School of Medicine, Japan
Submission: April 28, 2018; Published: May 22, 2018
*Corresponding author: Kazuo Yudoh, MD & PhD, Department of Frontier medicine, Institute of Medical Science, St. Marianna University School of Medicine, Japan.
How to cite this article: Naoko Y, Takanori K, Hajime K, Kamada T, Kazuo Y. The Osteoarthritis-Associated Catabolic Factor, Interleukin-1β, Down Regulates Chondrocyte Energy Metabolism by Modulating Adenosine Monophosphate-Activated Protein Kinase in OA. Nov Tech Arthritis Bone Res. 2018; 3(1): 555601. DOI: 10.19080/NTAB.2018.03.555601
Abstract
Objective:: The proinflammatory cytokine interleukin (IL)-1β is well known as a catabolic factor, inducing the degeneration of articular cartilage in osteoarthritis (OA). Numerous reports have already demonstrated that IL-1β alters chondrocyte activity and induces the progression of OA. However, few studies have examined how IL-1β affects chondrocyte energy metabolism in OA. To investigate this, we studied chondrocyte energy metabolism and its related regulatory mechanism in OA chondrocytes.
Methods:Human chondrocytes were isolated from samples of articular cartilage from patients with OA. The level of energy metabolism of OA chondrocytes was monitored based on the activity of the energy metabolic sensor, adenosine monophosphate-activated protein kinase (AMPK) and the level of production of adenosine triphosphate (ATP) in cultured chondrocytes in the presence or absence of IL-1β (10ng/ml). Effects of IL-1β on anabolic and catabolic activities of chondrocytes were analysed by levels of productions of the articular cartilage component, proteoglycan, and the marker of chondrocyte hypertrophy, matrix metalloproteinase (MMP)-13, respectively, in OA chondrocytes.
Results: Exposure to IL-1β significantly inhibited the activity of AMPK and production of ATP in OA chondrocytes. After an incubation period of at least 2 hours, the level of AMPK significantly decreased in the IL-1β-treated chondrocytes in comparison with the control chondrocytes. Treatment with IL-1β also significantly decreased proteoglycan production but significantly increased MMP-13 secretion by chondrocytes.
Conclusion: Our present study indicates that the OA-related catabolic factor IL-1β inhibits the AMPK-ATP energy metabolic pathway and the resultant cellular activity in OA chondrocytes.
Keywords: Energy metabolism; Adenosine Monophosphate-activated protein kinase; Adenosine Triphosphate; Chondrocyte; Osteoarthritis
Abbrevations: OA: Osteoarthritis; AMPK: Adenosine Monophosphate-Activated Protein Kinase; ATP: Adenosine triphosphate; MMP: Matrix Metalloproteinase; ADP: Adenosine Diphosphate; PBS: phosphate-buffered saline; DMEM: Dulbecco’s modified Eagle’s medium; HEPES: (2[4-(2-hydroxyethyl)-1-piperazinyl]
Introduction
Osteoarthritis (OA) is the most prevalent joint disease and has a complex pathogenesis and pathophysiology [1,2]. There is a consensus that the mechanical forces acting on articular cartilage, together with obesity, inflammation and aging are the critical catabolic factors in OA [3,4]. It has been demonstrated that both extrinsic and intrinsic catabolic stresses exerted on articular cartilage matrix and chondrocytes may be causal factors for the catabolic progression in OA [5,6]. The pro inflammatory cytokine interleukin (IL)-1β is well known as an important catabolic factor inducing the degeneration of articular cartilage, altering chondrocyte activity and inducing the progression of OA [4,6]. However, few studies have identified how OA-associated factors such as IL-1β, mechanical and chemical stresses affect chondrocyte energy metabolism in OA articular cartilage. We postulate that the change of chondrocyte energy metabolism during the progression of OA may down regulate chondrocyte activity and cartilage homeostasis, resulting in cartilage degradation. OA-related catabolic factors may disrupt cellular energy metabolism in cartilage, which may result in cellular catabolic stress, facilitating its degeneration.
To further understand how IL-1β induces the progression of OA, we focused on chondrocyte energy metabolism and its related regulatory mechanisms. Adenosine monophosphate-activated protein kinase (AMPK) is well recognized as an energy sensor monitoring the cellular ratios of adenosine monophosphate (AMP) to adenosine triphosphate (ATP) and/or adenosine diphosphate (ADP) to ATP [7,8]. Once switched on by various factors that increase these ratios, it activates catabolic pathways that generate ATP while switching off nonessential ATP-requiring processes, thus acting to restore energy homeostasis [7,9]. It has been demonstrated that AMPK is activated by intrinsic and extrinsic cellular stresses (such as nutrient deprivation, hypoxia, mechanical and chemical stresses) that increase the cellular AMP-ATP or ADP-ATP ratios [10,11]. Thus, it is now thought that AMPK may allow cells to adjust to changes in cellular energy demand. Chondrocytes are well-adapted to an avascular and hypoxic cellular microenvironment and to changing metabolic demands in response to inflammation, aging, mechanical and chemical stresses [12-16]. We postulated that the activity of AMPK and its regulated energy metabolism in chondrocytes may be influenced by OA-related catabolic stresses, such as IL-1β. In this study, we tested our hypothesis using human chondrocytes stimulated with IL-1β, and found that IL-1β profoundly disrupted chondrocyte energy metabolism, possibly via a mechanism involving interactions between ATP production and AMPK. Reduced activity of AMPK in OA chondrocytes may limit energy availability for chondrocyte/cartilage maintenance and homeostasis. We discuss the effect of IL-1β on energy metabolism through the regulation of the AMPK-ATP energy metabolic pathway.
Materials and Methods
Human Chondrocyte Cultures
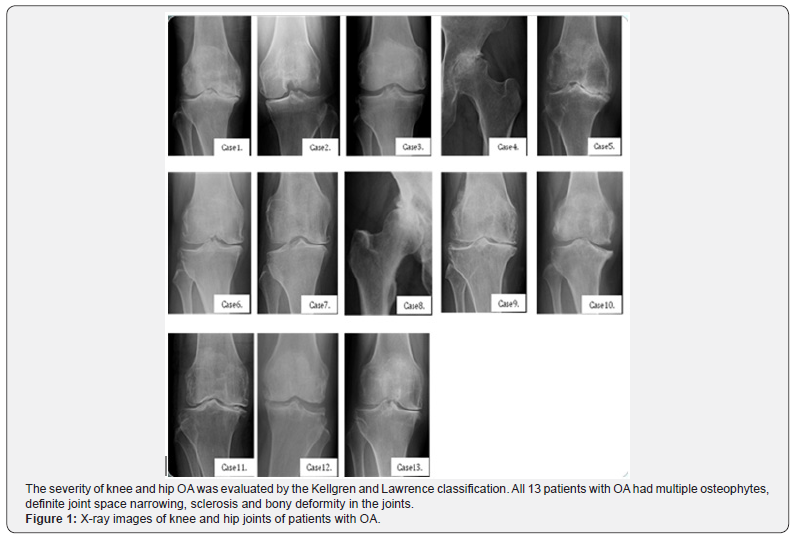
Human articular cartilage tissue samples were obtained from the joints of 13 patients with OA during arthroplastic knee and hip surgery after obtaining informed consent [mean age of 69.07 years (58-81 years)]. The protocol of this study was approved by the ethical committee of St. Marianna University School of Medicine (permission number: 1315). The clinical features of patients involved in the current study are summarized in Table 1. Representative pre-operative X-ray features of patients are shown in Figure 1. The severity of knee and hip OA was evaluated by the Kellgren and Lawrence classification [17].

Articular cartilage explants were cut into small pieces, washed with phosphate-buffered saline (PBS) and digested with 1.5mg/mL collagenase B (Sigma, St. Louis, MO, USA) in Dulbecco’s modified Eagle’s medium (DMEM) (Sigma) overnight on a shaking platform at 37 °C. Isolated chondrocytes were collected following centrifugation, washed three times with PBS, resuspended and cultured in DMEM supplemented with 10% heat-inactivated foetal calf serum (FCS), 2mM L-glutamine, 25mM HEPES (2[4-(2-hydroxyethyl)-1-piperazinyl] ethane sulfonic acid), and 100U/mL penicillin and streptomycin at 37 °C in a humidified atmosphere of 95% air and 5% CO2 as previously reported [18-20].
Effects of il-1β on Energy Metabolism in OA Chondrocytes
To study the effects of IL-1β on energy metabolism, we examined levels of ATP and AMPK production in chondrocytes in vitro. OA chondrocytes from patient number 1, 2, 3, 4, 5, 6 were used to analyse the production of ATP and from patient number 8, 9, 10, 11, 12, 13 were used to analyse the activity of AMPK in chondrocytes. Cultured chondrocytes were incubated in the presence or absence of IL-1β (10.0ng/mL) for 1, 2, 3, 4, 5, 6, 7, 8, 10, 12, 18, 24 hours at 37 °C in a humidified atmosphere of 95% air and 5% CO2. At the end of the culture period, the conditioned culture medium was collected and stored at -80 °C until analysis. The level of ATP production by chondrocytes was examined using an intracellular ATP assay kit (Toyo B-NET co., Ltd., Tokyo, Japan). The levels of AMPK in chondrocytes were measured using an AMPK assay kit (Thermo Fisher Scientific K.K., Kanagawa, Japan).
Effects of il-1β on Proteoglycan and mmp-13 Production in OA Chondrocytes
To study the effects of IL-1β on chondrocyte activity, we examined levels of production of the articular cartilage matrix component, proteoglycan, and the marker of chondrocyte hypertrophy, matrix metalloproteinase (MMP)-13, in chondrocytes in-vitro. OA chondrocytes from patient number 8,10,11,12,13 were used to analyse levels of production of proteoglycan and from patient number 3,4,6,7 were used to analyse levels of production of MMP-13 in chondrocytes. As described above, cultured chondrocytes were incubated with IL- 1β (10.0 ng/mL) for 1, 2, 3, 4, 5, 6, 7, 8, 10, 12, 18, 24 hours. The level of proteoglycan in chondrocytes was examined by ELISA (DIA source PG-EASIA kit, KAP1461, DIA source Immuno Assays S.A.). The levels of MMP-13 produced by chondrocytes were measured using an ELISA kit (MMP-13 assay kit; Amersham Biosciences, Little Chalfont, UK).
Statistical Analysis
Data were evaluated using Student’s test. Results are presented as mean ± 95% confidence interval. Analysis of variance was used for comparisons of more than two groups, and differences between two groups within the set were analysed by a Fisher’s protected least-significant difference test. Probability values of <0.05 were considered statistically significant.
Results
Severity of knee OA
The clinical features of the patients studied are summarized in Table 1. All 13 patients with OA had multiple osteophytes, definite joint space narrowing, sclerosis and bony deformity in the joints (Figure 1). According to the Kellgren and Lawrence system for classification of severity of OA, 1 patient with OA was classified as grade 2, 5 patients as grade 3 and 7 patients as grade 4 (Table 1).
IL-1β Regulates the Production of ATP and Activity of the Energy Metabolic Sensor AMPK in Human Chondrocytes from Patients with OA
After an incubation period of 24 hours, the level of ATP production by chondrocytes was significantly reduced in the IL-1β-stimulated group (P = 0.027 compared to control, Figure 2A). Regarding the level of ATP production in the presence of IL- 1β, there were few changes during 24 hours, and no significant difference was observed in the ATP production at the incubation period between 1 hour and 24 hour (Figure 2B). In addition, after an incubation period of 24 hours, the level of AMPK activity in chondrocytes was significantly inhibited in the IL-1β-stimulated group (P = 0.046 compared to control, Figure 3A).
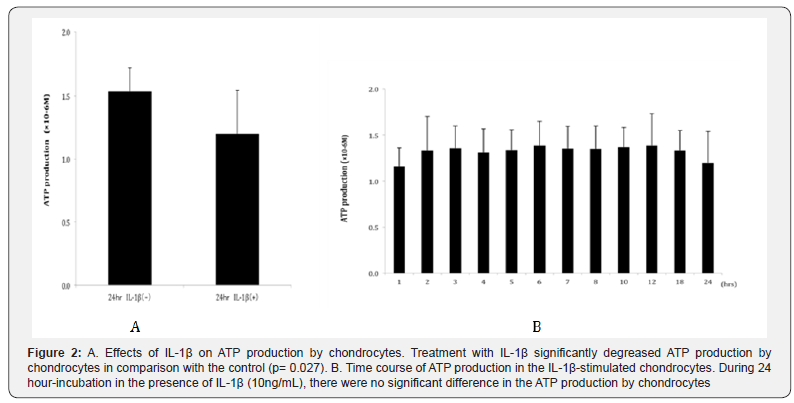

During 24 hours, the level of AMPK activity significantly decreased after an incubation period of 2 hours and more in the presence of IL-1β, indicating the tendency that AMPK activity gradually decreased by IL-1β stimulation. The level of AMPK activity after 24 hour-incubation was observed significantly lower than after 1 hour-incubation (Figure 3B, P=0.027). These findings indicate that the OA-associated catabolic factor, IL- 1β, may reduce the level of the energy sensor, AMPK, resulting in the downregulation of ATP production by chondrocytes. This suggests that energy metabolism is downregulated in osteoarthritic chondrocytes in comparison with control chondrocytes.
Effects of IL-1β on the Productions of Proteoglycan and MMP-13 by Human Chondrocytes from Patients with OA:
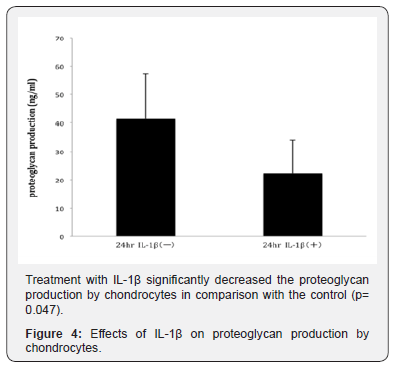
The production of proteoglycan by osteoarthritic chondrocytes was significantly decreased by treatment with IL- 1β (P = 0.047; control group vs. IL-1β-treated group, Figure 4).
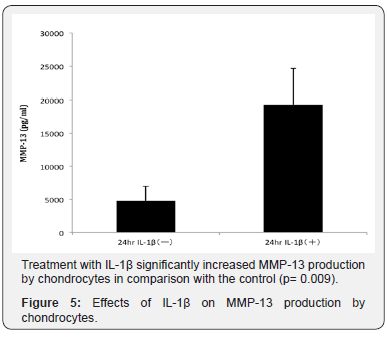
Meanwhile the production of MMP-13 by chondrocytes was significantly increased by IL-1β stimulation (P = 0.009 compared to control, Figure 5).
Discussion
Our present study indicates that the OA-associated catabolic factor IL-1β decreases the level of the energy sensor, AMPK, in OA chondrocytes. In addition, IL-1β causes chondrocytes to reduce the production of ATP. AMPK is known to be the energy sensor that regulates cellular energy metabolism [7,8,20,21]. Once activated, AMPK responds by phosphorylating downstream targets, which allow activation of the ATP production pathway [9]. Our findings suggest that the AMPK-ATP energy metabolic pathway in chondrocytes may be reduced by OA-related factors during disease progression. Previous studies also suggested that the activity of AMPK in chondrocytes may be reduced in aged human and mouse OA cartilage [11,22]. Our finding of the decrease in the level of the AMPK-ATP energy metabolic pathway in OA chondrocytes is consistent with their findings. From the results of our present study, we conclude that the OA-induced catabolic factor IL-1β could lead to the downregulation of AMPK and its related energy production (ATP) by chondrocytes.
This change in energy metabolism may result in an imbalance between anabolic and catabolic responses to OA-related catabolic stresses and cause progression of the cartilage degradation in OA. Thus AMPK, as an energy sensor, plays a critically-important role in maintaining articular chondrocytes and consequently cartilage. IL-1β also caused chondrocytes to decrease production of a key component of articular cartilage, proteoglycan, indicating that IL-1β induced downregulation of maintenance of articular cartilage components in OA. Our findings of IL-1β- induced reduction in energy metabolism in chondrocytes may contribute to the decreased level of proteoglycan production by chondrocytes. Our study also indicates that IL-1β causes increased secretion of MMP-13 by chondrocytes. MMP-13 is well known as a marker of chondrocyte hypertrophy, degeneration state linked with OA progression [23,24]. Although further studies are needed to clarify the correlation between energy metabolism and the level of MMP-13 in OA chondrocytes, the change of chondrocyte energy metabolism may cause chondrocyte hypertrophy and influence cartilage homeostasis with the advance of OA. Bandow et al. demonstrated that AMPK activation inhibited gene expression of sox6, sox9, col2a1 and aggrecan core protein, suggesting that AMPK activity negatively regulates chondrocyte differentiation [25].
Conclusion
In conclusion, our present study indicates that the OArelated catabolic factor, IL-1β, inhibits the AMPK-ATP energy metabolic pathway in OA chondrocytes. Our findings also show that IL-1β causes chondrocytes to decrease the production of the cartilage matrix component, proteoglycan, and the secretion of the marker of chondrocyte hypertrophy, MMP-13. Reduced activity of AMPK in chondrocytes may limit energy availability for chondrocyte/cartilage maintenance in OA. IL-1β could affect energy balance and homeostasis in chondrocytes/cartilage, resulting in the progression of OA.
References
- Sellam J, Berenbaum F (2010) The role of synovitis in pathophysiology and clinical symptoms of osteoarthritis. Nat Rev Rheumatol 6(11): 625-635.
- Loeser RF, Goldring SR, Scanzello CR, Goldring MB (2012) Osteoarthritis: a disease of the joint as an organ. Arthritis Rheum 64(6): 1697-1707.
- Martin JA, Buckwalter JA (2002) Aging, articular cartilage chondrocyte senescence and osteoarthritis. Biogerontology 3(5): 257‐264.
- Liu Bryan R (2015) Inflammation and intracellular metabolism: new targets in OA. Osteoarthritis Cartilage 23(11): 1835-1842.
- June RK, Liu-Bryan R, Long F, Griffin TM (2016) Emerging role of metabolic signaling in synovial joint remodeling and osteoarthritis. J Orthop Res 34(12): 2048-2058
- Berenbaum F, Griffin TM, Liu Bryan R (2017) Metabolic Regulation of Inflammation in Osteoarthritis. Arthritis Rheumatol 69(1): 9-21.
- Witczak CA, Sharoff CG, Goodyear LJ (2008) AMP-activated protein kinase in skeletal muscle: from structure and localization to its role as a master regulator of cellular metabolism. Cell Mol Life Sci 65(23): 3737-3755.
- Steinberg GR, Kemp BE (2009) AMPK in health and disease. Physiol Rev 89: 1025-1078.
- Jensen TE, Wojtaszewski JF, Richter EA (2009) AMP-activated protein kinase in contraction regulation of skeletal muscle metabolism: necessary and/or sufficient? Acta Physiol (Oxf) 196(1): 155-174.
- Salminen A, Kaarniranta K (2012) AMP-activated protein kinase (AMPK) controls the aging process via an integrated signaling network. Ageing Res Rev 11(2): 230-241.
- Terkeltaub R, Yang B, Lotz M, Liu-Bryan R (2011) Chondrocyte AMPactivated protein kinase activity suppresses matrix degradation responses to inflammatory cytokines IL-1β and TNFα. Arthritis Rheum 63(7): 1928-1937.
- Pfander D, Gelse K (2007) Hypoxia and osteoarthritis: how chondrocytes survive hypoxic environments. Curr Opin Rheumatol 19(5): 457-462
- Murphy CL, Thoms BL, Vaghjiani RJ, Lafont JE (2009) Hypoxia. HIFmediated articular chondrocyte function: prospects for cartilage repair. Arthritis Res Ther 11(1): 213.
- Gibson JS, Milner PI, White R, Fairfax TP, Wilkins RJ (2008) Oxygen and reactive oxygen species in articular cartilage: modulators of ionic homeostasis. Pflugers Arch 455(4): 563-573.
- Henrotin YE, Bruckner P, Pujol JP (2003) The role of reactive oxygen species in homeostasis and degradation of cartilage. Osteoarthritis Cartilage 11(10): 747-755
- Loeser RF (2009) Aging and osteoarthritis: the role of chondrocyte senescence and aging changes in the cartilage matrix. Osteoarthritis Cartilage 17(8): 971-979.
- Brandt KD, Fife RS, Braunstein EM, Katz B (1991) Radiographic grading of the severity of knee osteoarthritis: relation of the Kellgren and Lawrence grade to a grade based on joint space narrowing, and correlation with arthroscopic evidence of articular cartilage degeneration. Arthritis Rheum 34(11): 1381-1386.
- Yui N, Yoshioka H, Fujiya H, Musha H, Beppu M, et al. (2014) The DNA repair enzyme apurinic/apyrimidinic endonuclease (Apex nuclease) 2 has the potential to protect against down-regulation of chondrocyte activity in osteoarthritis. Int J Mol Sci 15(9): 14921-14934.
- Terauchi K, Kobayashi H, Yatabe K, Yui N, Fujiya H, et al. (2016) The NAD-Dependent Deacetylase Sirtuin-1 Regulates the Expression of Osteogenic Transcriptional Activator Runt-Related Transcription Factor 2 (Runx2) and Production of Matrix Metalloproteinase (MMP)- 13 in Chondrocytes in Osteoarthritis. Int J Mol Sci 17(7).
- Cantó C, Auwerx J (2009) PGC-1alpha, SIRT1 and AMPK, an energy sensing network that controls energy expenditure. Curr Opin Lipidol 20(2): 98-105.
- Cantó C, Gerhart-Hines Z, Feige JN, Lagouge M, Noriega L, et al. (2009) AMPK regulates energy expenditure by modulating NAD+ metabolism and SIRT1 activity. Nature 458(7241): 1056-1060.
- Petursson F, Husa M, June R, Lotz M, Terkeltaub R, et al. (2013) Linked decreases in liver kinase B1 and AMP-activated protein kinase activity modulate matrix catabolic responses to biomechanical injury in chondrocytes. Arthritis Res Ther 15(4): 77.
- Neuhold LA, Killar L, Zhao W, Sung ML, Warner L, et al. (2001) Postnatal expression in hyaline cartilage of constitutively active human collagenase-13 (MMP-13) induces osteoarthritis in mice. J Clin Invest 107(1): 35-44.
- Kamekura S, Kawasaki Y, Hoshi, K, Shimoaka T, Chikuda H, et al. (2012) Contribution of runt-related transcription factor 2 to the pathogenesis of osteoarthritis in mice after induction of knee joint instability. Arthritis Rheum 54(8): 2462-2470.
- Bandow K, Kusuyama J, Kakimoto K, Ohnishi T, Matsuguchi T (2015) AMP-activated protein kinase (AMPK) activity negatively regulates chondrogenic differentiation. Bone 74: 125-133.






























