Cell-to-Cell Interactions of Activated T Cells with Adjacent Cells in Inflamed Tissue in Inflammatory Diseases
Kazuo Yudoh1*, Naoko Yui2, Takanori Kumai2 and Rie Karasawa1
1Department of Frontier Medicine, St. Marianna University School of Medicine, Japan
2Department of Sports Medicine, St. Marianna University School of Medicine, Japan
Submission: November 24, 2017; Published: December 12, 2017
*Corresponding author: Kazuo Yudoh, Department of Frontier Medicine, Institute of Medical Science, St. Marianna University School of Medicine, Kawasaki, Japan, Tel: 044-977-8111, Email: yudo@mariannau.ac.jp
How to cite this article: Kazuo Y, Naoko Y, Takanori K, Rie K. TCell-to-Cell Interactions of Activated T Cells with Adjacent Cells in Inflamed Tissue in Inflammatory Diseases. Nov Tech Arthritis Bone Res. 2017; 2(3) : 555588. DOI: 10.19080/NTAB.2017.02.555588
Abstract
Importance of inflammatory cytokine cascade has been pointed out in the pathogenesis of arthritis. Recent clinical studies have already shown the benefit in suppressing proinflammatory cytokines in arthritis, indicating the central role of those cytokines, particularly tumor necrosis factor (TNF)- a and interleukin (IL)-6, in the progression of bone and joint deterioration. Several candidate pathways are currently being investigated in the pathogenesis of joint deterioration. In consequence, considerable interest has arisen in those pathways that in turn regulate proinflammatory cytokine productions, because they may offer further possible clinical trial against bone and joint diseases.
Recently, the attention has been attracted the cell-to-cell interaction that drive proinflammatory cytokine productions, metalloproteinase secretions, and the differentiation of bone resorbing osteoclasts. The cell-to-cell membrane contact may have a crucial important role in the progression of inflammation/arthritis; synovial hyperplasia: infiltration of inflammatory cells into the tissue; bone resorption; and cartilage degeneration.
It has been reported that the direct cell membrane contact between synovial T cells, adjacent macrophages and synovial fibroblasts induces the production of proinflammatory cytokines from macrophages and synovial fibroblasts. Also, recent reports indicating the role of receptor activator of NF-kB (RANK) signaling pathway through T cell-osteoclast precursor cell contact in the maturation of osteoclast have revealed the mechanism of bone and joint destruction in arthritis. More recently, we have demonstrated that the cell-cell interactions between activated T cells and chondrocytes may induce articular cartilage destruction through the mechanism involving the acceleration of proteolytic enzyme secretion, suggesting the T cell-mediated destruction of articular cartilage through pathways dependent on cell contact.
Recent advances in our understanding of the events ongoing in inflamed joint tissues; synovial membrane, bone and cartilage tissue, have shed a light to the elucidation of mechanisms of joint destruction in arthritis. We here reviewed the potential implication of "cell-to-cell interactions” in the joint deterioration in arthritis. These novel pathways may represent exciting potential therapeutic targets.
Abbreviation: TNF- α : Tumor Necrosis Factor- α; IL: Interleukin; PI-3 kinase: Phosphatidylinositol-3 kinase; NFkB: Nuclear Factor kB; MMPs: Metalloproteases; IFN: Interferon; RANK: Receptor Activator of NF-kB
Opinion
Inflammatory arthritis such as rheumatoid arthritis (RA) is characterized by the synovial hyperplasia, neoangiogenesis, the infiltrating lymphocytes and macrophages, and bone and articular destruction [1,2]. A variety of cytokines and chemokines that are mainly produced from synovial infiltrating T cells, macrophages and synovial fibroblasts form a network and deeply participate in the pathogenesis of arthritis. Recent clinical and basic intervention studies have shown the therapeutic effectiveness of the agents, which specifically inhibit the activity of proinflammatory cytokines [3,4]. The predominance of macrophage-derived cytokines, particularly TNF- α, interleukin (IL)-6 and IL-18 in the hierarchy of synovial inflammation has been clearly demonstrated in several recent clinical studies, indicating that those inflammatory cytokines are closely involved in the development of arthritis [4,5].
It has been also revealed that macrophage-derived cytokines are closely involved in the osteoclastogenesis and degeneration of articular cartilage, indicating its implication in the articular degeneration in arthritis [3-6]. The factors that promote such macrophage activation could represent effective therapeutic targets, however, the mechanisms whereby macrophages participate in the proinflammatory cytokine productions remain still unknown in arthritis.
It becomes clear that not only the relationship between individual cytokines and their target cells but also the cellular cross-talk may be involved in the cytokine networks and the pathogenesis of inflammatory arthritis [3,5,7]. In inflamed synovial tissue, numerous numbers of lymphocytes and macrophages are infiltrated and form cellular aggregates [8,9]. The aggregates have a part as a germline center in the synovial tissue. McInnes et al. demonstrated that RA synovial fibroblasts show enhanced production of proinflammatory cytokines in response to the direct cell membrane contact between T cells and synovial fibroblasts through specific adhesion molecules, suggesting the importance of "cell contact induced inflammation” in synovitis/arthritis [3,5,10]. We have also elucidated the regulatory mechanism whereby the direct cell contact contributes to the production of macrophage-derived cytokines, TNF-α and IL-1 [11]. IL-15 has a stimulatory effect on the cell contact-induced production of proinflammatory cytokines by macrophages [10,12]. Our findings clearly demonstrated that IL-18 also accelerates the macrophage-derived cytokines productions through the direct cell membrane contact [11]. Also, we have studied the synovial infiltrating T cell-derived destructions of bone and articular cartilage tissues though the mechanism involving cell-cell contact pathway.
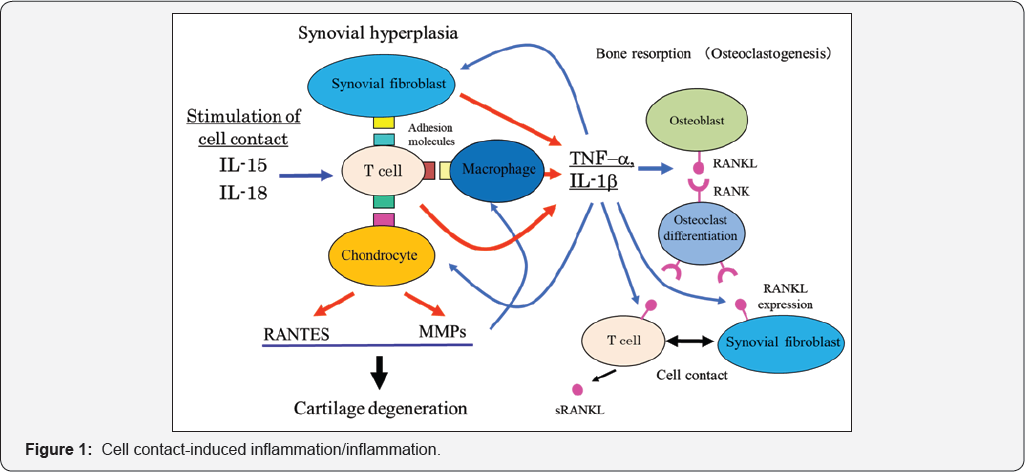
Recent advances in our understanding of the evidences ongoing in inflamed synovial membrane have revealed that synovial infiltrating T cells may drive inflammation, osteoclastogenesis, and destruction of articular cartilage through pathways dependent on cell-to-cell contact [1,3,4,5,1013]. We have reviewed the mechanism of tissue deterioration in inflammatory diseases from the point of view of the cell-to-cell interactions and the resultant deterioration of inflamed tissue/ organ in arthritis (Figure 1).
Characteristics of Inflamed Synovial Membrane
Inflammatory synovial membrane is characterized by hyperplasia of synovial cells, especially synovial fibroblasts type B cells, neoangiogenesis, and infiltration of T cells and macrophages in the synovium [1-3]. It is well known that the synovitis is mainly regulated by cooperation of various cytokines and chemokines [1-5]. Inflamed synovial membrane has higher levels of proinflammatory cytokines, TNF-α, IL-1, IL-15, IL-18 and IL-21, and lower level of inhibitory cytokines, IL-4 and IL-10 expressions [1-3,6-10-13]. Compatible with these observations, higher level of NFkB translocation is evident in RA than in osteoarthritis arthritis synovium. Further distinctions in the neoangiogenesis and the expression of matrix degrading proteolytic enzymes have also been described [14,15]. These studies are important, because they may prove informative as to those immune pathways in synovial membrane that represent common pathways to articular destruction as opposed to those that more closely reflect underlying potentially distinct causes.
Cytokines and proteases are mainly produced from synovial infiltrating macrophages and synovial fibroblasts [1,2,3,5]. TNF-α and IL-1 act on macrophages and T cells as autocrine/ paracrine factors and can induce their secretions each other. Thus, recent studies have paid attention to the mechanism whereby macrophages and synovial fibroblasts are activated for productions of secretary factors.
In inflamed synovial tissue, infiltrating T cells and macrophages lie in juxtaposition within and adjacent to cellular aggregates in the synovial tissue in arthritis, providing for reciprocal cellular cross-talk [5]. It has attracted attention that the cell-to-cell contact of T cells with macrophages or synovial fibroblasts induces the production of proinflammatory cytokines with no requirement of other cytokine stimulation [10,11]. Since there is a variety number of infiltrating T cells, macrophages as well as synovial fibroblasts in the synovial tissue, direct cell contact-induced cytokine productions could have a crucial important role in the progression of synovitis. The observation indicated that a fundamental property of synovial infiltrating T cells may be the activation of adjacent cells mediated by cell membrane contact.
We have postulated that the cell-to-cell interaction may modulate not only inflammation but also bone and cartilage destruction through the osteoclastogenesis and proteolytic enzyme production that are induced by the cell membrane contact between T cells and osteoclast precursors or between T cells and chondrocytes.
Interaction of T Cells and Macrophages with Endothelial Cells and Inflammation
Numerous reports have already demonstrated that interactions of T cells and macrophages with vascular endothelial cells play an important role in the pathogenesis of inflammation [16-18]. Circulating macrophages and T cells can attach the vascular endothelial cells and migrate from the circulatory system to an extravascular compartment such as inflammatory organs. T cell- or macrophage-attachment, invasion, and subsequent degradation of the subendothelial extracellular matrix are crucial important steps to develop the inflammation. It is thought that the ability of activated T cells/ macrophages of the immune system to attach and invade the vascular endothelium and to degrade extracellular matrix are very similar to that reported for highly metastatic tumor cells.
cell-macrophage contact and proinflammatory cytokines
Proinflammatory cytokines, especially TNF-α and IL-1, are mainly produced from macrophages. The levels of cytokine production are lesser from T cells than from macrophages. It is very important to elucidate the mechanism whereby infiltrating T cells induce cytokine productions from macrophages in arthritis.
McInnes et al. [3] demonstrated that the cell membrane contact of T cells with macrophages or synovial fibroblasts induce the progression of proinflammatory cytokines [3,5,10]. The cell contact is thought to induce the production of proinflammatory cytokines with no stimulation by other cytokines and growth factors. These findings indicate that the direct cell contact of the synovial infiltrating T cells with adjacent macrophages in the synovial tissue accelerates the inflammation in synovitis. Furthermore, it has been reported that the cell contact-mediated inflammation is enhanced by IL-15 [12,13,19]. IL-15 stimulated the expression of CD69 or ICAM-1 on the cell surface of activated T cells and their adhesion molecules-mediated membrane contact between T cells and macrophages accelerate the production of proinflammatory cytokines by macrophages [13,19]. Based on the properties of the cell contact induced inflammation, to date, it has been considered the inhibitory effect of neutralizing antibody for IL-15 against spondilytis or arthritis [19].
It has been reported that IL-17 or IL-18 with NF k B activity is closely involved in the pathogenesis of arthritis [11,20,21]. We observed the high levels of these cytokines in the synovial fluid of patient with RA. It has been also reported that IL-18 induces the production of IFN- γ from activated T cells and differentiation of T-helper type 1 cells, suggesting the potential implication of the cytokines in the pathogenesis of arthritis [21,22]. Our findings suggest that IL-18 has a potential to modulate the direct cellular contact-induced production of proinflammatory cytokines, TNF-α and IL-1, from macrophages in synovitis. T cell-macrophage contact also accelerates the expression of IL-18 receptor and IL-18 itself in macrophages, suggesting that IL-18 acts on as an autocrine factor for the cell membrane contact- mediated inflammation [11]. In arthritis, cell contact of synovial infiltrating T cells with macrophages may induce the secretion of proinflammatory cytokines, IL-6, IL-8, IL-15, IL-17 and IL-18 as well as TNF- a and IL-1, in the synovial tissue. These cytokines secretions through the cell contact form the cytokine network and participate in the pathogenesis of arthritis. We have found that the cell contact-induced inflammation is mediated by the activation of NF kB and PI-3 kinase of macrophages [11]. Specific inhibitors of both signal transductions completely inhibited the cell contact-mediated production of proinflammatory cytokines in vitro. Based on the properties of cell-to-cell interactions in arthritis, we have demonstrated the inhibitory effects of IL-18 neutralizing antibody on the cell contact-mediated inflammation in arthritis. Inhibition of the cell contact may have a potential to develop a novel therapeutic strategy to arthritis.
Regarding the inhibition of T cell-monocyte contact-induced production of inflammatory cytokines, it has been reported that serum factor displays an inhibitory effect on the cell contact- mediated activation of monocytes as a homeostatic system. Hyka N. et al. [23] demonstrated that high density lipoprotein- associated apolipoprotein A-I inhibits contact-mediated activation of monocytes by binding activated T cells, inhibiting production of TNF-α and IFN- β . These findings suggest that anti-inflammatory effect of serum factor, apolipoprotein A-I, may lead to therapeutic approaches in inflammatory diseases.
T cell-synovial fibroblast contact and arthritis
It has been demonstrated that T cells can modulate the activity of a variety of cell types through cell contact [1,4,11-13]. Interactions between T cells and synovial fibroblasts result in reduced synthesis of collagen type II and increased production of MMPs, suggesting that the cell contact may be a crucial means of promoting articular cartilage degeneration. Identification of those ligands mediating T cell-synovial fibroblast interactions is of crucial importance. We have also found that accelerated productions of proinflammatory cytokines from activated synovial fibroblasts through the direct contact with T cells [unpublished data]. These findings provide evidence to support that interactions of synovial fibroblasts with T cells could contribute significantly to the development of arthritis.
Adhesion molecules have been implicated in a number of interactions between T cells and synovial fibroblasts. Intercellular adhesion molecule (ICAM)-1, leukocyte function antigen-1, CD40L, or CD69 have been proposed as adhesion molecules promoting the cell contact [1,4,11,23]. Since the adhesion molecule can mediate the cell-cell interactions, such molecules represent legitimate therapeutic targets has been proposed. Previous reports demonstrated that IL-18 enhanced the expression of VCAM-1, ICAM-1 and E-selectin on the cell surface of synovial fibroblasts [24,25]. We also found that the blocking antibodies to the ligands of VCAM-1 or ICAM-1 inhibited the stimulatory effects of IL-18 on the T cell-synovial fibroblast contact as well as T cell-macrophage contact [11]. These results suggest that VCAM-1 and ICAM-1 may mediate the stimulatory effects of IL-18 on the cell contact of T cells with macrophages or synovial fibroblasts.
Miranda-Carus M-E. et al. demonstrated that RA synovial fibroblasts induces the upregulation of proinflammatory cytokines TNF-α, IFN- γ, IL-6, IL-8, IL-17, CD25 and CD69 expression through a cell contact-dependent system [26]. They found that these proinflammatory cytokines stimulate the further production of IL-6, IL-8, and IL-15 in synovial fibroblasts, thereby; creating a feedback loop that favors persistent synovial inflammation. Especially, the induction of IL-17 in cell contact is an important contributor to the feedback loop. A blocking antibody to IL-17 significantly inhibited T cell contact-dependent expression of IL-6, IL-8, IL-15 and CD54 by synovial fibroblasts. It is well known that IL-17 is a crucial important cytokine in pathogenesis of RA. Elevated levels of IL-17 have been described in synovial fluids in patients with RA but not OA [27,28]. Other report also revealed that methotrexate may disrupt this loop by inhibiting cell contact between T cell-to-synovial fibroblasts [26].
T cell- skin fibroblast contact and systemic sclerosis
In systemic sclerosis, it is well known that T cells infiltrate fibrotic tissues or organs and may be involved with dysregulated production of collagen by fibroblasts. It has been reported that T cell-skin fibroblast contact has a role in the pathogenesis of systemic sclerosis. Chizzolini et al. [29] demonstrated that T cell-fibroblast contact significantly inhibits collagen production by fibroblasts via membrane-associated TNF-α [29]. Their findings revealed that T cells infiltrating the skin of patients with systemic sclerosis deliver inhibitory signals to skin fibroblasts via direct cell-to-cell contact. Also, they mentioned that the cell contact dependent inhibition of collagen production by skin fibroblasts is a powerful characteristic of Th2 cells and is mediated by membrane-bound TNF-α. These findings suggest the therapeutic strategies based on TNF-α blockade aimed at controlling fibrosis in systemic sclerosis.
T cell-chondrocyte interactions and articular cartilage deterioration
Recently, we examined whether T cell-chondrocyte interactions influence the articular deterioration. We postulated that chondrocytes, as well as infiltrating macrophages and synovial fibroblasts, also act on the joint destruction in response to the cell-cell contact with infiltrating lymphocytes. Our findings of the T cell-immune response to chondrocytes clearly demonstrated the autologous T cell-stimulating properties of OA chondrocytes [30,31]. Co-culture of T cells with irradiated autologus chondrocytes induced the acceleration of T-cell proliferation in vitro, suggesting the presence of immune reaction to articular cartilage. The direct contact between infiltrating T cells and articular chondrocytes may participate in the immune-mediated mechanisms of OA. Interestingly, this response was partially blocked by antibodies for HLA class I and II, CD4 and CD8. These findings provide evidence to support that immune response occurs in response to chondrocytes that are exposed during the progression of OA.
Furthermore, we have found that cell contact of activated T cells with chondrocytes induces the secretion of MMP-1, -3, and -13, suggesting the effect of the cell contact on articular cartilage deterioration [31]. The direct T cell-chondrocyte contact via specific adhesion molecules may result in the accelerated secretions of matrix degrading enzymes from chondrocytes, consequently leading to the cartilage degeneration.
Indeed, it is rare occurrence that infiltrating T cells directly contact to chondrocytes in normal articular cartilage. However, in the degenerated cartilage during the progression of OA, articular chondrocytes may be exposed to synovial fluid with numerous number of infiltrating T cells. Nakamura et al. observed the presence of degenerated cartilage fragment with articular chondrocytes in the joint fluid of patient with osteoarthritis [31]. It has been demonstrated the CD3 positive mononuclear cells and chondrocyte-like cells in synovial fluid of patients with OA. Destructed cartilage fragments (cartilage matrix component) with articular chondrocytes may be shaved off and be floating in the joint fluid with advance of degeneration of articular cartilage. Thus, articular chondrocytes that are attached on fragmented articular cartilage tissue may contact with the infiltrating lymphocytes in the joint fluid. Also, chondrocytes in degenerated cartilage zone may be exposed out and be influenced by several factors in the joint fluid.
Although further studies are needed to clarify the exact implication of T cell-chondrocyte contact in the pathogenesis of OA, the direct contact between activated T cells and chondrocytes may induce the immune response and cartilage- matrix degradation.
Osteoclast Differentiation Through Cell Contact- Induced Rank/Rankl System
A number of cytokines and factors produced by RA synovial membrane and by pannus tissue are known to have the ability to induce the differentiation of monocyte/macrophage lineage cells into functional osteoclasts [1,32]. These factors are either directly on osteoclast precursor cells or on osteoclasts, for example macrophage colony stimulating factor (M-CSF), TNF- α and IL-1, or indirectly on bone lining/osteoblast lineage cells IL-1, IL-11, IL-17 and TNF-α, which in turn contribute to the differentiation of osteoclast precursors [33-35]. The essential and direct acting factor for osteoclast differentiation has been cloned and identified as receptor activator of NF κ B ligand (RANKL) [36-39].
RANKL is a member of the TNF ligand superfamily of cytokines that binds to its signal transducing receptor, RANK [36]. In addition to its required role in the differentiation of osteoclasts from their precursor cells, RANKL also augments osteoclast activity and survival. Synovial membrane provides a source of RANKL that could influence osteoclastogenesis [35]. Synovial fibroblasts from patients with RA produce mRNA and protein for RANKL [40]. RANKL is also expressed by infiltrating T cells in RA synovial membrane. Activated T cells expressing RANKL induce osteoclasts from autologus infiltrating macrophages [41-43]. Synovial tissues may also provide a source of osteoclast precursor cells, as macrophages isolated from RA synovial tissues differentiate into osteoclasts in the presence of M-CSF plus RANKL. Taken together, cell contact between T cells and osteoclast precursors or between synovial fibroblasts and osteoclast precursors through the pathway depending on the RANK/RANKL binding is a crucial important for osteoclastic bone resorption in arthritis.
Conclusion
We reviewed the potential involvement of cell-to-cell interactions of activated T cells with adjacent cells in inflamed articular tissue in the joint deterioration (Figure 1). These novel pathways may represent exciting potential therapeutic targets in arthritis.
Acknowledgement
This study was supported by grants from the Ministry of Education, Culture, Sports, Science and Technology of Japan, the Ministry of Health, Labour and Welfare of Japan, and the Japan Rheumatism Foundation.
References
- Angelotti F, Parma A, Cafaro G, Capecchi R, Alunno A, et al. (2017) One year in review 2017: pathogenesis of rheumatoid arthritis. Clin Exp Rheumatol 35(3): 368-378.
- Burger D, Dayer JM (2002) Cytokines, acute-phase proteins, and hormones: IL-1 and TNF-αlpha production in contact-mediated activation of monocytes by T lymphocytes. Ann N Y Acad Sci 966: 464-473.
- Firestein GS, McInnes IB (2017) Immunopathogenesis of Rheumatoid Arthritis. Immunity 46(2): 183-196.
- Mori M, Hashimoto M, Matsuo T, Fujii T, Furu M, et al. (2017) Cell- contact-dependent activation of CD4+ T cells by adhesion molecules on synovial fibroblasts. Mod Rheumatol 27(3): 448-456.
- McInnes IB, Leung BP, Liew FY (2000) Cell-cell interactions in synovitis: interactions between T lymphocytes and synovial cells. Arthritis Res 2(5): 374-378.
- Bernardini G, Benigni G, Scrivo R, Valesini G, Santoni A (2017) The Multifunctional Role of the Chemokine System in Arthritogenic Processes. Curr Rheumatol Rep 19(3): 11.
- Kinne RW, Brauer R, Stuhlmuller B, Palombo-Kinne E, Burmester GR (2000) Macrophages in rheumatoid arthritis. Arthritis Res 2(3): 189202.
- Brennan FM, Hayes AL, Ciesielski CJ, Green P, Foxwell BM, et al. (2002) Evidence that rheumatoid arthritis synovial T cells are similar to cytokine-activated T cells: involvement of phosphatidylinositol 3-kinase and nuclear factor B pathways in tumor necrosis factor production in rheumatoid arthritis. Arthritis Rheum 46(1): 31-41.
- van de Sande MG, Baeten DL (2016) Immunopathology of synovitis: from histology to molecular pathways. Rheumatology (Oxford) 55(4): 599-606.
- McInnes IB, Leung BP, Sturrock RD, Field M, Liew FY (1997) Interleukin-15 mediates T cell-dependent regulation of tumor necrosis factor-alpha production in rheumatoid arthritis. Nat Med 3(2): 189195.
- Dai SM, Matsuno H, Nakamura H, Nishioka K, Yudoh K (2004) Interleukin-18 enhances monocyte tumor necrosis factor alpha and interleukin-1beta production induced by direct contact with T lymphocytes: implications in rheumatoid arthritis. Arthritis Rheum 50(2): 432-443.
- McInnes IB, Gracie JA (2004) Interleukin-15: a new cytokine target for the treatment of inflammatory diseases. Curr Opin Pharmacol 4(4): 392-397.
- Miranda-Carus ME, Balsa A, Benito-Miguel M, Perez de Ayala C, MartinMola E (2004) IL-15 and the initiation of cell contact-dependent synovial fibroblast-T lymphocyte cross-talk in rheumatoid arthritis: effect of methotrexate. J Immunol 173(2): 1463-1476.
- Martel-Pelletier J, McCollum R, Fujimoto N, Obata K, Cloutier JM, et al. (1994) Excess of metalloproteases over tissue inhibitor of metalloproteases may contribute to cartilage degradation in osteoarthritis and rheumatoid arthritis. Lab Invest 70(6): 807-813.
- Arend WP, Dayer JM (1995) Inhibition of the production and effects of interleukin-1 and tumor necrosis factor alpha in rheumatoid arthritis. Arthritis Rheum 38(2): 151-160.
- Savion N, Vlodavsky I, Fuks Z (1984) Interaction of T lymphocytes and macrophages with cultured vascular endothelial cells: attachment, invasion, and subsequent degradation of the subendothelial extracellular matrix. J Cell Physiol 118(2): 169-178.
- Jungo F, Dayer JM, Modoux C, Hyka N, Burger D (2001) IFN-beta inhibits the ability of T lymphocytes to induce TNF-αlpha and IL-1beta production in monocytes upon direct cell-cell contact. Cytokine 14(5): 272-282.
- Elshabrawy HA, Chen Z, Volin MV, Ravella S, Virupannavar S, et al. (2015) The pathogenic role of angiogenesis in rheumatoid arthritis. Angiogenesis 18(4): 433-448.
- Kasyapa CS, Stentz CL, Davey MP, Carr DW (1999) Regulation of IL- 15-stimulated TNF-αlpha production by rolipram. J Immunol 163(5): 2836-2843.
- Jovanovic DV, Di Battista JA, Martel-Pelletier J, Jolicoeur FC, He Y, et al. (1998) IL-17 stimulates the production and expression of proinflammatory cytokines, IL-beta and TNF-αlpha, by human macrophages. J Immunol 160(7): 3513-3521.
- Nakanishi K, Yoshimoto T, Tsutsui H, Okamura H (2001) Interleukin-18 regulates both Th1 and Th2 responses. Annu Rev Immunol 19: 423 474.
- Morel JC, Park CC, Zhu K, Kumar P, Ruth JH, et al. (2002) Signal transduction pathways involved in rheumatoid arthritis synovial fibroblast interleukin-18-induced vascular cell adhesion molecule-1 expression. J Biol Chem 277(38): 34679-34691.
- Hyka N, Dayer JM, Modoux C, Kohno T, Edwards CK, et al. (2001) Apolipoprotein A-I inhibits the production of interleukin-lbeta and tumor necrosis factor-alpha by blocking contact-mediated activation of monocytes by T lymphocytes. Blood 97(8): 2381-2389.
- Morel JC, Park CC, Woods JM, Koch AE (2001) A novel role for interleukin-18 in adhesion molecule induction through NF- B and phosphatidylinositol (PI) 3-kinase-dependent signal transduction pathways. J Biol Chem 276(40): 37069-37075.
- Yoshida A, Takahashi HK, Nishibori M, Iwagaki H, Yoshino T, Morichika T, et al. (2001) IL-18-induced expression of intercellular adhesion molecule-1 in human monocytes: involvement in IL-12 and IFN- gamma production in PBMC. Cell Immunol 210(2): 106-115.
- Miranda-Carus ME, Benito-Miguel M, Llamas MA, Balsa A, Martin-Mola E (2005) Human T cells constitutively express IL-15 that promotes ex vivo T cell homeostatic proliferation through autocrine/juxtacrine loops. J Immunol 175(6): 3656-3662.
- Ziolkowska M, Koc A, Luszczykiewicz G, Ksiezopolska-Pietrzak K, Klimczak E, et al. (2000) High levels of IL-17 in rheumatoid arthritis patients: IL-15 triggers in vitro IL-17 production via cyclosporin A-sensitive mechanism. J Immunol 164(5): 2832-2838.
- Kotake S, Udagawa N, Takahashi N, Matsuzaki K, Itoh K, et al. (1999) IL-17 in synovial fluids from patients with rheumatoid arthritis is a potent stimulator of osteoclastogenesis. J Clin Invest 103(9): 13451352.
- Chizzolini C, Parel Y, De Luca C, Tyndall A, Akesson A, et al. (2003) Systemic sclerosis Th2 cells inhibit collagen production by dermal fibroblasts via membrane-associated tumor necrosis factor alpha. Rheum 48(9): 2593-2604.
- Sakata M, Masuko-Hongo K, Nakamura H, Onuma H, Tsuruha JI, et al. (2003) Osteoarthritic articular chondrocytes stimulate autologous T cell responses in vitro. Clin Exp Rheumatol 21(6): 704-710.
- Nakamura H, Tanaka M, Masuko-Hongo K, Yudoh K, Kato T, et al. (2006) Enhanced production of MMP-1, MMP-3, MMP-13, and RANTES by interaction of chondrocytes with autologous T cells. Rheumatol Int 26(11): 984-990.
- Gravallese EM, Manning C, Tsay A, Naito A, Pan C, et al. (2000) Synovial tissue in rheumatoid arthritis is a source of osteoclast differentiation factor. Arthritis Rheum 43(2): 250-258.
- Morony S, Capparelli C, Lee R, Shimamoto G, Boone T, et al. (1999) A chimeric form of osteoprotegerin inhibits hypercalcemia and bone resorption induced by IL-1, TNF-α, PTH, PTHrP and 1,25(OH)2D3. J Bone Miner Res 14(9): 1478-1485.
- Nakashima T, Kobayashi Y, Yamasaki S, Kawakami A, Eguchi K, et al. (2000) Protein expression and functional difference of membrane- bound and soluble receptor activator of NF-B ligand: modulation of the expression by osteotropic factors and cytokines. Biochem Biophys Res Commun 275(3): 768-775.
- Gravallese EM, Harada Y, Wang JT, Gorn AH, Thornhill TS, et al. (1998) Identification of cell types responsible for bone resorption in rheumatoid arthritis and juvenile rheumatoid arthritis. Am J Pathol 152(4): 943-951.
- Yasuda H, Shima N, Nakagawa N, Yamaguchi K, Kinosaki M, et al. (1998) Osteoclast differentiation factor is a ligand for osteoprotegerin/ osteoclastogenesis-inhibitory factor and is identical to TRANCE/ RANKL. Proc Natl Acad Sci U S A 95(7): 3597-3602.
- Lacey DL, Timms E, Tan HL, Kelley MJ, Dunstan CR, et al. (1998) Osteoprotegerin ligand is a cytokine that regulates osteoclast differentiation and activation. Cell 93(2): 165-176.
- Wong BR, Rho J, Arron J, Robinson E, Orlinick J, et al. (1997) TRANCE is a novel ligand of the tumor necrosis factor receptor family that activates c-Jun N-terminal kinase in T cells. J Biol Chem 272(40): 25190-25194.
- Kotake S, Udagawa N, Hakoda M, Mogi M, Yano K, Tsuda E, et al. (2001) Activated human T cells directly induce osteoclastogenesis from human monocytes: possible role of T cells in bone destruction in rheumatoid arthritis patients. Arthritis Rheum 44(5): 1003-1012.
- Ukai T, Hara Y, Kato I (1996) Effects of T cell adoptive transfer into nude mice on alveolar bone resorption induced by endotoxin. J Periodontal Res 31(6): 414-422.
- John V, Hock JM, Short LL, Glasebrook AL, Galvin RJ (1996) A role for CD8+ T lymphocytes in osteoclast differentiation in vitro. Endocrinology 137(6): 2457-2463.
- Kong YY, Feige U, Sarosi I, Bolon B, Tafuri A, et al. Activated T cells regulate bone loss and joint destruction in adjuvant arthritis through osteoprotegerin ligand. Nature 402(6759): 304-309.
- Horwood NJ, Kartsogiannis V, Quinn JM, Romas E, Martin TJ, et al. (1999) Activated T lymphocytes support osteoclast formation in vitro. Biochem Biophys Res Commun 265(1): 144-150.






























