Effect of Aphanizomenon Flos-Aquae (Afa) on Endogenous Mesenchymal Stem Cells Proliferation in African Adult Donkeys (Equus africanus) during Fracture HEALING
Ochube GE1*, Hassan AZ1, Kudi CA2, Fadason ST1, Bada AA1, Emmanuel EG3, Usman B3, Augustine A1, Bappa MN1 and James AA3
1Department of Veterinary Surgery & Radiology, Ahmadu Bello University, NigeriaBello University, Nigeria
2Department of Veterinary Medicine, Ahmadu Bello University, Nigeria
3 Veterinary Teaching Hospital, Ahmadu Bello University, Nigeria
Submission: March 28, 2017; Published: July 25, 2017
*Corresponding author: Ochube GE, Department of Veterinary Surgery & Radiology, Ahmadu Bello University, Zaria, Nigeria, Email: gabrielochube2000@gmail.com
How to cite this article: Ochube GE, Hassan A, Kudi C, Fadason ST, Bada AA. Effect of Aphanizomenon Flos-Aquae (Afa) on Endogenous Mesenchymal Stem Cells Proliferation in African Adult Donkeys (Equus africanus) during Fracture HEALING. Nov Tech Arthritis Bone Res. 2017; 1(3) : 555564 DOI: 10.19080/NTAB.2017.01.555564
Abstract
The aim of this study which lasted sixteen weeks was to study the effects of feeding Aphanizomenon flos-aquae (stem enhance®] on stem cell proliferation and haematologic parameters in fractured African Adult Donkeys. Nine donkeys with clinical cases of mid shaft open metacarpal and mid shaft open metatarsal fractures were used for this experiment. Animals were divided into groups A&B. Group A comprised six donkeys (n=6) and was further divided into A1c and A2. A1 comprised of A1a, A1b and A1c (n=3) while A2 consists of A2a, A2b and A2c (n=3).A1 was the study group fed with stem enhance®, while A2 was the control group that was untreated. Group B had three donkeys that were tagged B1 and B2 (n=3). B1 was made up of B1a and B1b (n=2) while B2 was the only animal in the group of B2 (n=1). B1 was the study group fed with stem enhance®, while B2 was the control that was untreated. Group A was managed by external reduction using fiber cast, while group B was managed by internal reduction using Sherman’s compression bone plates. Both study groups were fed two capsules of stem enhance® (2.5mg/capsule) each day for two weeks a month and two weeks off (alternatively). Stem cell count was carried out for both groups’ pre and post operatively. Data obtained were analyzed and findings showed that stem cell counts for the group treated with stem enhance® was significant (p<0.05). It was concluded that stem enhance ® is a potent stem cell enhancer and may be of value in reduction of healing time of fracture in animals thereby facilitating early return of the study group to active physical exercise. From this experiment, the study group was shown to have a superior healing time of 13±0.5weeks as against the control group had a healing time of 27±0.5 weeks.
Keywords: Donkeys; Fracture; Healing time; Stem cells; Stem enhance
Introduction
Stem enhance is a patented blend of migratose® and mobilin™-proprietary natural concentrations of an edible aquatic botanical known as Aphanizomenon Flos Aquae (AFA) [1,2]. Aphanizomenon Flos Aquae (AFA) has been growing in a unique, pristine environment in the North Western United States of America for long; it has been safely consumed for over three decades [3,4]. Stem enhance is the world’s first ever natural stem cell enhancer, the only natural supplement in the world proven to support the natural release of one’s adult stem cells from ones bone marrow [5,6]. Two capsules of stem enhance (2.5mg/ kg) support an average 25% increase in the natural release of adult stem cell [7,8] this is equivalent to 3 to 4 million stem cells in circulation. Stem cells are defined as cells with the unique ability to self replicate into various cell types of the body [9]. Generally speaking, there are two types of stem cells. Embryonic stem cells and Adult stem cells, [10]. Embryonic stem cells (ESC) are extracted within 5-10 days from an embryo called blastula [11,12]. Once isolated ESC can be grown in-vitro and led to differentiate into various type of tissue cell (such as heart cells, nervous cells, kidney cells) [13] after which they are injected in specific tissues in order to regenerate the tissue [14].
Adult stem cells are found in any living organism after birth [15,16]. Umbilical cord stem cells and placental stem cells are considered as Adult stem cells [12,17]. The confirmation that Adult stem cells are primarily found in any living organism after birth was further buttressed by Revishchin et al. [18]. Also confirmed umbilical cord stem cells and placental stem cells are Adult stem cells. Adult stem cells are mainly found in the bone marrow and in the blood, although many tissues contain their own specific population of tissue stem cells [19,20]. Tissues stem cells are traditionally believed to be limited in their ability to differentiate into other tissues, however bone marrow stem cells (BMSC) was recently shown to have significant capacity to become cells of other tissues [9].
Stem cells are produced by the red marrow present in the ribs, the vertebral column, pelvis bones and skull [21]. There are roughly about 125 million stem cells in the Adult human bone marrow and around 10 million in blood circulation at any time [22-24]. In the bone marrow, stem cells duplicate using a process known as "asymmetrical cellular division" according to which two daughter cells are not identical, one cell obtains original DNA and remains in the bone marrow whereas the other contains DNA copies and is released into the blood stream where it migrates into various tissues in need of repair [25,26].
Comminuted long bone fractures in large animals, certain small animal species and poultry (ostriches and peacocks) have proved difficult to manage in most cases; hence the trend to salvage them is popular [27, 28]. If fracture is properly corrected in such animal species and the animal is fed on stem enhance in the normal doses, natural healing time for these long bones could be reduced thus putting an end to complications that hitherto arise from previous management procedures.
Ethical Committee Permission
Ethical permission was sought from the Ahmadu Bello University Zaria ethical committee on animal welfare before commencement of experiment.
Material and Methods
Blood samples from 9 clinical cases of fractured African Adult donkeys (Equus Africanus) that were fed two capsules of stem enhance® (2.5mg/capsule) each day for two weeks a month and two weeks off (alternatively) were obtained from the jugular vein using 23" needle (Discard II, brenton, England), a pack of sterile glass slides, automatic cell counter (Phillips®), light microscope, a carton of 5mls, Randox Calcium kit (Randox® USA) and (Cadiff, UK).
Mesenchymal Stem Cell determination was carried out by the procedure described by Huss et al. [29], Ojeda-Uribe et al. [30]. Photomicrographs for stained Stem Cells was obtained by mounting the glass slide on a mounting solution (Sigma® Israel, Rehovot) and pictures from microscopy was taken with a Nikon Coopix camera with a Nikon TE200 microscope with Nomarsky optics and fluorescence set up. Stem cell count was achieved by use of the automatic cell counter (Phillips®).
Statistical Analysis
Graph Pad Prism version 6.0 for windows 7 (Graph Pad software, San Diego, California, USA) was used for the Analysis. Results obtained were expressed as mean±standard deviation (Mean±SD) and subjected to students T test to determine the difference between the treated and control groups. Values of p<0.05 were considered significant.
Result
The results of stem cell determination between A1 & A2 and B1 & B2 during the study showed that there is significant difference in favor of A1 (p<0.05) and significant difference in favor of B1 (p<0.05) with no difference between A1 and B1. The graph (Figure 1) shows a decrease of mean stem cell count at 4 weeks for A1, while the A2 group that was untreated recorded and increase in week 4, mean stem cell of experimental cases (Figure 2) peaked at 14 weeks for the treated group and the untreated group, however for the rest of the period the pattern was normal. The treated group recorded a dramatic decline at 6 weeks.
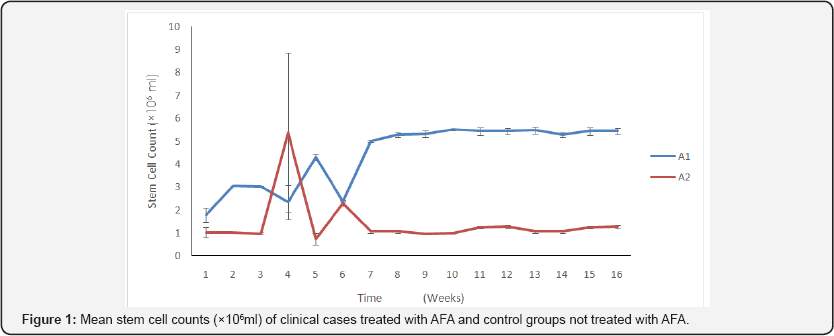
A1 : Treated, A2: Untreated
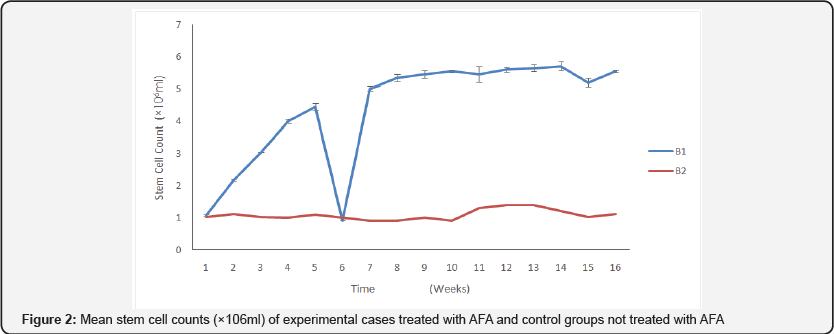
B1: Treated, B2: Untreated
Discussion
Mobilization of bone marrow mesenchymal stem cells (BMSC) to the area of challenge (fracture sites) actually was shown to have reduced healing time considerably as treated animals returned to the use of their limbs earlier than the untreated ones [9]. Healing process of the fractures resulted in some observable clinical signs which included pain, abducted limb, swelling, slight limping at the onset of the study and finally, normal gait, stance and good posture for the study group at the end of the study. These are in conformity with previous reports. This is because all the cardinal signs of inflammation, pain, poor gait noticed at onset of the study had abated and coupled with radiographic evidence of fracture and wound healing seen physically and good locomotion assessment test seen. Stem enhance has also been shown to cause increase in inflammatory cells which form free radicals which these inflammatory cells need in order to function properly and thus kill micro-organism and increase the efficacy of stem enhance. Appreciable drop in PCV in individual cases treated with stem enhance was noticed , this was shown by some level of anaemia recorded but as the study progressed the values returned to normal after they were treated. Transient anemia has been recorded in humans, using the human form of this product.
The groups treated with AFA,( A1a,A1b, A1c) and (B1a, B1b) healed much earlier because the use of AFA caused mobilization of mesenchymal stem cells from the bone marrow to the fracture site, this led to faster healing as the MSC differentiated from their primitive forms to become osteoblasts [19]. This led increased osteoclasis and faster bone resorption and remodeling as the excess osteoblasts "die" by laying down a matrix around itself to become osteoclasts [28]. The more osteoclasts, the faster remodeling occurs.
From the study, the donkeys treated with AFA had appreciable rise in stem cell count which coincided with the period when very high osteogenic activities were noticed radio graphically (Week 4, 8, 12 and 16). For these group of donkeys treated with AFA, healing time averagely was 12.5±0.5 weeks, confirming the work done by Goh et al. [8] and Efrat [7] that stem enhance stimulates the release of Adult MSC which can facilitate quick healing of injured/fractured bone.
There was enhancement of inflammatory response in the group treated with AFA which aided healing of the injured tissue [21,22]. This is so because in large wounds where blood supply have been compromised, there is no post surgical complications despite the scanty tissue as it occurs in the metacarpal area.
The post surgical infection in the control could be attributed to the absence of AFA which is known to stimulate lymphocytocytosis which are classified as immunocytes and therefore facilitate the formation of antibodies to fight infection.
The group treated with AFA had their healing time shortened (12.5±0.5) because of the use of stem enhance, while the control that was not treated with AFA had a longer healing time (20.9±0.2).
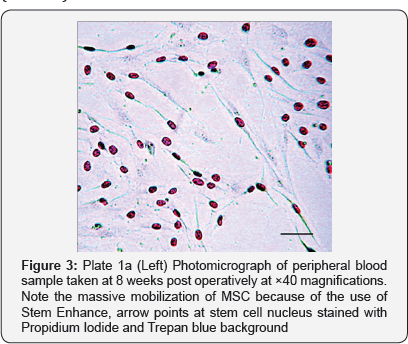
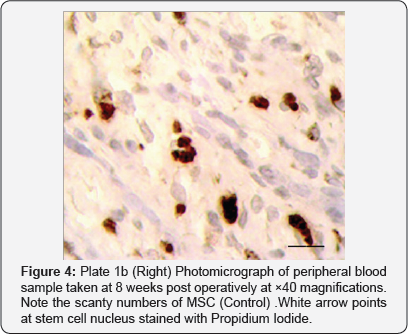
From the study, the donkeys treated with Aphanizomenon Flos Aquae had appreciable rise in stem cell count (Figure 3 & 4) which coincided with period when heightened osteogenic activities were noticed radio graphically (week 4, 6, 12 & 16). For these group of donkeys treated with Aphanizomenon Flos Aquae, healing time averagely was 12.5±0.5 weeks, confirming the work done by Goh et al. [8] & Efrat [7] that stem enhance stimulates the release of Adult MSC which can facilitate quick healing of injured/fractured bone.
Acknowledgment
We deeply acknowledge and appreciate the contributions of the following; Mr. Clement Ochayi of the department of chemical pathology, ABUTH, Shika, Zaria; Technical staff of the chemical pathology Laboratory UCH, Ibadan and all casualstaff of equine unit of Ahmadu Bello University Veterinary Teaching Hospital, Samaru Zaria, Kaduna State.
References
- Kicic A, Shem WY, Wilson AS, Constable IJ, Robertson T, et al. (2003] Differentiation of marrow stromal cells into photoreceptors in the rat eye. J Neurosci 23(21): 7742-7749.
- Knag S, Gillespie MA, Rudnicki MA (2008) Niche regulation of muscle satellite cell, self renewal & differentiation. Cell Stem Cell 2(l): 22-32.
- Abedi M, Greer DA, Calvin GA, Demers DA, Dooner MS, et al. (2004) Robust conversion of marrow cells to skeletal with formation of marrow derive muscle colonies: a multifunctional process. Experimental Hematology 32: 426-434.
- Bianco P, Riminucci M, Gronithos S, Robey PG (2003) Bone marrow stromal stem cells: nature, biology and potential applications. Stem Cells 9(3): 180-192.
- Burke ZD, Thowfeeqa S, Peran L, Tosh D (2007) Stem cells in the adult pancreas & liver. Biochemistry Journal 404(2): 169-178.
- Dawn B, Tiwari S, Kacia MJ, Zuba-Surma EK, Guo Y, et al. (2008) Transplantation of bone marrow derived cells of very small embryonic- like stem cell attenuated by left ventricular dysfunction and remodeling after myocardial infection. Stem Cells 26(6): 1646-1655.
- Efrat S (2004) Generation of insulin-producing cells from stem cell replacement therapy of type i diabetes. Israeli Medical Association 6(5): 265-267.
- Goh EL, Ma D, Ming GL, Song H (2003) Adult neural stem cells and repair of the adult central nervous system. J Hematother Stem Cell Res 12(6): 671-679.
- Chen LB, Jang XB, Yang L (2004) Differentiation of rat marrow mesenchymal stem cells into pancreatic islet beta-cells. World J Gastroenterol 10(20): 3016-3020.
- Branshi LK, Ganglitz GG, Herndon DN, Jeschke MG (2009) A review of gene and stem cell therapy in cutaneous wound healing. Burns 35(2): 171-180.
- Amoh Y, Li L, Katsuoka K, Hoffman RM (2008) Multipotent hair follicle stem cells promote repair of spinal cord injury and recovery of walking function. Cell Cycle 7(12): 1865-1869.
- Raghvacher A, Steinbach KH, Prumrher O, Grilli G, Fliedner TM (1983) Survival of transfused cryopreserved granulocytic progenitor cells (CFU-C) in recipient circulation cell. Tissue Kinet 16(3): 303-311.
- Asahara T, Masuda H, Takahashi T, Kalka C, Pastore C, et al. (1999) Bone marrow origin of endothelial progenitor cells responsible for postnatal vasculogeneration in physiological and pathological neovascularization. Circ Res 85(3): 221-228.
- Cui HF, Bai ZL (2003) Protective effects of transplanted and mobilized bone marrow stem cells mice with severe acute pancreatitis. World J Gastroenterol 9(10): 5227-5232.
- Maloney MA, Dorie M, Lamela RA (1978) Hematopoietic stem cell regulatory volumes as revealed in studies of big chimera. Journal of Experimental Veterinary Medicine 4: 1189-1197.
- Krause DS, Theise ND, Collector MI, Henegariu O, Hwang S, et al. (2001] Multi-Organ, Multi-Lineage Engraftment by a Single Bone Marrow- Derived Stem Cell. Cell 105(3): 369-377.
- Kreutzmann H, Fliedner TM (1979) Studies on the presence & possible oscillations of granulocytic progenitor cells (CFU-C) in human blood. European Journal of Haematology 23(5): 360-366.
- Revishchin AV, Korochkin LI, Okhotin VE, Pavlova G (2008] Neural stem cells in the mammalian brain. Advanced histology 265: 55-109.
- Jang YY, Collector MI, Baylin SB, Diehl AM, Sharkis SJ (2004] Hematopoietic stem cells convert into liver cells within days without fussion. Nat Cell Biol 6(6): 532-549.
- Maloney MA, Pah HM (1978) Marrow stem cell release in the auto re-population. Experimental Hematology 6(2): 227-232.
- Bossolasco P, Cova L, Calzarossa C, Rimoldi SG, Borsotti C, et al. (2005) Neuro-glial differentiation of human bone marrow stem cells in vitro. Exp Neurol 193(2): 312-325.
- Bongo Y, Araki H, Kato J, Nakamura K, Kawano Y, et al. (2005) Human mesenchymal stem cells xenografted directly to rat liver are differentiated into human hepatocytes without fusion. Blood 106(2): 756-763.
- Chan-Ling T, Baxter L, Afzal A, Sengupta N, Caballero S, et al. (2006) Hematopoietic stem cells provide functions after laser-induced bruch's man-brain model of choroidal neovascularization. Am J Pathol 168(3): 1031-1044.
- Dezawa M, Ishikawa H, Hoshino M, Itokazu H, Nabeshima Y (2005) Potential of bone marrow stromal cells in applications for neuro- degenerative, euro-traumatic and muscle degenerative diseases. Current Neuropharmacology 3(4): 257-266.
- Reyes M, Verfaillie CM (2001) Characterization of multipotent adult progenitor cells, a subpopulation of mesenchymal stem cells. Ann N Y Acad Sci 938: 231-233.
- Sanchez-Ramos JR (2002) Neural cells derived from adult bone marrow and umbilical cord Blood. J Neurosci Res 69(6): 880-893.
- Encarta Reference Library (2004) Microsoft Corporation.
- Harris WH (1996) Bone Healing Principles: Clinical Orthopedic. Related Research Journal 33: 155-157.
- Huss R, Lange C, Weissinger EM, Kolb HJ, Thalmeier K (2002) Fvidence of peripheral blood-derived, plastic-adherent cd34(-/ low) hematopoietic stem cell clones with mesenchymal stem cell characteristics. Stem Cells 18: 252-260.
- Ojeda-Uribe M, Brunot A, Lenat A, Legros M (2000) Failure to detect spindle-shaped fibroblastic cell progenitors in pbpc collections. Acta Haematol 90: 139-143.






























