Effectivenness of PRP on Pain, Function and Quality of Life in Chondromalacia and Patellofemoral Pain Syndrome: A Pretest-Postest Analysis
Olga Susana Pérez Moro1, María Jesús Albaladejo Florín1, Beatriz Entrambasaguas Estepa2 and Marcos Edgar Fernández Cuadros1,3*
1Rehabilitation Department, Santa Cristina´s University Hospital, Spain
2Rehabilitation Department, La Princesa University Hospital, Spain
3Rehabilitation Department, Santísima Trinidad General Foundation´s Hospital, Spain
Submission: April 28, 2017; Published: May 30, 2017
*Corresponding author: Marcos E Fernández-Cuadros, Hospital Universitario Santa Cristina, Calle del Maestro Vives, 2, CP 28009, Madrid , Spain,Tel:+34-620314558; Email:marcosefc@hotmail.com;
How to cite this article:Perez-Moro, O; Albaladejo-Florin, M; Entrambasaguas-Estepa, B; Fernandez-Cuadros, M.. Effectivenness of PRP on Pain, Function and Quality of Life in Chondromalacia and Patellofemoral Pain Syndrome: A Pretest-Postest Analysis. Nov Tech Arthritis Bone Res. 2017; 1(1) : 555554.
Abstract
Objectives:
- To demonstrate the effectiveness of a 3-doses PRP treatment protocol on pain, function and quality of life (QoL) in patients with Patellofemoral Pain Syndrome (PFPS) and Chondromalacia.
- To apply PRP as a conservative treatment option with a demonstrable level of scientific evidence (2b).
Material and Methods: Prospective quasi-experimental before-after study (non-randomized control-trial) on 24 patients with PFPS and Chondromalacia grade 2 or more, who attended to Santa Cristina’s University Hospital, from January 2014 to March 2017. The PRP-protocol consisted of 3-sessions (1 session/week) of an intra articular infiltration of 3ml of platelet-rich plasma (PRP). To get the PRP, Accelerate Concentration System Device® from EXACTECH, EmCyte Anticoagulant Sodium Citrate Solution U.S.P. and Drucker Centrifuge (Series Performance) were used. Pain and Quality of Life were measured by Visual Analogical Scale (VAS) and Western Ontario and Mc Master Universities Index for Osteoarthritis (WOMAC) at the beginning / end of treatment.
Results: Average age was 40.3±9.5 years. Women 37.5% (n=9), men 62.5% (n=15). The most frequent Chondromalacia grade was 2nd degree (n=11, 45.8%), followed by 3rd (n=9, 37.5%) and 4th (n=4, 16.6%). Post-puncture erythema and inflammation 4.1% (n=1).
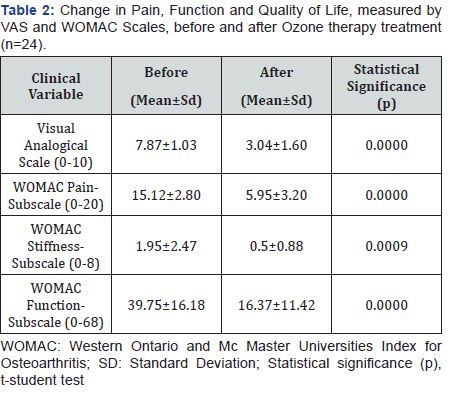
Pain measured by VAS significantly decreased (p<0.0001) from 7.87 to 3.04 points. The WOMAC-pain, WOMAC-stiffness and WOMAC-function subscales decreased significantly (p<0.0001) from 15.12 to 5.95 points, 1.95 to 0.5 and 39.75 to 16.37, respectively.
Conclusion: PRP is safe and improves significantly pain relief, stiffness and function in patients with Patellofemoral Pain Syndrome and Chondromalacia.
The study shows a good level of evidence and grade of recommendation that allows us to consider PRP as a conservative therapeutic option in the treatment of PFPS and chondromalacia.
Keywords: Patellofemoral pain syndrome; Chondromalacia; Pain; PRP therapy; Quality of life
Abbreviations: PFPS: Patellofemoral Pain Syndrome; W Pain, WOMAC pain. W Rigidity, WOMAC Rigidity. W Function, WOMAC Function. WOMAC, Western Ontario and Mc Master Universities Index for Osteoarthritis
Introduction
Patellofemoral pain syndrome (PFPS) is present as anterior knee pain and is a frequent over use disorder that affects the patellofemoral region. PFPS is the most common cause of knee pain seen by primary care physicians, traumatology, rehabilitation and sports medicine specialists [1,2]. PFPS involves pain on the patella and retinaculum once intra-articular and peri-patellar pathology is excluded [3]. For those reasons, the diagnosis is difficult, because there is no consensus about the pathophysiology and the treatment remains a challenge. Frequently chondromalacia is a term used to describe PFPS.Chondromalacia patella describes the pathologic changes in the articular cartilage of the patella, such as softening, erosion and fragmentation [4]. Moreover, some authors consider chondromalacia patella as a precursor of Osteoarthritis (OA) [5]. PFPS describes complex symptoms and is an exclusion diagnosis, while chondromalacia patella is a pathologic diagnosis [4].
PFPS is the most common diagnosis in sports medicine, is present in active individuals in up to 25-40% with knee pain; although, the true incidence is unknown [6]. PFPS is multifactorial, resulting from an interaction of intrinsic and extrinsic factors. Overuse, malalignment and trauma are some causative factors [7]. Abnormal tissue homeostasis that include inflamed synovial lining and fat pad tissues, retinacular neuromas, increased intraosseous pressure and increased osseous metabolic activity of the patella are believed to cause pain and dysfunction[8].
The etiology, diagnosis and treatment of PFPS and chondromalacia constitute a challenge. In an initial study, Kon et al. [9] have stated good outcomes of intra-articular PRP in early degenerative cartilage lesions. Posteriorly, when compared to knee OA, Kon et al. [9] observed that patients with degenerative chondropathy had better results than patients of early OA, who in turn had better results than advanced OA [10]. In both studies, Kon et al. [9] stressed on better results in younger patients, low BMI patients and those with lower degree of cartilage degeneration [9,10]. Since chondromalacia and PFPS share common features, we believe PRP could be an effective treatment option for both entities. PFPS diagnoses are based on clinical history, examination and the exclusion of alternative diagnosis [11]. PFPS has no pathognomonic sign or symptom; however, the combination of pain with resisted quadriceps contraction and pain with squatting are the maneuvers with more diagnostic validity [12]. Plain radiographies are not necessary for initial management. They are helpful to rule out any other sources of anterior knee pain (bipartite patella, OA, loose bodies and occult fractures) [13].
The treatment goals for PFPS are to reduce pain (on the acute phase), improve patellofemoral tracking and alignment, and return the patient to as a high level of function as possible (on recovery phase). Physical therapy focused on improving the strength and flexibility of the lower extremity and core muscles, included hip adductors and quadriceps, are effective in the treatment of PFPS [14-18]. NSAIDs (non-steroidal antiinflammatory drugs) are only valid for short term pain relief (two to three weeks) [19].
With respect to physical therapies, ice applied to the anterior knee relieves pain associated to PFPS. There is no empiric evidence to support the use of ultrasound, iontophoresis, phonophoresis or electrical stimulation in the treatment of PFPS [19-22]. Surgical intervention for PFPS is considered when all previous therapies have failed. It is a last resort treatment; then, non-operative therapy should be pursued for 6 to 12 months prior to considering operative intervention [2]. The main objective of this study is to postulate PRP as a conservative therapeutic option for pain relief, function and quality of life on PFPS and chondromalacia, since its treatment remains a challenge. A second objective is to provide clinical-based-evidence on PRP as a feasible treatment for such entities.
Material and Methods
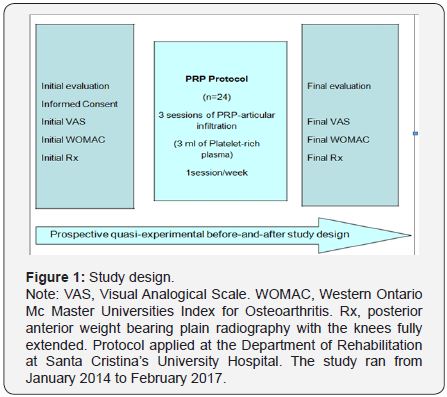
A prospective quasi-experimental before-and-after study (non-randomized control trial) is been performed on 24 patients with PFPS or Chondromalacia (Figure 1). The study period run from January 2014 to February 2017 and involved patients who attended the Rehabilitation Department at Santa Cristina’s University Hospital. Patients were referred from Traumatology, Rheumatology and Familial Medicine specialists and received non-surgical conservative treatment for at least 6 months without success (analgesics, non-steroidal anti-inflammatory drugs, steroidal/hyaluronic acid/Ozone injections, viscosupplementation and/or physical medicine). The study was approved by the ethical Committee of the Hospital.
Inclusion criteria
- Patients with PFPS/Chondromalacia grade 2 or more diagnosed by MRI (magnetic Resonance Imaging).
- With pain greater than 3 on Visual Analogical Scale (VAS).
- Older than 18 years of age.
- Who have failed any other conservative treatment (analgesics, non-steroidal anti-inflammatory drugs, steroidal/hyaluronic acid/Ozone injections, viscosupplementation and/or physical medicine).
- Unwilling or not candidate for knee arthroplasty replacement.
Exclusion criteria
At initial evaluation, age, comorbidities, occupation and other demographic data were recorded. An explanation of the PRP-protocol with indications and adverse effects was made. Informed consent was signed and VAS and WOMAC (Western Ontario and Mc Master Universities Index for Osteoarthritis) validated scales were filled prior to treatment (Figure 1). The proposed PRP-protocol consisted of 3-sessions (1session/ week) of an intra articular infiltration of 3ml of autologous blood, composed mainly on platelet-rich plasma. To get the PRP, Accelerate Concentration System Device® from EXACTECH, EmCyte Anticoagulant Sodium Citrate Solution U.S.P. and Drucker Centrifuge (Series Performance) were used. Main steps were based on blood draw, blood processing and blood infiltration.
The Accelerate II Platelet concentrating System PRP-S 30ml® is a concentrating System manufactured by EmCyte Corporation and distributed by EXACTECH Inc. This device permits the rapid preparation of platelet-rich plasma from 30ml of the patient’s whole blood drawn at the time of treatment. This device is for one single use only, and standard aseptic techniques must be applied during the whole procedure.
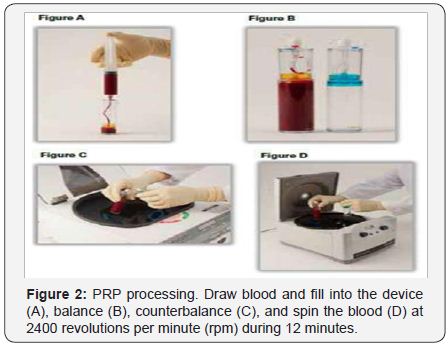
For whole blood draw procedure: draw 3ml of Anticoagulant Sodium Citrate Solution U.S.P. into the 30ml syringe. Attach a syringe to the apheresis needle and prime the needle with the anticoagulant. Slowly draw 27ml of blood from the patient (by venipuncture) filling the syringe to 30ml. Gently but thoroughly mix the blood and anticoagulant upon collection to prevent coagulation (Figure 2). For blood processing, load, balance, spin and PRP-extraction maneuvers must be executed. To load, using the concentrating device, unscrew the cup and slowly add the 30ml of anticoagulated whole blood into the concentrating device. Screw the cap onto the device and load into the Drucker Centrifuge (Series Performance). To balance, counterbalance the concentrating device.
Place the counterbalance and Concentrating Device directly opposite to each other in the centrifuge rotor and make sure they contain the same amount of volume. To spin, close the centrifuge lid. Set RPM (revolutions per minute) to 2400, and the time to 12 minutes. Press the start bottom. Once the centrifuge processing is complete, open centrifuge and removes the Concentrating Device. To extract PRP, unscrew the cap from the Port (Figure 2). Using the Aspirating accessory aspirate the plasma into the 30ml syringe until the disc touches the red cell interface, trapping the platelet buffy coat. Open the stopcock to the 12ml syringe and aspirate 3ml of PRP. Dilute the PRP with plasma to adjust the platelet concentrations. Remove sterile syringe and apply sterile cap (Figure 3).
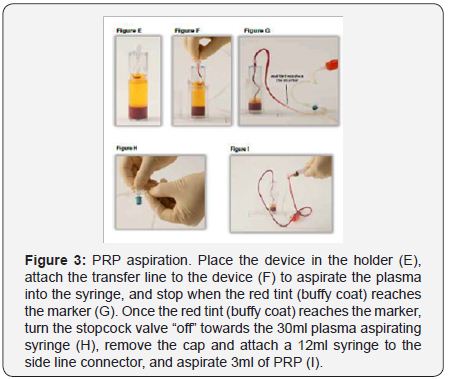
For blood infiltration, the 3ml of PRP obtained from previous processing was applied into the lateral aspect of the knee joint. To infiltrate the knee joint, the patient lies on supine position, with mild flexion of the knee and slight external displacement of the patella to expose the articular joint space. To clean the skin, previous to infiltration, 1% antiseptic chlorhexidine solution was used. After 3 sessions are performed, a clinical evaluation by VAS and WOMAC scales was performed one-two months after treatment, and adverse effects (if any), are recorded. From that point of, evaluation every 6 months were accomplished depending on the clinical symptoms and weight –bearing radiographies were taken.
The symptoms severity of knee OA was evaluated using the Western Ontario and Mac Master Universities Index for Osteoarthritis (WOMAC) [20-22] The WOMAC Index contains 24 questions in a total of three sections, namely pain, stiffness and function [20-22]. Each section has five response options (none, mild, moderate, severe and extreme); and subtotal scores for pain (5 items), stiffness (2 items) and function (17 items), which range from 0-20, 0-8 and 0-68 respectively [20-22]. It is considered a difference greater than 6% of the maximum scores of the WOMAC Index as being clinically important. That is, for the WOMAC sub-scales: pain=1.2, stiffness=0.5 and function=4.1 [23]. Outerbridge classified Chondromalacia in grades as follows: Grade 0, no features of Chondromalacia; Grade 1, edema and softening of cartilage; Grade 2, fibrillation and fissure lower than 1cm; Grade 3, fibrillation and fissure between 1-2cm; and Grade 4, fibrillation greater than 2cm and exposure of subchondral bone [24,25].
Statistical analysis was performed using the SPSS ® version 20.0. Initially, to evaluate quantitative variables means and standard deviations were used; while for qualitative variables, frequencies and percentages were used. To evaluate a significant change in before-and-after the treatment for quantitative variables, the t-student test was used. For the evaluation of qualitative variables (if needed), x2 test was used. The level of significance was 95% with an α-error of 0.5 (p<0.05). If a new treatment protocol was needed, repeated measurements for statistical analysis were applied.
Results
The mean age of the patients was 40.3±9.5 years. The women studied corresponded to 37.5% (n=9) and men 62.5% (n=15). The female to male ratio was 0.6:1 (Table 1). The most frequent Chondromalacia grade was 2nd degree (n=11, 45.8%), followed by 3rd (n=9, 37.5%) and 4th (n=4, 16.6%). Post-puncture erythema and inflammation occurred in 4.1% of patients (n=1) (Table 1). Pain measured by VAS significantly decreased (p<0.0001) from 7.87 to 3.04 points. The WOMAC-pain, WOMACstiffness and WOMAC-function subscales decreased significantly (p<0.001) from 15.12 to 5.95 points, 1.95 to 0.5 and 39.75 to 16.37, respectively (Table 2) and (Figure 3). Post-puncture erythema and inflammation was present as a complication of intra articular PRP protocol in 4.1% (n=1) of patients.
Discussion
PRP is a regenerative therapy that has gained popularity in musculoskeletal medicine for its potential to augment repair of tissues with low healing ability, cartilage is an example [26]. Clinical studies have investigated PRP for tendon, ligament, muscle and cartilage repair, yielding limited Level-I evidence supporting the use for knee OA [26]. PRP has gained popularity in regenerative medicine and other specialties since the earliest reports of its clinical use in the 1980s and 1990s, with applications traced to the fields of cardiac, dental and maxillofacial surgery [27,28].
Today, in musculoskeletal and sports medicine, PRP has become highly attractive for its potential benefit and influence on repairing injured tissue, treating a wide range of degenerative disorders, and accelerating return to sport, finding its role as an injectable biologic used to augment healing of tendon, ligament, muscle and cartilage [29]. The utility of PRP in promoting healing is specially significant for tendons, ligaments and cartilage, in which the process of repair can be particularly slow and poor due to their limited blood supply and slow cell turnover [30,31]. The physiologic progression through the phases of wound healing is orchestrated by growth factors and cytokines, many of which are released and modulated by blood components in PRP, mainly stored in platelet alpha-granules [26].
PRP therapy involves the direct introduction within the joint of an autologous product enriched in growth factors and cytokines that may be capable of limiting the inflammatory response and promoting healing over a fairly long period. These theoretical considerations provide a rationale for investigating PRP as a treatment for cartilage disorders, including focal lesions and the diffuse damage, as it is seen in chondromalacia and PFPS [32]. To the best of our knowledge, several trials have been focused on use of PRP for management of knee and hip OA [26]; but scarce studies, if any, have been published on the study and management of knee cartilage, as in chondromalacia and PFPS. There subsides the importance and novelty of this study.
To date, there are controversial results of PRP on musculoskeletal pathology, including tendon, bone and cartilage repair. No clear conclusions were obtained on these studies because of heterogeneity on PRP preparation methods, application techniques and outcome measures, and low-level studies [26]. The present quasi-experimental before-and-after study, also known as a non-randomized control trial, tries to solve such weaknesses, by the use of an established protocol (3-doses of 3ml PRP intra articular infiltration, 1 session/week), the use of validated outcome measures (VAS and WOMAC Index) and a before-and-after follow-up analysis, all of that gives a good level of evidence (IIb, according to the Canadian Task Force) [33], that let us postulate PRP as a promising option for Chondromalacia and PFPS.
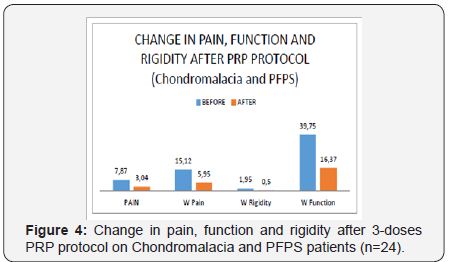
The importance to show our clinical experience in a series of patients is to provide more clinical evidence of an established PRP-treatment protocol on knee cartilage degeneration; because of the scarce of the studies on these issues (mainly Chondromalacia and PFPS). Patellofemoral pain Syndrome (PFPS) and Chondromalacia are among the most common etiologies of anterior knee pain in the general population [34]. The etiology, diagnosis and treatment are still a challenge. Ficat believes there is a degradation of articular cartilage, secondary to mechanical factors, releasing proteolytic enzymes, such as catepsines, that are responsible for the fibrillation and breakdown of cartilage [34]. The release of growth factors and cytokines by PRP could modify the intra articular environment to reduce inflammation, preserve cartilage and or induce cartilage regeneration [35]. This is the reason why we postulate PRP as a promising alternative in the management of PFPS and Chondromalacia (Figure 4).
PRP is an autologous derivative of whole blood that contains high concentrations of growth factors including transforming growth factor beta (TGF-β), insulin-like growth factor (ILGF) platelet derived growth factor (PDGF), basic fibroblast growth factor (BFGF), epidermal growth factor (EGF), and vascular endothelial growth factor (VEGF), as well as bioactive proteins that influence the healing of tendon, ligament, muscle and bone [36]. These growth factors serve to promote local angiogenesis, modulate inflammation, inhibit catabolic enzymes and cytokines, recruit local stem cells and fibroblasts to sites of damage or injury, and induce healthy nearby cells to manufacture greater number of growth factors [36]. Thus, the local use of PRP directly at the site of cartilage injury is thought to stimulate a natural healing cascade and accelerate the formation of cartilage repair tissue [36]. These combined effects of PRP make it a potential injectable option for the management of knee cartilage disease.
In our series, the mean age of was 40.3±9.5 years. It is similar to the series reported by Chahla et al. [35], and Fernández- Cuadros et al. [34]. This is in accordance with several studies which state that PFPS/Chondromalacia is very frequent in the second and third decades of life [37]. Wiles states that the process often begins during the second decade of life, and by the age of thirty nearly everyone is affected. It is, however, in only a few individuals that the changes cause symptoms, and in a fewer still that they progress to Osteoarthritis (OA) [38].
PFPS/Chondromalacia affects more commonly women, with a female to male ratio of nearly 2:1 [34,37,38]. However, in our study, the female to male ratio was lower (0.6:1). The natural history of PFPS/Chondromalacia is controversial. Wiles consider this entity as a pre-stage of OA [38]. Conversely, Karlson [39] states that the conversion of Chondromalacia to OA is very uncommon, that in a 20 years follow-up, he found no incidence in OA [38-40]. In our study, we have found the prevalence is greater in lower Chondromalacia degrees according to Outerbridge classification [24] (2º was the most frequent and 4º the least common), probably because of the slow progression of this entity [38-40].
When a histological study is performed on Chondromalacia patients, the synovial membrane shows some hyperemia and proliferation in almost all cases. In a few, usually near the fissuring, a synovial pannus spread over the outer rim of the articular cartilage. This pannus consists of relatively vascular connective tissue covered by flattened synovial cells, and it is so firmly adherent to the cartilage as to be inseparable [38]. This suggests that inflammation subsides under this entity [34].
As a recent meta-analysis suggests, the rationale use for PRP is that the supraphysiological release of platelet derived factors directly at the site of cartilage disease may stimulate the natural healing cascade and the regeneration of tissue and further mediate the anti-inflammatory response [41]. This could be the reason why PRP was effective in the treatment of pain, function and quality of life in such patients. PFPS/Chondromalacia affect pain, function and quality of life in young populations, as OA affects pain, function and quality of life in old populations [34,42-45]. As pain and inflammation are common features in both entities, PRP is an effective therapeutic weapon, as it is being demonstrated improving WOMAC and VAS pain scales on this study.
In our study, a PRP-protocol of 3 injections has shown effectiveness in improving pain relief, function and rigidity in PFPS/Chondromalacia globally and in all grades of Chondromalacia. Considering that Chondromalacia is a pre-stage of OA [38], our findings are in accordance with most present studies that show moderate quality of evidence supporting the use of PRP on knee OA treatment [32,35,36]. PRP is capable to improve pain and function measured by VAS and WOMAC [26,36]. But, when it comes to see whether PRP is effective in all grades of severity, is still a matter of controversy.
Wu et al. [26] states that most of trials suggest the efficacy of PRP to improve functional outcomes only for mild knee OA [26]. Spaková et al. [46] concluded that autologous PRP is effective and safe in early knee OA (K-L 1º, 2º and 3º grades). Moreover, Zlotnicki et al. [47] stated that multiple studies suggest that advanced knee OA implies ineffective response to PRP injection therapy. Most of researchers state that PRP is more effective in milder than in severe lesions of the knee, as it was the case in our chondromalacia patient series.
Study Limitations
A limitation of the study is the small size of the series (n=24). Fifty months were needed to collect such a number of patients, since this is a prospective study. Another limitation of the study is the lack of control group. This is mainly due to the limited number of cases. As the effectiveness of PRP on knee OA is supported by several RCT, it was not ethical to deny the intervention on chondromalacia (a pre-stage of OA), when other conservative non-surgical treatments had already failed. A quasi-experimental before-and-after study, also known as a non-randomized control trial is applied to this specific ethical situation, in order to solve the lack of control give and to give clinical based-evidence. Besides, this type of study tries to evaluate the impact of an intervention, and since there is only one group, no randomization is possible. Despite all previous considerations, this is a kind of experimental study with a good level of evidence; in fact, the Canadian Task Force on Preventive Health Care gives these studies a II-b level of evidence [33].
Conclusion
PRP Infiltrated in a 3-doses-protocol is effective in the treatment of PFPS/Chondromalacia symptoms such as pain and stiffness, improving function and QoL measured by validated outcome scales such as VAS and WOMAC. PRP alleviates PFPS/ Chondromalacia symptoms in all Outerbridge grades. The study shows a good level of evidence and grade of recommendation that allows us to consider PRP as a conservative therapeutic option in the treatment of PFPS and chondromalacia.
References
- Thomeé R, Augustsson J, Karlsson J (1999) Patellofemoral pain syndrome. Sports med 28(4): 245-262.
- Dixit S, DiFiori JP, Burton M, Mines B (2007) Management of patellofemoral pain syndrome. Am Fam Physician 75(2): 194-202.
- O’Connor FG, Mulvaney SW (2016) Patellofemoral pain Syndrom. Wolters Kluwer, USA.
- Cutbill JW, Ladly KO, Bray RC, Thorne P, Verhoef M (1997) Anterior knee pain: a review. Clin J Sport Med 7(1): 40-45.
- Wiles P, Andrews PS, Devas MB (1956) Chondromalacia of the patella. Bone & Joint Journal 38(1): 95-113.
- Witvrouw E, Callaghan MJ, Stefanik JJ, Noehren B, Bazett-Jones DM, et al. (2014) Patellofemoral pain: consensus statement from the 3rd International Patellofemoral Pain Research Retreat held in Vancouver, September 2013. British Journal of sports medicine 48(6): 411-414.
- Fulkerson JP (2002) Diagnosis and treatment of patients with patellofemoral pain. The American Journal of sports medicine 30(3): 447-456.
- Dye SF (2005) The pathophysiology of patellofemoral pain: a tissue homeostasis perspective. Clin Orthop Relat Res 436: 100-110.
- Kon E, Mandelbaum B, Buda R, Filardo G, Delcogliano M, et al. (2011) Platelet-rich plasma intra-articular injection versus hyaluronic acid viscosupplementation as treatments for cartilage pathology: from early degeneration to osteoarthritis. Arthroscopy 27(11): 1490-1501.
- Kon E, Buda R, Filardo G, Di Martino A, Timoncini A, et al. (2010) Platelet-rich plasma: intra-articular knee injections produced favorable results on degenerative cartilage lesions. Knee Surg Sports Traumatol Arthrosc 18(4): 472-479.
- Post WR (1999) Current concepts clinical evaluation of patients with patellofemoral disorders. Arthroscopy: The Journal of Arthroscopic & Related Surgery 15(8): 841-851.
- Cook C, Hegedus E, Hawkins R, Scovell F, Wyland D (2010) Diagnostic accuracy and association to disability of clinical test findings associated with patellofemoral pain syndrome. Physiother Can 62(1): 17-24.
- Haim A, Yaniv M, Dekel S, Amir H (2006) Patellofemoral pain syndrome: validity of clinical and radiological features. Clin Orthop Relat Res 451: 223-228.
- Bizzini M, Childs JD, Piva SR, Delitto A (2003) Systematic review of the quality of randomized controlled trials for patellofemoral pain syndrome. J Orthop Sports Phys Ther 33(1): 4-20.
- van der Heijden RA, Lankhorst NE, van Linschoten R, Bierma-Zeinstra SM, van Middelkoop M (2015) Exercise for treating patellofemoral pain syndrome. Cochrane Database Syst Rev 1: CD010387.
- Crossley K, Bennell K, Green S, McConnell J (2001) A systematic review of physical interventions for patellofemoral pain syndrome. Clin J Sport Med 11(2): 103-110.
- Oliveira VC, Henschke N (2013) Multimodal physiotherapy is effective for anterior knee pain relief Br J Sports Med 47(4): 245-246
- Crossley KM, van Middelkoop M, Callaghan MJ, Collins, NJ, Rathleff MS, et al. (2016) 2016 Patellofemoral pain consensus statement from the 4th international patellofemoral pain research retreat, manchester. Part 2: recommended physical interventions (exercise, taping, bracing, foot orthoses and combined interventions). Br J Sports Med 50 (14): 844- 852.
- Heintjes E, Berger MY, Bierma-Zeinstra SM, Bernsen RM, Verhaar JA, et al. (2004) Pharmacotherapy for patellofemoral pain syndrome. Cochrane Database Syst Rev 3: CD003470.
- Cho HJ, Chang CB, Yoo JH, Kim SJ, Kim TK (2010) Gender differences in the correlation between symptom and radiographic severity in patients with knee osteoarthritis. Clin Orthop Relat Res 468(7): 1749- 1758.
- Bellamy N, Buchanan WW, Goldsmith CH, Campbell J, Stitt LW (1988) Validation study of WOMAC: a health status instrument for measuring clinically important patient relevant outcomes to antirheumatic drug therapy in patients with osteoarthritis of the hip or knee. J Rheumatol 15(12): 1833-1840.
- Wright RW (2005) Knee sports injury outcome measures. J Knee Surg 18(1): 69-72.
- Angst F, Aeschlimann A, Stucki G (2001) Smallest detectable and minimal clinically important differences of rehabilitation intervention with their implications for required sample sizes using WOMAC and SF‐36 quality of life measurement instruments in patients with osteoarthritis of the lower extremities. Arthritis Rheum 45(4): 384- 391.
- Outerbridge RE (1961) The etiology of chondromalacia patellae. J Bone Joint Surg Br 43-B: 752-757.
- Helms CA (1992) Fundamentos de radiología del esqueleto. España, Spain.
- Wu PI, Diaz R, Borg-Stein J (2016) Platelet-rich plasma. Phys Med Rehabil Clin N Am 27(4): 825-853.
- DelRossi AJ, Cernaianu AC, Vertrees RA, Wacker CJ, Fuller SJ, et al. (1990) Platelet-rich plasma reduces postoperative blood loss after cardiopulmonary bypass. J Thorac Cardiovasc Surg 100(2): 281-286.
- Ferrari M, Zia S, Valbonesi M, Henriquet F, Venere G, et al. (1987) A new technique for hemodilution, preparation of autologous platelet-rich plasma and intraoperative blood salvage in cardiac surgery. Int J Artif Organs 10(1): 47-50.
- Nguyen RT, Borg-Stein J, McInnis K (2011) Applications of platelet-rich plasma in musculoskeletal and sports medicine: an evidence-based approach. PM R 3(3): 226-250.
- Boswell SG, Cole BJ, Sundman EA, Karas V, Fortier LA (2012) Plateletrich plasma: a milieu of bioactive factors. Arthroscopy 28(3): 429-439.
- Sánchez M, Anitua E, Azofra J, Andía I, Padilla S, et al. (2007) Comparison of surgically repaired Achilles tendon tears using platelet-rich fibrin matrices. The American Journal of sports medicine 35(2): 245-251.
- Ornetti P, Nourissat G, Berenbaum F, Sellam J, Richette P, et al. (2016) Does platelet-rich plasma have a role in the treatment of osteoarthritis? Joint Bone Spine 83(1): 31-36.
- Mirón-Canelo JA (2013) Sistema de Información Sanitaria. Indicadores de Salud, Bienestar y Calidad de Vida. En: Guías para la elaboración de trabajos científicos, Grado, Máster y Posgrado. España, Spain.
- Fernández-Cuadros ME, Pérez-Moro OS, Albaladejo-Florin MJ (2016) Patellofemoral Pain Syndrome and Chondromalacia: The Effect of Ozone on Pain, Function and Quality of Life. A Non-Randomized Control-Trial. JSM Physical Med Rehabil 1(1): 1002.
- Chahla J, Piuzzi NS, Mitchell JJ, Dean CS, Pascual-Garrido C, et al. (2016) Intra-articular cellular therapy for osteoarthritis and focal cartilage defects of the knee. J Bone Joint Surg Am 98(18): 1511-1521.
- Dai WL, Zhou AG, Zhang H, Zhang J (2017) Efficacy of platelet-rich plasma in the treatment of knee osteoarthritis: a meta-analysis of randomized controlled trials. Arthroscopy 33(3): 659-670.
- DeHaven KE, Lintner DM (1986) Athletic injuries: comparison by age, sport, and gender. Am J Sports Med 14(3): 218-224.
- Wiles P, Andrews PS, Devas MB (1956) Chondromalacia of the patella. Bone & Joint Journal 38(1): 95-113.
- Karlson S (1939) Chondromalacia patellae. Acta Chir Scand 83: 347- 381.
- Castillo HD, Alonso JA, López FQ, Torres DL, Campuzano RS (2000) Correlación clínico-artroscópica de pacientes con síndrome de dolor anterior de la rodilla. Rev Mex Ortop Trauma 14(2): 137-52.
- Campbell KA, Saltzman BM, Mascarenhas R, Khair MM, Verma NN, et al. (2015) Does intra-articular platelet-rich plasma injection provide clinically superior outcomes compared with other therapies in the treatment of knee osteoarthritis? A systematic review of overlapping meta-analyses. Arthroscopy 31(11): 2213-2221.
- Fernández-Cuadros ME (2013) Análisis de la Calidad de Vida en pacientes con prótesis de rodilla. Tesis Doctoral. Universidad de Salamanca, España, Spain.
- Fernández-Cuadros ME, Pérez-Moro OS, Mirón-Canelo JA (2016) Could Ozone Be Used as a Feasible Future Treatment in Osteoarthritis of the Knee? Diversity & Equality in Health and Care 13(3): 232-239
- Fernández-Cuadros ME, Perez-Moro OS, Miron-Canelo JA (2016) Age and comorbidities affect quality of life in patients with osteoarthrtitis and knee replacement. Middle East Journal of Rehabilitation and Health 3(4).
- Fernández-Cuadros, ME, Pérez-Moro OS, Miron-Canelo JA (2016) Knee osteoarthritis: Impact on quality of life and effectiveness of total knee arthroplasty. Diversity and Equality in Health and Care 13(4): 278-283.
- Spaková T, Rosocha J, Lacko M, Harvanová D, Gharaibe A (2012) Treatment of knee joint osteoarthritis with autologous platelet-rich plasma in comparison with hyaluronic acid. American Journal of Physical Medicine & Rehabilitation 91(5): 411-417.
- Zlotnicki JP, Watson J, Rothrauff BB, Van Eck CF, Musahl V (2016) current state for clinical use of stem cells and platelet-rich plasma. Operative techniques in orthopaedics 26(2): 89-97.






























