Main Types of HPV with High Oncogene Risk: A Systematic Review
Bruna Campagnin Luiz1,2, Bruna Maria Carvalho1,2, Ricardo Cervini1,3,4, Kennyel André Velozo1,4, Lucas Ortiz Ledur1,4, Filipe de Matias Wagner1, Gustavo Colombo Dal-Pont1,4, Claudriana Locatelli1,3,4,5, Ariana Centa1,2,3,4,5*
1Medicine Course, Universidade Alto Vale do Rio do Peixe - UNIARP
2Academic League of Gynecology and Obstetrics (LAGO)- UNIARP
3Academic League of Oncology (LAOn)- UNIARP
4Health Translational Research Laboratory-UNIARP
5Postgraduate Program in Development and Society- UNIARP
Submission:February 19, 2023;Published:March 01, 2024
*Corresponding author:Ariana Centa, Universidade Alto Vale do Rio do Peixe - UNIARP, Caçador, Brazil
How to cite this article:Bruna Campagnin L, Bruna Maria C, Ricardo C, Kennyel André V, Lucas Ortiz L, et al. Main Types of HPV with High Oncogene Risk: A Systematic Review. J Tumor Med Prev. 2024; 4(3): 555638.DOI: 10.19080/ JTMP.2024.04.555638
Summary
HPV (Human Papillomavirus) is the virus that causes a sexually transmitted infection, of which five of its 53 genera infect human beings and can develop cervical cancer. Among the genera, more than 200 types of HPV are still classified, 15 of which have a preference for the anogenital tract and some of them have a high carcinogenic risk, such as subtypes 16, 18, 45 and 59. The present study seeks, through a review, systematic knowledge of which HPV subtypes are the most oncogenic, and was based on the study of 122 articles found in two databases. Therefore, of the 529 articles initially selected, 169 were read in full and 122 were properly included in this review, which identified as the most oncogenic HPV subtypes described in the literature: HPV-16, HPV-18, HPV-31, HPV- 58.
Abstract
The HPV (Human Papilomavirus), is the virus that causes a sexually transmitted infection, which five of its 53 genera infect humans and can develop cervical cancer. Among the genera, more than 200 subtypes of HPV are still classified, of which 15 prefer the anogenital tract and some of them have a high carcinogenic risk, such as subtypes 16, 18, 45 and 59. The present study seeks, through a systematic review, to find out which are the most oncogenic HPV subtypes,and is based on the study of 122 articles found in two databases. Thus, of the 529 articles selected, 169 were read in the integration and 122 were properly contemplated in this review, which pointed out as the most undesirable oncogenic HPV subtypes in the literature: HPV-16, HPV-18, HPV-31, HPV-58.
Keywords: Human Papillomavirus; HPV; High Grade Intraepithelial Lesion; Cervical Cancer; Prevalence; Genotypes.
Introduction
The Human Papillomavirus (HPV), identified as causing a sexually transmitted infection (STI), is part of the Papillomaviridae (PV) family. It is divided and cataloged into 53 genera, but only five of them are capable of infecting humans and are linked to different pathologies: alphapapillomavirus, betapapillomavirus, gammapapillomavirus, mupapillomavirus and nupapillomavirus. These five genera differ from each other by epithelial tropism. HPVs of the beta, gamma, mu, and nu genera have a cutaneous tropism causing warts on the hands or feet, while members of the alpha genus have a mucosal preference and may be associated with more serious diseases, including cancers [1]. Among the different types of HPV, 40 of them have a preference for the human genitoanal tract. Of these, 15 present a potential oncogenic risk and end up causing cervical neoplasms in 98% of cases. Therefore, the types of HPV viruses at high risk for the development of cervical cancer most described in the literature so far are: 16, 18, 33, 45, 58. Types 16 and 18 may be linked to up to 70% of cases of this neoplasm. two regarding its biological characteristics, it is known that HPV is a double-stranded, non-enveloped DNA virus with high tropism for stratified squamous (squamous) epithelium [2]. The squamous epithelium is distributed in layers and is part of the epithelial tissue, which performs the main functions of lining and secreting substances in different locations of the human body, including the vagina, allowing, after friction and mechanical forces, replacement cell phone. Furthermore, it is known that the walls of the vagina do not have mucous glands, with mucus secreted into the vaginal lumen coming from the cervical glands of the uterus [3].
Therefore, for HPV and its tropism for epithelial cells to actually occur, one of the necessary factors described in current literature is the interaction between the virus and an unbalanced vaginal microbiota, which can lead to the development of cervical cancer [4,5]. HPV infections occur mainly sexually, requiring contact between mucous membranes. In this way, the number of sexual partners a woman has during her life can be linked to the prevalence of the pathology among this portion of the population [6]. The HPV virus reaches the epithelium through microcracks or metaplastic cells and can reach the deep layers of the cervix. After that, the virus has the ability to evade the host’s immune system and remain latent for years or thrive immediately in the squamous epithelium, maturing and differentiating. Viral action causes squamous intraepithelial lesions, which can be considered pioneers in the development of a cancerous pathology [7]. Therefore, chronic infections with oncogenic types of HPV are the main risk factor for the development of cervical cancer. However, even if necessary, HPV infection in more than 99% of cases is not sufficient for the development of this type of cancer. Some factors increase the risk of neoplastic development, such as smoking, use of hormonal contraceptives, alcohol abuse and inadequate nutrition with an intake of simple carbohydrates, as well as saturated fats [8].
An important relationship is established between the development of cervical cancer and HPV infection, with this link being essential for the progression of lesions of the cervical epithelium, which precede the neoplasia itself [9]. Therefore, this neoplasia occurs due to a succession of cellular and molecular modifications preceded by contamination with HPV [10]. Furthermore, the misadjusted HPV production cycle is associated with the development of cervical cancer. Therefore, when the viral genome attaches to that of the human cell, it ends up losing genes E4 and E2, which are considered controls for the transcription of countless other viral genes, such as E6 and E7. As the function of the E2 gene decreases, there is an amplification in the genetic expression of E6 and E7, which ends up blocking the usual viral cycle [11-13].
The HPV E6 and E7 oncoproteins, considered essential for high risk, are attached to the host’s genome and act with different mechanisms of action. E6 binds to the tumor suppressor protein (p53), deactivating it. At the same time, E7 acts with the pRb protein (retinoblastoma) and provides the E2F transcription factor, which will facilitate the progress of the infected cell in its cell cycle, granting it the power of immortalization. In this way, the maturation of host cells does not occur, creating tissue mutations that individualize the HPV lesions induced in their different degrees [11,14,15]. HPV, in its most oncogenic subtypes, tends to have a long incubation time, usually close to 15 years from latent infection to cancer expression [15-17]. Among the subtypes, those that are most oncogenic can be highlighted, which belong to the species alpha-9 (HPV 16, 31, 33, 35, 52, 58 and 67), alpha-5 (HPV 51), alpha-6 (HPV 53, 56 and 66) and alpha-7 (HPV 18, 39, 45, 59 and 68). Alpha-9 HPV types are persistent and more likely to progress to CIN 3 or worse. HPVs 16, 31 and 33 are associated with an increased risk of CIN 2/3 and cervical cancer. Types 52 and 58 are only associated with a higher risk of CIN 2 [18].
Epidemiologically, cancer cases are expected to increase by 81% in poor countries by 2040 due to the growing increase in socioeconomic inequality, showing that this is an important determining factor in the development of some neoplasms, including cervical cancer (CA) [19]. In this sense, cervical cancer, also called cervical cancer, is the fourth cause of mortality in women due to neoplasms in Brazil and the third most common malignancy among women [20]. Being a public health problem, this neoplasm is diagnosed in approximately 17 thousand Brazilian women annually. In 2021, the estimated risk of cervical cancer was 15.38/100,000 women [21]. Furthermore, worldwide, the pathology is considered one of the main causes of mortality among women. In 2020, 604 thousand diagnoses of cervical cancer were recorded, of which 342 thousand resulted in death [22]. The HPV vaccine is a preventive measure against persistent infection by the virus, as well as a prophylaxis of precursor lesions that could transform into malignant neoplasms. The first vaccine worldwide was approved by the US Food and Drug Administration (FDA) in 2006 [23]. In this regard, there are different vaccines in the world with coverage of more or less subtypes of HPV, including the bivalent vaccine which guarantees protection against HPV 16 and HPV 18, the quadrivalent (qHPV) which provides protection against subtypes 06, 11, 16 and 18 and also other vaccines with wider coverage such as the nonavalent which, in addition to the protection offered by the quadrivalent, encompasses subtypes 31, 33, 45, 52 and 58 [24,25].
In Brazil, the vaccine was implemented into the national vaccination calendar in 2014, free of charge through the Unified Health System (SUS). Therefore, the available vaccine is quadrivalent, recombinant and covers subtypes: 6 and 11, which are considered low risk for neoplastic lesions, and also 16 and 18, which are more frequently linked to pre-cancerous lesions. The National Immunization Program (PNI) indication is for all female adolescents aged 9 to 14 years and males aged 11 to 14 years, two doses with 6 months between applications [26]. The nonavalent vaccine is not available through the PNI, but it is approved by the National Health Surveillance Agency (ANVISA) for use in Brazilian territory and is available for sale in pharmacies. In view of the above, the research is justified given the importance of controlling cervical cancer through the adoption of public policies for the prevention and treatment of the disease. However, knowledge of the oncogenic subtypes of HPV, that is, those that have greater potential for the development of cervical cancer, is important due to the possibility of the study contributing to the implementation of preventive measures for the disease. Therefore, understanding the individual oncogenic risk of different types of HPV can provide basic data to evaluate the success rate of vaccination in cervical cancer prophylaxis [27]. Furthermore, the information compiled from these data can help in the analysis of potential risks, as identifying the genotypes with the highest incidence and their relationship with certain populations can contribute to studies and development of new therapeutic approaches in order to improve the prognosis and quality of life of patients diagnosed with HPV. Therefore, the article aims to develop a systematic review regarding the subtypes of the Human Papillomavirus (HPV) with greater oncological potential and relate them to the risk of developing cervical CA.
Methodology
The study aims to develop a systematic review on the types of HPV involved in the development of cervical cancer following the methodological recommendations of the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (Prisma). The systematic review was carried out in July 2022 with a search for scientific articles through the electronic platforms PubMed and ScienceDirect. The articles used were those published in full since 2012. The descriptors that were used in the search were ‘ ‘High-risk Human papillomavirus’’ OR “hrHPV” AND ‘’uterine cervical neoplasms’’ OR “cervical cancer” AND “carcinogenisis” OR “carcinogenicity” OR “genotyping” OR “genotype”. For the evaluation, original articles on the topic published in the electronic databases mentioned above, in the English language, and which address issues related to the objective of the study were included in vitro and animal studies, clinical trials, book chapters, theses, dissertations, conference annals, technical reports and ministerial documents were excluded.
Experimental Design
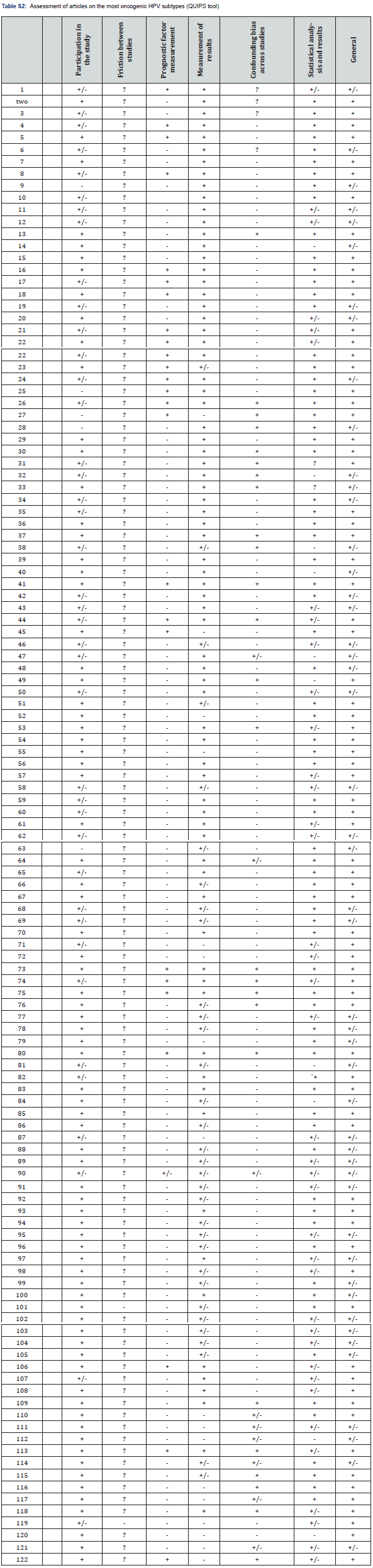
Data extraction from the selected articles took place by searching the chosen databases. Subsequently, the first selection of articles occurred with evaluation of the title and abstract, assessing whether they were, in fact, related to the review question and applying the appropriate inclusion and exclusion criteria. From this, the articles were read in full, once again applying the study criteria and the relationship with the topic addressed. The remaining articles were evaluated for quality and risk of bias according to the Quality in Prognosis Studies (QUIPS) classification criteria. The following items were evaluated: participation in the study, abandonment of the study, evaluation of the result, clarity and objectivity of the study and statistical analysis. After this classification, the articles obtained a qualification based on the following criteria: high quality (+), acceptable (+/-), low quality (-) and unsafe (?) [28].
Results
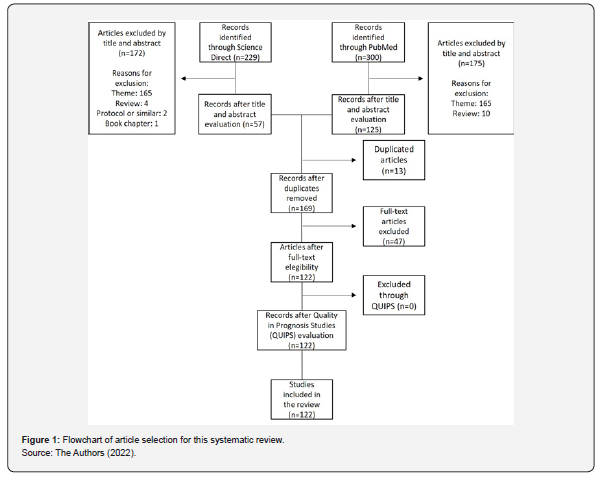
529 articles were found in the two databases selected using the methodology described above. After applying the inclusion and exclusion criteria, 57 articles remained in the ScienceDirect database and 125 in PubMed, totaling 182 articles. Of these, 13 were duplicates, leaving 169 articles for analysis. After reading the 169 articles in full, 47 were discarded as they did not address the theme proposed in this systematic review. Therefore, 122 articles were qualified for the necessary data collection as shown in Figure 1. The QUIPS instrument was used to assess the quality and risk of bias of the articles chosen for analysis. No articles were excluded from the systematic review, as none presented a high risk of bias. The main characteristics of each included article are presented in Table S1 & S2. Table 1 was created to account for those types of HPV that appeared most in the review. Thus, it was found that HPV 16 appeared in 119 articles (97.54%), followed by HPV 18 with 112 appearances (91.80%) and HPV 31 and 58, both appeared in 95 articles (77.87%). In relation to the less frequent types, HPV 70 appeared 8 times (6.56%) and the HPV 88 and HPV 89 types appeared only once (0.82%). The oncogenic HPV subtypes analyzed in the selected articles, according to the geographic location by country or region of the sample evaluated, are presented in Table 2.
Source: The Authors (2022).
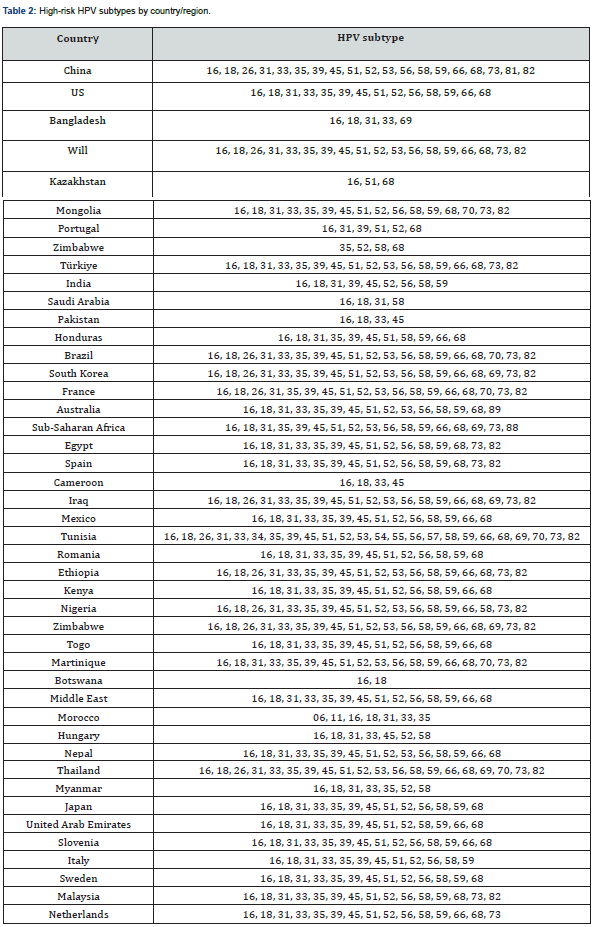
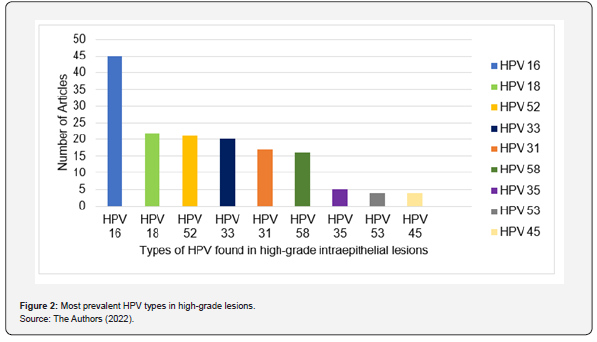
Of the 122 articles analyzed, 46 included studies on the type of HPV in high-grade intraepithelial lesions, considering the importance of HPV infection for cervical cancer. The most frequent HR-HPV (high risk -HPV) in high-grade lesions are described in Figure 2, with HPV subtype 16 being the most found, followed by subtypes 18, 52 and 33. Furthermore, the subtype that was least found in high-grade intraepithelial lesions was HPV 45. The nonavalent type HPV vaccine encompasses protection against HPV viruses 06, 11, 16, 18, 31, 33, 45, 52 and 58. These were shown, through of this review, highly prevalent in studies. However, as shown in Figure 2, there are some types of HPV frequently present in high-grade lesions that do not have vaccination coverage, among these the most frequent are types 35 and 53. As for the quadrivalent vaccine, used in the PNI, which encompasses types 06, 11, 16 and 18 some additional virus types are left without protection, these are types 52, 33, 31, 58 and 45. In Brazil, three studies were found, number #21, #95 and #117 (Appendix 1) and included in the review. In studies carried out in the Brazilian population, subtypes 16, 18, 31, 33, 34, 39, 45, 51, 52, 58, 66, 68 and 82 appeared unanimously. Furthermore, subtypes 53, 56, 59 and 73 were mentioned in two studies (#95 and #117). In China, 41 studies on the topic were found, totaling around 31.6% of the articles included in the review. In the Asian country, the presence of subtype 16 in the evaluations was unanimous. Subtype 18 was not verified in only 2 (4.88%) articles. Other highly prevalent subtypes, according to Chinese studies, were subtype 58 (92.68%), 33 and 52 (90.24%), 45 and 31 (85.37%) and 35 and 39 (82.93%).
Source: The Authors (2022).

(+) High Quality, (+/-) Acceptable, (-) Low Quality, (?) Unsafe.
Source: The Authors (2022).
Discussion
HPV, in its high-risk types such as HPV-16 and HPV-18, causes neoplastic and pre-neoplastic lesions in the uterine cervix. In this sense, virus infection can be considered as an initial stage of a potentially malignant lesion, which is a major cause of mortality among women worldwide [8]. Since there are few studies in the national literature compiling the most oncogenic subtypes of HPV in Brazil, and given their strong connection with cervical neoplasia, it is extremely important to understand what these subtypes are and relate them to the most common ones in the world. As seen in the review of articles, most of the studies on the topic are from the Asian continent, 41 of them (31.6%) from China. Therefore, with the large number of studies in the Asian country, the reliability regarding the types of the virus most prevalent in that region is high. Among the Chinese studies, the one with the largest sample of patients reveals that of the 961,029 women screened, 197,367 tested positives for HPV and of these, the most prevalent highrisk oncogenic types were HPV 16, followed by 52 and 58 [29]. The same pattern is repeated in other studies where the most prevalent subtypes were HPV 16, followed by 52. Therefore, when dealing with HPV in Chinese territory, the nonavalent vaccine is effective against the main viral subtypes of HPV that pose a high risk of neoplasms in the country [30].
In this sense, despite being a source of large studies regarding the virus, the Asian country only started vaccinating against HPV in 2016 when the bivalent vaccine was approved for subtypes HPV 16 and HPV 18. The nonavalent vaccine began to be applied later, in 2018, when it was approved by the China Food and Drug Administration (CFDA) and is used in the population between 11 and 26 years old in Chinese territory. 10 Thus, it is expected that there will be a reduction in the number of HPV infections in its subtypes covered by vaccination, as well as a reduction in mortality from cervical cancer. Among the HPV types with the greatest oncogenic potential are HPV 16, 31, 33, 52 and 58. 27 In a global scenario, HPV subtype 16 is the most prevalent in highgrade lesions, generally followed by 56 and 51 [31]. This scenario coincides with studies 32 that show that in approximately 60.3% of cervical neoplasms there is the presence of HPV 16 DNA.
In Brazil this is also a reality and HPV 16 is present in around 37% of high-grade lesions and in 2/3 of patients with invasive cervical neoplasia. 31 In 70% of cases of cervical neoplasia, types 16 or 18 are present. However, subtypes 52, 31 and 58 were also found in the sample in decreasing order of prevalence. 20,31 Another study 33 shows that the most frequent high-risk HPV types in the sample were different, demonstrating that high-risk subtypes 56 and 51 are more common. Finally, a study 34 shows subtypes 16, 31, 33, 52 and 58 present in the sample studied. Thus, it is noted that the Brazilian population already has contamination by subtypes of HPV not covered by the quadrivalent vaccine, which provides protection against HPV types 06, 11, 16 and 18. Since the nonavalent vaccine, which protects against all subtypes that tetravalent has coverage and, also, against subtypes 31, 33, 45, 52 and 58, it is not available through the SUS, it appears that the Brazilian population is at risk of infection by the other types found in the studies and which could be avoided by integrating this new vaccine into the PNI. Furthermore, although not available free of charge to the population, the nonavalent vaccine was approved for use in the national territory by the National Health Surveillance Agency 35 and is available for purchase to the population with indications for boys and girls aged between 9 and 26 years. However, there is an important limitation to its use since the cost is high and there are few incentive campaigns that demonstrate the importance of extra coverage. Therefore, this situation shows the importance of these studies to serve as a basis for the adoption by the PNI of the nonavalent vaccine that encompasses the other carcinogenic types of HPV that live in the national territory.The relationship between the development of cervical cancer and the HPV virus is well defined as a primary scenario for improvement and progression of lesions, which are important messengers that precede cancer [9].
The lesions that precede cervical cancer have had several changes in their terminologies over the years. Therefore, cervical intraepithelial neoplasias were systematized, histologically, into 3 grades: CIN I, qualified by cellular atypia located in the lower third of the squamous epithelium, considered mild dysplasia; CIN II, where atypia is covering two thirds of the squamous epithelium, with the category of moderate dysplasia; CIN III, typifying cells that involve more than two thirds or the entire thickness area of the epithelium, indicated in marked dysplasia/carcinoma in situ [32]. Therefore, an updated cytological classification was established, the Bethesda System, which introduced numerous cytological and histological concepts and established the following classifications: CIN I, low-grade squamous lesions (LSIL); CIN II and CIN III, highgrade intraepithelial lesions (HSIL) grouped together, all related to Human Papilloma Virus (HPV) infection. As we know, each HPV genotype has a tendency to develop different lesions on the cervix. HPV types 16, 31 and 33 are associated with a higher risk of cervical cancer, while types 52 and 58 are associated with a higher risk of CIN2. 18 Therefore, it is expected that, in a population where HPV 16 is prevalent, such as the Brazilian population, we may have higher rates of neoplasms in relation to CIN2 lesions, especially if there is no adequate prevention and screening program [33].
Furthermore, it is noted that there is a trend where, regardless of the country studied, most studies have documented the presence of several of the HR-HPV subtypes. Therefore, it is inferred that the type of HPV is not only related to the location of a population itself, but shows that it is also linked to the particularities of each individual within that population [34]. Therefore, within the same population, several oncogenic subtypes of HPV are distributed. After all, it is known that HPV infection is a necessary factor, but not sufficient, for the development of cervical cancer. Other factors, mainly related to lifestyle such as smoking, prolonged use of hormonal contraceptives and diet also influence this condition [35].
When analyzing studies with larger samples from each continent, it is possible to observe the variability of HPV types that can infect the same population. In the Asian population, in 197 thousand Chinese women, subtypes 16, 52, 58, 31, 33, 45, 66, 73 and 82 were found. 29 On the European continent, with a sample of 10,665 women, subtypes 16 were observed, 39, 31, 68, 52 and 51 [36]. North America, with a sample of 15,040 Mexican women, brought subtypes 16, 18, 31, 33, 35, 39, 45, 51, 52, 56, 58, 59, 66 and 68 [37]. In South America, with a sample of 443 women, HPV types 56 and 51 are most common, followed by 53, 18, 58, 52 and 16 [38]. On the African continent, sample of 1020 women, the most prevalent subtypes were HPV 16, 18, 31, 33, 45, 53, 58. 38 Finally, in Oceania, a study with 1013 women infected by HPV, the most prevalent were 16, 51, 53, 62, 89 and 52 [39]. Therefore, it is noted that knowledge about HPV subtypes can serve as a foundation for the adoption and development of more effective prevention and therapeutic approaches to HPV infections and, consequently, reduce mortality from this neoplasm.
Conclusion
This systematic review, using appropriate search criteria, found 122 articles related to HPV subtypes at high risk for developing cervical cancer. After analyzing the articles, it is concluded that, in general, the most prevalent HR-HPV infections are HPV 16, 18, 31, 58, 52, 45, 35, 51, 39 and 33. Furthermore, it is clear that the most prevalent HR-HPV types in high-grade lesions are HPV 16, followed by HPV 18, 52, 33, 31, 58, 35, 53 and 45. Therefore, it is inferred that studies on the types of HPV that are most related to cervical neoplasms are of paramount epidemiological importance for public health policies, especially vaccines that protect a population against the main types found in their region.
References
- Pippa Cosper F, Samantha Bradley, Lexi Luo, Randall Kimple J et al. (2021) Biology of HPV Mediated Carcinogenesis and Tumor Progression. In: Seminars in Radiation Oncology. WB Saunders pp. 265-273.
- Calumby RJN, Silva RAS, Suárez JAG, Lôbo TLGF, Vieira DS, et al. (2020) Human Papilloma Virus (HPV) and cervical neoplasia: importance of vaccination. BJR 3(2):1610-1628.
- Junqueira LCU, Silva Filho JC (2012) Cellular and molecular biology. Rio de Janeiro: Guanabara Koogan.
- Kovachev SM (2020) Cervical cancer and vaginal microbiota changes. Arch Microbiol 202(2):323-327.
- Usyk M, Zolnik CP, Castle PE, Porras C, Herrero R, et al. (2020) Cervicovaginal microbiome and natural history of HPV in a longitudinal study. PLoS Pathology 16(3): e1008376.
- Martinez GG, Nunez JT (2014) Natural history of human papilloma virus infection: an update. Invest. Clin 55(1): 82-92.
- Cardial Mf, Roteli-Martins CM, Naud P, Fridman FZ (2017) Human papillomavirus (HPV). In: Vaccination program for women. São Paulo: Brazilian Federation of Gynecology and Obstetrics Associations, Brazil.
- Busnardo DK, Silva GF, Centa A, Locattelli C (2022) Risk factors associated with human papillomavirus-induced cervical cancer: an integrative review. Conjec 22(2): 913-927.
- Koshiyama M (2019) The effects of the dietary and nutriente intake on gynecologic cancers. Healthcare 7(3): 88.
- Li Y, Yu T, Yan H, Li D, Yu T, et al. (2020) Vaginal microbiota and HPV infection: novel mechanistic insights and therapeutic strategies. Infect Drug Resist 13:1213.
- Ferraz LC, Santos ABR, Discacciati MG (2012) Cell cycle, HPV and evolution of cervical intraepithelial neoplasia: selection of biological markers. J Health Sci Inst 30(2): 107.
- Wentzensen N, Vinokurovaet S, Doeberitz MVK (2004) Systematic review of genomic integration sites of human papillomavirus genomes in epithelial dysplasia and invasive cancer of the female lower genital tract. Cancer Res 64(11): 3878 -3884.
- Doorbar J (2005) The papillomavirus life cycle. J Clin Virol 32: S7-S15.
- Burley M, Roberts S, Parish JL (2020) Epigenetic regulation of human papillomavirus transcription in the productive virus life cycle. Semin Immunopathol 42(2): 159-171.
- Penson RT,Lee LT (2021) Câncer cervical. BMJ Best Practice.
- Watson RA (2005) Human papillomavirus: confronting the epidemic- a urologist's perspective. Rev Urol. Summer 7(3): 135-144.
- Schwarz E, Freese UK, Gissmann L, Mayer W, Roggenbuck B, et al (1985) Structure and transcription of human papillomavirus sequences in cervical carcinoma cells. Nature 314: 111-114.
- Liu F, Chang L, Bai T, Liu X, Hu J (2021) Association of human papillomavirus genotype distribution and cervical cytology: a cross-sectional study. Epidemiol Infect 149:
- World Health Organization (2020) Focal Points Brazil.
- José Alencar Gomes da Silva (2019) National Cancer Institute. 2020 estimate: incidence of Cancer in Brazil. Rio de Janeiro: INCA, Brazil.
- National Cancer Institute José Alencar Gomes da Silva Estimate (2020) incidence of Cancer in Brazil. Rio de Janeiro: INCA, Brazil.
- World Health Organization (2021) Who guideline for screening and treatment of cervical pre-cancer lesions for cervical cancer prevention: web annex A: syntheses of evidence.
- Biselli-Monteiro M, Ferracini AC, Sarian LO, Derchain SFM (2020) Influence of gender and undergraduate course on the knowledge about HPV and HPV vaccine, and vaccination rate among students of a Public University. Rev Bras Ginecol Obstet 42(2): 96-105.
- Nadal SR, Manzione CR (2006) Vaccines against human papillomavirus. Rev bras colo-proctol 26: 337-340.
- National vaccination calendar (2022) Brasília: Ministry of Health, Brazil.
- So KA, Lee IH, Lee KH, Hong SR, Kim YJ, et al. (2019) Human papillomavirus genotype-specific risk in cervical carcinogenesis. J Gynecol Oncol 30(4): e52.
- Hayden JA, Côté P, Bombardier C (2006) Evaluation of the quality of prognosis studies in systematic reviews. Ann. Intern. Med. 144(6): 427-437.
- Chen X, Xu H, Xu W, Zeng W, Liu J, et al. (2017) Prevalence and genotype distribution of human papillomavirus in 961,029 screening tests in southeastern China (Zhejiang Province) between 2011 and 2015. Sci Rep 7(1): 1-8.
- Wang R, Guo X, Wisman GBA, Schuuring E, Wen-feng Wang, et al (2015) Nationwide prevalence of human papillomavirus infection and viral genotype distribution in 37 cities in China. BMC infectious diseases 15(1): 1-10.
- Castellsagué X, Alemany L, Quer M, Halec G, Quirós B, et al. (2016) HPV involvement in head and neck cancers: comprehensive assessment of biomarkers in 3680 patients. J Natl Cancer Inst 108(6): djv403.
- Martins TR, Oliveira CM, Rosa LR, Centrone CC, Rodrigues CLR, et al. (2016) HPV genotype distribution in Brazilian women with and without cervical lesions: correlation to cytological data. Virol J 13(1): 1-9.
- Sanjose S, Serrano B, Tous S, Alejo M, Lloveras B, et al. (2018) Burden of human papillomavirus (HPV)-related cancers attributable to HPVs 6/11/16/18/31/33/45/52 and 58. JNCI 2(4): pky0.
- Lorenzi AT, Fregnani JH, Villa LL, Sichero L, Nunes MN, et al. (2019) Diversity of human papillomavirus typing among female population living in rural and remote areas of Brazilian territory. Papillomavirus Res 8: 100186.
- Paesi S, Correa L, Tregnago MC, Mandelli J, Roesch-Ely M (2015) Human papillomavirus among women with atypical squamous cells of undetermined significance in southern Brazil. Int J Gynecol Obstet 128(1): 23-26.
- Resolution of the RDC Collegiate Board No. 197, 2017. Brasília: Anvisa, Brazil.
- Sousa H, Tavares A, Campos C, Marinho Dias J, Brito M, et al. (2019) High-Risk human papillomavirus genotype distribution in the Northern region of Portugal: Data from regional cervical cancer screening program. Papillomavirus Res 8: 100179.
- Torres-Poveda K, Ruiz-Fraga I, Madrid-Mariana V, Chavez M, Richardson V (2019) High risk HPV infection prevalence and associated cofactors: a population-based study in female ISSSTE beneficiaries attending the HPV screening and early detection of cervical cancer program. BMC cancer 19(1): 1-12.
- Catarino R, Vassilakos P, Jinoro J, Croquet C, Benski AC, et al. (2016) Human papillomavirus prevalence and type-specific distribution of high-and low-risk genotypes among Malagasy women living in urban and rural areas. Cancer Epidemiol 42: 159-166.
- Tabrizi SN, Brotherton JML, Kaldor JM, Skinner, SR, et al. (2014) Assessment of herd immunity and cross-protection after a human papillomavirus vaccination programme in Australia: a repeat cross-sectional study. Lancet Infect Dis 14(10): 958-966.






























