Study to Evaluate the Effectiveness and Safety of a Product for the Restoration of Physiological Hydration, and of a Product for the Control of Tissue Hyperactivation During Radiotherapy in Subjects Affected by Cancer
Raffaele Migliaccio*
NEILOS SRL, 80063 Piano di Sorrento, Italy
Submission:September 08, 2023;Published:October 02, 2023
*Corresponding author:Raffaele Migliaccio, NEILOS SRL, 80063 Piano di Sorrento, Italy
How to cite this article:Raffaele M. Study to Evaluate the Effectiveness and Safety of a Product for the Restoration of Physiological Hydration, and of a Product for the Control of Tissue Hyperactivation During Radiotherapy in Subjects Affected by Cancer. J Tumor Med Prev. 2023; 4(2): 555634. DOI: 10.19080/ JTMP.2023.04.555634
Abstract
The radiotherapy is a therapy that uses ionizing ra- diations for medical purposes, particularly during the cancer therapies for the control of malignant cells growth, that could develop in cancer, sometime is wrongly confused with radiology (that despite is the use of radiation for imaging and medical diagnosis). The sensitivity of tumors to radiotherapy is highly variable. A certain kind of tumor is defined ‘sensible’ when it is more vulnerable to the radiation effect in comparison with the normal tissues that surround it. When a cancer is easily approachable, for example, a superficial tumor or a tumor localized in a precise location in an organ like uterus, in which is possible to introduce a radiation source, could be treated with radiotherapy. As the radiotherapy tries to avoid the normal tissues, it is useful when the cancer cannot be removed surgically because the surrounding tissues could be damaged, or because the tumor could have been penetrated in the near tissues that cannot be touched. It is important to pay attention to the fact that the radiotherapy has many side effects, that cause a re- ally pathology: the radiation induced pathology. The symptomatology of this disease is nausea, diarrhea, vomit, hair loss and anemia.
Keywords:Diarrhea; Anemia; Bacterial Infection; Ulcerations; Radiotherapy; Pathology; Breast Cancer; Radiodermatitis
Introduction
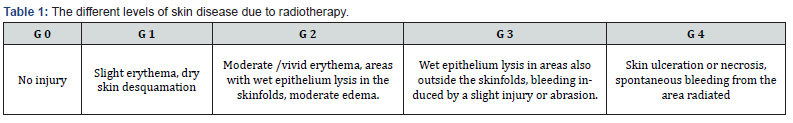
The solution could be in decreasing the dosage or if the symptomatology does not decrease also the therapy cessation. The radiotherapy is not painful. In the case in which it is used a low dosage (palliative treatments), it has any side effect. In the case in which a high dosage is used, there are many side effects. Some could arise during the treatment (acute side effects), or in the following mon- ths and/or years after the treatment (long term side effects). The type of side effects depends on the organ treated, on the therapy applied, but overall on the dosage of radiation administered and absorbed by the tissues (Table 1). Each person has its proper reaction in comparison with the absorbed dosages, moreover in the next tre- atments made on the same area could arise different problems: each kind of tissues has its proper maxi- mum tolerance to radiation, for this reason treating in different periods some different tissues that had reached their maximum dosage could cause many problems.
Many side effects are expected and waited:
Acute side effects:
i.Damaging of epithelial tissues (precocious radio dermatitis)
ii.Inflammation and swelling of the radiated area
iii.Infertility
iv.Fatigue
Long term side effects:
i.Later radiodermatitis
ii. Fibrosis
iii. Hair loss
iv. Dry mout
The different levels of skin disease due to radiotherapy could be linked as in the following table:
G 0 No damage
G 1 Slight eruption, edema desquamation
G 2 Erythema, desquamation, edema, small vesicles,
ulceration
G 3 Vesicles, ulcerations, edema with impact on the
normal activity
G 4 Local or wide process, with bacterial infection
Clinical Report
To submit the patients to radiotherapy, that to be efficacy has to be finished in totally as provided in the therapeutically scheme, it is very important the status of the skin in the area that is going to be radiated because it is necessary that should be kept more intact as possible to be able to complete the radiotherapy treatment. In fact the skin alteration due to radiation, which it is never G0 or G1, can cause the interruption of the therapy, or at least has a bad influence on the quality of life of patients.
In order to conclude the therapy it is important to maintain the skin in G0 -G1.
In our hospital the cancer patients are submitted to radiotherapy both adjuvant and neo-adjuvant.
The therapeutically effect of the treatment is important for the improve of survival of patients.
The side effects that could arise are both systemic and topic.
The topic side effect is very important for two reasons:
i. The patient is compelled to interrupt the therapy for the
relevant skin alteration that could not permit the going on with
therapies.
ii. Negative effect in psychophysical level for the outside
looking of his skin, and overall for the pain that this treatment
could cause.
The skin submitted to radiation is easily attacked by pathogens and it is very important to protect in the correct way. The presence in the market of two products DerLife cream and RaLife cream, of Again Life Italia, has attracted our attention in order to evaluate the effects on cancer patients submitted to radiotherapy with the goal to observe and evaluate the skin status during and at the end of treatment. Derlife cream is characterized by a great capability of hydrating. This characteristic is very important in subjects submitted to radiotherapy because the physio- logical hydration allows a correct therapy efficacy. RaLife cream is characterized for the presence of a specific group of fatty acids FAG to be used in the patients undergoing radiotherapy; the goal is to control the inflammatory process of the skin and the skin hydration. We decided to use these two products in some patients, properly informed, to be submitted to radiotherapy with the purpose of maintaining the skin layers in normal condition in patients with damaged skin, and in patients with ascertained skin hyperactivity with the intention to repair and normalize the skin layers. The main goal is to carry out the therapy. On this basis 94 patients had been selected, submitted to radiotherapy during the period from June 2012 to January 2013, in Radiotherapy UOC of AORN, SG Moscati, Avellino Italy.
The inclusion criteria of patients to topic therapy with the
purpose of prove the products efficacy was:
i. Nobody had been submitted to a dermatological
treatment in the six months before, due to a dermato- logical
pathology
ii. Nobody should have had diffusive pathologies or
allergies to drugs, food, cosmetic products and personal hygiene
products (soaps etc.)
iii. Nobody should present skin discoloration
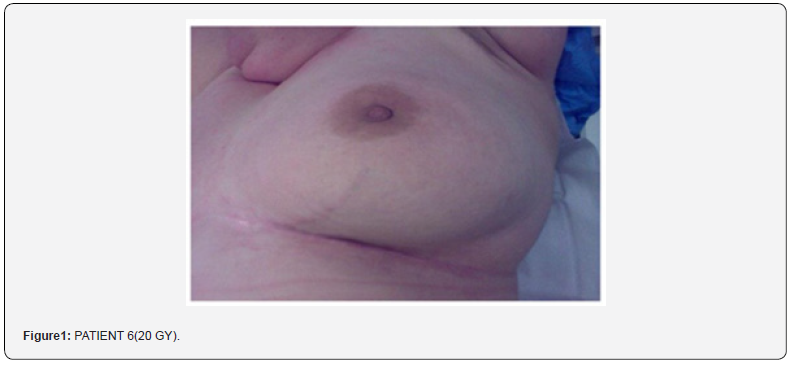
Everybody had been presented the treatment that they were going to be submitted to, and everybody signed an ‘informed consent’. These patients were 94 females (Breast cancer). The middle age is 54 years old, (range between 32 and 87). The skin tropisms were normal in 63 patients and hypo trophic in the other 31. The total dosage given, and the treatment timing of radiotherapy is 50/60 Gy as dosage, and 5/6 weeks of treatment. All patients had been trained to adopt some simple personal hygiene treatments, as for example the usage of soap with neutral pH (ex. ClinLife wash), to avoid rubbing with abrasive presides (the only permitted had been the natural sponge), and to avoid to rub the skin. It had also been requested to not wear synthetic clothing in direct contact with skin, with seams, lace etc. A particular care had been advised to patients for the skinfolds in this case had been suggested Der- Life milk (Figure 1).
The product RaLife cream had been given to patients at the beginning of the skin irritation G1, the number of cream packs given had been related to the treatment timing, and generally at 10/15 sessions (20/30 Gy).
The patients went on with the treatment with DerLife cream one week after the end of the radiotherapy.
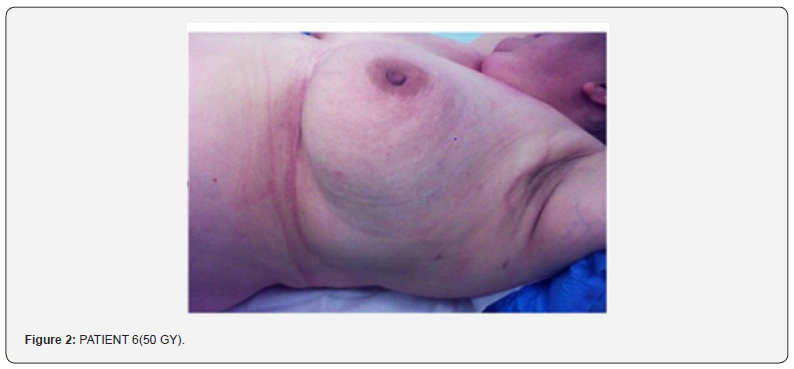
Photos had been made to some patients at the first treatment and at the treatment end to evaluate the skin conditions in the radiated area. All patients had been submitted to an objective examination when started the radiotherapy, and one per week until the end of treatment. Then after 15,30, 45 day after the treatment end. Nobody needed any further evaluation for skin toxicity (Figure 2). Patients had been submitted to a six-monthly follow up. Nobody had skin fibrosis, everybody had a nor- mal trophic skin and with a slight hyper chromatic, the personal evaluation of the skin looking had been good/elevated.
The products had been given in this way:
i. DerLife cream: 2 applications per day with an interval
of 8/10 hours, on dry skin, for two weeks before radiotherapy
start, with a suspension 3/4 days before treatment
ii. RaLife cream: 2 applications per day with an interval
of almost 10 hours, on red skin, avoiding applying immediately
before the radiotherapy session.
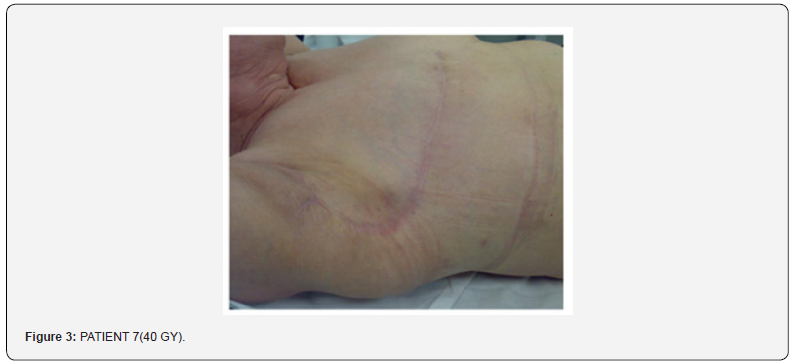
Nobody had intolerance or skin reactivity when applying the examined products, nobody stopped the radiotherapy. Nobody had used other products outside the given ones. The product RaLife had been given at the arising of the symptomatology, in 92 patients the grade of erythema due to therapy did not increase, in just 2 patients, had a G2 erythema, and regressed within 10/12 days from radiotherapy end (Figure 3).
Conclusion
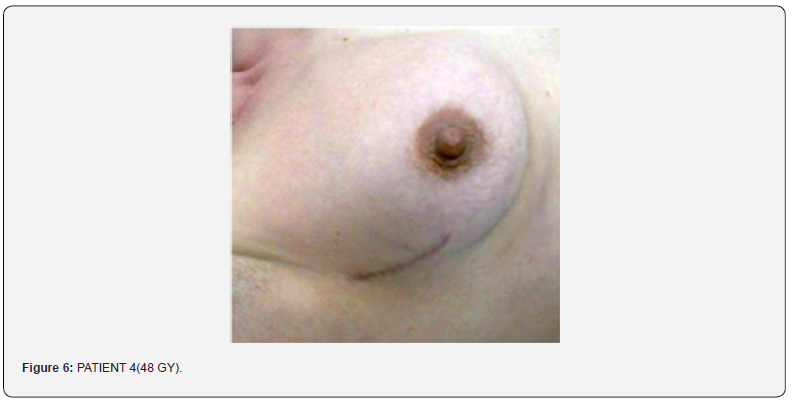
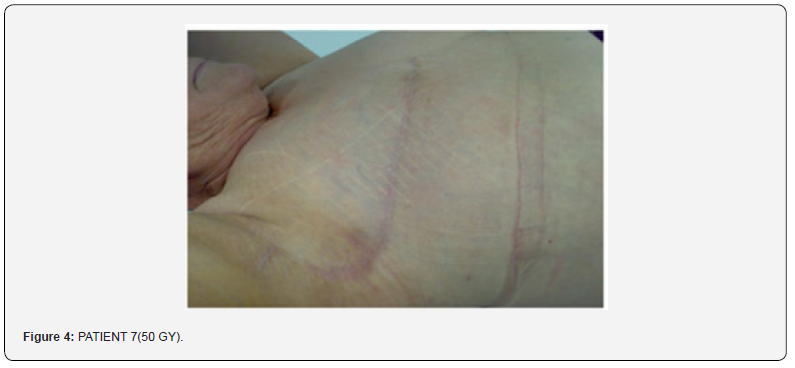
Nobody had skin toxicity signals, and/or of idiosyncrasy. In patients treated therapeutically this products allowed to go on with therapy without being compelled to stop for skin toxicity., in fact the slight erythema did not increase during the treatment and despite the ongoing of the exposure to radiation. Nobody has interrupted the radiotherapy, in 92 of the 94 patients the slight erythema did not increase (G1) during all the therapy, in 2 patients the increasing of the erythema reached G2, (slight erythema, without epithelium damages). The patients submitted to six-monthly follow up, at the examination nobody presented skin fibrosis and the personal evaluation of the skin looking had been good/elevated (Figure 4).
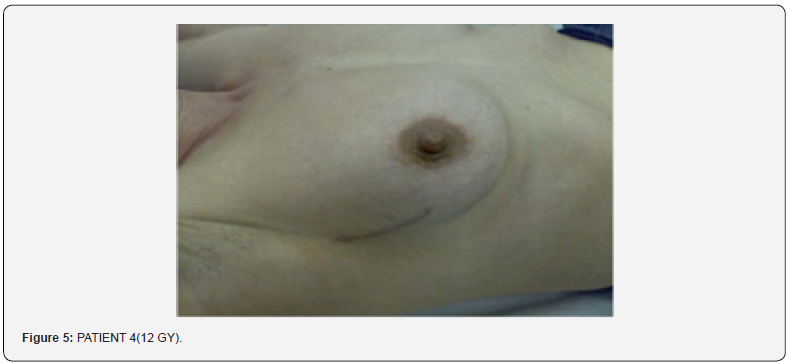
In conclusion the products had demonstrated a very good tolerability profile, as further confirm in respect to the cytotoxicity test, skin irritations, and allergic sensitization in comparison with the current regu- lations related to Medical Devices. In the same time they had demonstrated to protect the skin against the radiation exposure, preventing the side effects in the case of patients with skin alteration and as helper in order to restore the physiological skin normalization (Figure 5). In any case the product did not act as screen to the action of radiotherapy and for this reason it never had interfered with the therapy. Here under there is the table to which we referred to for damages measurement and satiations of the radiotherapy injury (Figure 6).