Immunization with Hpv16e7d Vaccine to Tumor Bearing Mice: Changes of Cytokines Patters in the Tumor Microenvironment
Pariya Mahin Samadi1, Mohammad Hossein Yazdi2,3*, Setareh Haghighat1 and Mehdi Mahdavi3,4*
1Department of Microbiology, Pharmaceutical Sciences Branch, Islamic Azad University, Tehran, Iran
2Biotechnology Research Center, Faculty of Pharmacy, Tehran University of Medical Sciences, Tehran, Iran
3Immunotherapy Group, The Institute of Pharmaceutical Sciences (TIPS), Tehran University of Medical Sciences, Tehran, Iran
4Recombinant Vaccine Research Center, Tehran University of Medical Sciences, Tehran, Iran
Submission: January 28, 2019; Published: March 07, 2019
*Corresponding author: Mohammad Hossein Yazdi, Biotechnology Research Center and Recombinant Vaccine Research Center, Faculty of Pharmacy, Tehran University of Medical Sciences, Tehran, Iran and Evidence-Based Medicine Group, Pharmaceutical Sciences Research Center, Tehran University of Medical Sciences, Tehran, Iran Mehdi Mahdavi, Recombinant Vaccine Research Center, Faculty of Pharmacy, Tehran University of Medical Sciences, Tehran, Iran
How to cite this article:Pariya M S, Mohammad H Y, Setareh H, Mehdi M. Immunization with Hpv16e7d Vaccine to Tumor Bearing Mice: Changes of Cytokines Patters in the Tumor Microenvironment. J Tumor Med Prev. 2019; 3(5): 555621. DOI: 10.19080/ JTMP.2019.03.555621
Abstract
Immune response patter in the tumor environment determines the outcome of tumor prognosis. Here, tumor-bearing mice were immunized with HPV17E7d vaccine at a distant area of the tumor and balance of cytokines were assessed in the tumor microenvironment. Tumor was established in C57BL/6 mice and mice were vaccinated subcutaneously, three times with two weeks intervals with 20 μg of E7d protein. Two weeks after final immunization, tumor homogenate was prepared and the quantity of IL-2, IL-12, IL-17 and TNF-α cytokines were evaluated with commercial ELISA kits. In addition, lymphocyte proliferation of spleen cells was determined with BRDU method. Results show that immunization with vaccine candidates increased IL-2, IL-12, IL-17 and TNF-α cytokines and lymphocyte proliferation versus un-vaccinated PBS control group. As regard to results of cytokines and lymphocyte proliferation could conclude that tumor microenvironment has been modified by changing cytokines patterns to defeat tumor cell using tumor vaccination even at a distant area of the tumor.
Keywords: HPV16E7d; Vaccine; Cytokines; Tumor micro-environment
Introduction
Cervical cancer is the second most common cancer in the worldwide [1]. Over these last 25 years, proved the direction relation between cervical cancer and the persistent infection by specific genotypes of the HPV is the most important discovers in the etiologic researches [2]. In developing countries, statistics show that more than half of women who suffered by Human Papilloma Viruses (HPVs) lose their lives [3]. Papilloma virus is a DNA virus, which has three main protein regions containing early, late and non-coding areas [4]. Actually, E6 and E7 are two oncoproteins of HPVs that are related to early region. Among the tasks of these two proteins can be mentioned to disturb tumor prognosis and cell cycle of infection [5]. These small proteins have powerful connection to the regulatory proteins of host cells [3]. Protein E6 could attach to p53 and E7 protein by connecting to pRb can inactivate these proteins and thereby the cells transformed and divided without controlling mechanisms [4-8]. Many candidate vaccines such as DNA vaccine, peptide or protein vaccines, whole cell vaccine, viral and bacterial vectors are some of wide variety procedure of producing vaccine versus HPV; however, they are not indicate enough efficacy and potency for therapeutic purpose. Because protein vaccines are a series of candidate vaccines and at the same time, they are not sufficient immunogen, therefor needed to change their formulations. Adjuvants increase vaccine immunogenicity and efficacy via various mechanisms. Some mechanisms of adjuvants act at systematic levels and some mechanisms act at local levels of a host. For example adjuvants are able to increase antibody responses versus an organism in serum as systematic level and also at an specific organ such as lung as a local and thereby increases the clearance of the invader in this organ [9,10]. In recent years, adjuvants due to the effect of raising capacity and efficacy of the vaccination are under consideration. Various adjuvants are able to modify immune responses versus of the organisms in different immunologic sites. Because of the importance of immune responses at the tumor microenvironment site, and its effect on the tumor prognosis, here we mainly focused on the cytokines patterns in the tumor microenvironment [11].
Herein, we hypothesized that maybe vaccination at a distant area of the tumor, changes the immunologic profiles of immune responses in the tumor microenvironment. By this viewpoint, we focused on the cytokines patterns of tumor milieu after immunization course of experimental mice and observed that immunization of mice with vaccines far away from the tumor site changed the cytokines patterns in the tumor microenvironment.
Material and Methods
Mice
A total of 100 mice (C57BL/6, Inbred female) with age of Six to eight weeks-old were purchased from Pasteur Institute of Iran (Karaj, Iran) and kept in standard condition (temperature, 20-22 °C, 12/12 light/dark) with free access to food and water. After one-week adaptation, the experiments began and carried out with professional technician. All experiments on mice were carried out in agreement with the Animal Care and Use Protocol of Pasteur Institute of Iran.
Cell Culture
TC1 cell line that predominantly express E6 and E7 proteins of HPV was purchased from Cell Bank of Pasteur Institute of Iran (Tehran, Iran) and cultured in RPMI-1640 medium containing 10% FBS with 1 mM non- essential amino acids, 1 mM sodium pyruvate, and 2 mM L-glutamine. After culture of cells and enough growth, cells were harvested with trypsin enzyme and used for tumor induction.
Tumor induction
A suspension of TC1 cell line (1×107 cells per milliliter) in PBS buffer were prepared and 100 μl of cell suspension were injected into the flank of C57BL/6 mice to develop stock tumor mice for further use. After 14-21 days, tumor in the flank of the mice was appeared and these mice were used as stock for tumor modeling in naïve mice via transplantation method.
Tumor transplantation
Because of low tumor size variation in tumor transplantation method, this method is used for tumor modeling in C57BL/6 mice. Stock tumor bearing mice were dislocated and tumor were harvested and putted in cold PBS containing penicillin and streptomycin. Then tumor was cutted to the pieces between 3-5 mm in length and used for transplantation. Mice were anesthetized with injection of 100 mg/kg of Ketamine and 20 mg/kg of Xylosine via peritoneal injection and tumor piece was placed at flank of anesthetized mice. After surgery, the local was disinfected with povidone iodine and three days after surgery mice were used for immunization program.
Experimental groups and immunization
After tumor transplantation the mice were divided into five groups (n=20). The first and second groups of mice were immunized with HPV-16-E7d candidate vaccine formulated in Montanide ISA 206 and alum adjuvants respectively. The third, fourth and fifth as the control groups, alum, Montanide ISA 206 and PBS were injected. These vaccines were subcutaneously injected three times (20μg) with an interval of two weeks. In order to evaluate the immunologic parameters, two weeks after the last immunization, mice were dislocated, and tumor samples and spleen cells were used for immunoassay.
Lymphocyte proliferation assa
In other to assay the lymphocyte proliferation, two weeks after the last shot, spleen cell suspension prepared in sterile condition and used for lymphocyte proliferation assay by BrdU method. Single cell suspension was adjusted to 3×106 splenocytes per milliliters in RPMI-1640 (Gibco) containing 10% FBS, 4 mM L glutamine, 1 mM non-essential amino acid, 1 mM sodium pyruvate, 100 μg/ml of streptomycin and 100 IU/ml of penicillin. Then, 100 μl of cell suspension were dispensed into 96-well plates and stimulated with 10μg/ml of antigen. Un-stimulated wells were used as negative controls and PHA (5 μg/ml) was used as a positive control. The final volume of wells was reached to 200 μl and incubation at 37 °C for three days. Then 20 μl of BrdU labeling solution was added to each well and the plates were incubated for 18 hrs again. The plates were centrifuged at 300g for 10 minutes, supernatant were discarded, and the plates kept at 60 °C for 30 minutes to be completely dry. Then, 200μl of fixation/denaturation buffer was added to each well for 30 min and then 100 μl of anti-BrdU conjugate were added and incubated again for 90 minutes. The next step, the plated washed 4 times with PBS, 100μl of TMB substrate was added, and reaction was stopped by H2SO4. Eventually, OD450 was read using ELISA plate reader.
Preparation of tumor homogenate
Two weeks after the last immunization, experimental mice were dislocated and tumor sample were removed, dissected, and mechanically homogenized in PBS containing 1 mM of PMSF. Then the samples centrifuged at 14000 RPM for 10 minutes and the concentration of protein determined with Bradford method and frozen at - 70 °C for further assay.
Measurement of IL-2, IL-17, IL-12 and TNF-α Cytokines in the tumor microenvironment
ELISA test by using commercial kits allocated to measurement of IL-2, IL-17, IL-12 and TNF-α cytokines (Mabtech, Sweden) that was performed according to the manufacture instruction. Based on the cytokines standards, the results were reported quantitatively as pg/ml.
Statistical Analysis
After data collection, using Non-parametric methods and Mann Whitney U test, statistical analysis was performed, and finally, the figures and tables based on the SD ± Mean, were plotted by using software Prism Version 6.01.
Results
Proliferation assay
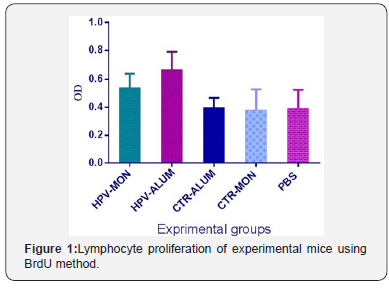
The results of proliferation assay in experimental groups demonstrate that immunization of HPV16E7d vaccine candidate with Montanide/alum as adjuvants have led to increase of lymphocyte proliferation in HPV-alum group versus control groups (P<0.0392). Immunization with HPV-alum does not show significant differences versus HPV-Montanide group (P>.0.05) (Figure 1).
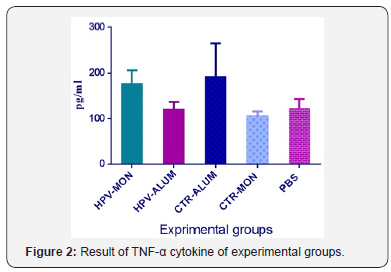
TNF-α cytokine assay
The results of TNF-α cytokine assay show that immunization with HPV-16 E7d vaccine formulated in Montanide ISA 206 cause to significant increase of TNF-α cytokine versus CTR-MON and PBS control groups (P< 0.0461) (Figure 2).
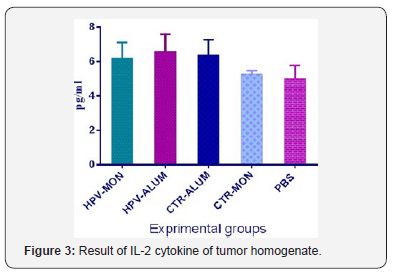
IL-2 assay
The results of IL-2 cytokine assay show that immunization of HPV-16 E7d vaccine formulated in Montanide/alum as adjuvants cause to significant increase of IL-2 versus Montanide and PBS control groups (P<0.0347). However, there were not any significant differences among E7d vaccinated experimental groups (P>.0.05) (Figure 3).
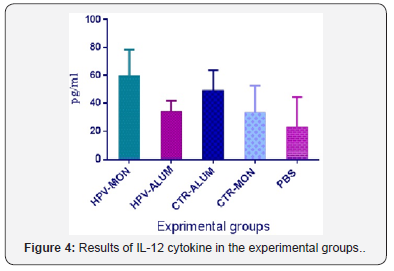
IL-12 assay
The results of IL-12 cytokine assay show that injection of HPV-16 E7d vaccine formulated in Montanide ISA 206 shows an increase versus other experimental groups but shows significant differences versus PBS control group (P=0.0246). No noticeable differences were observed among vaccinated experimental groups (P> 0.05) (Figure 4).
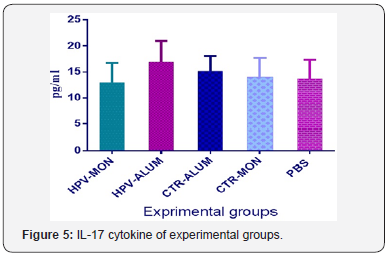
IL-17 assay
The results of IL-17 cytokine assay show that injection with HPV-16 E7d-Alum shows an increase versus HPV-16 E7d-Monta nide group (P= 0.0876) (Figure 5). No other differences were observed among experimental groups (P>0.05).
Discussion
The nature of tumor microenvironment correlates with its local immune responses and tumor prognosis [12]. Therefore, modulation of tumor microenvironment by immunological methods can result in tumor controlling and suppression. Vaccine as an approach can induce specific effector T cells against tumor antigen and thereby can modulate tumor microenvironment by production of cytokines and immunologic bioactive molecules that change outcome of tumor.
Vaccine as an effective immunologic method can modulate immune response versus tumors and due to ability to move of effector and memory T cells, this modulatory effect can be translocated too far away than immunization site. Herein, we hypothesized that may be subcutaneous immunization of tumor bearing mice with a vaccine change the nature of immune responses at the local of tumor.
Here, we used tumor bearing mice model that induced by TC1 cell line and also HPV16 E7d vaccine that target this antigen at the surface of this tumor [13-16]. Herein, our results showed that administration with HPV16E7d vaccine candidates could increase lymphocyte proliferation and also IL-2, IL-12, IL-17 and TNF-α cytokines in the tumor microenvironment in comparison to un-vaccinated PBS group. The increase in lymphocyte proliferation meaning improvement of cellular immune response that is at the first line of adaptive immune response against tumor in the controlling of tumor as reported by other researchers [17-19]. Assessment of cytokines pattern in the tumor homogenate show that vaccination at a distant area from the tumor changed immunologic cytokines at the tumor microenvironment. In fact, the level of IL-2, IL-12, IL-17 and TNF-α cytokines in vaccinated groups increased versus un-vaccinated PBS group. These finding show that immunization at a local far away from tumor environment can modulate immune responses in the tumor microenvironment. It is clear that the microenvironment of tumor, determines the outcome of tumor in immunotherapy toward tumor suppression or progression [20]. According to our finding it is possible that by immunization at a local far away from a tumor, modulate tumor microenvironment to combat with tumor. Studies show that microenvironment of tumor has critical in tumor immunotherapy and modulation of this milieu can change the prognosis of tumor [12]. In overall, our findings indicate that administration with HPV16 E7d vaccine candidate can modify tumor microenvironment via increase of IL-2, IL-12, IL-17 and TNF-α cytokines. Consequently, as regard to results of cytokine and lymphocyte proliferation could conclude that tumor microenvironment have been modified by changing cytokine pattern to defeat tumor cell in cancer network. However, immunization and also effector and memory T cell formation constitute far away from local of tumor.
Our findings indicate that administration with HPV16 E7d vaccine candidates can modify tumor microenvironment via increase of IL-2, IL-12, IL-17 and TNF-α cytokines as vital cytokines to control tumor growth and lymphocyte proliferation in comparison to un-vaccinated PBS control group. Consequently, as regard to results of cytokines and lymphocyte proliferation could conclude that tumor microenvironment have been modified by changing cytokines patterns to defeat tumor cell using tumor vaccination even at a distant area of the tumor.
Conflict of Interest
The authors declare no conflicts.
Acknowledgment
We like special thanks to Ms. Fatemeh Asgar Halvaei because of her assistance.
References
- W.G.o.t.E.o.C.R.t (2010) Humans, IARC monographs on the evaluation of carcinogenic risks to humans. Ingested nitrate and nitrite, and cyanobacterial peptide toxins, IARC monographs on the evaluation of carcinogenic risks to humans/World Health Organization. International Agency for Research on Cancer 94.
- Bosch FX, Lorincz A, Munoz N, Meijer CJ, Shah KV (2002) The causal relation between human papillomavirus and cervical cancer. J Clin Pathol 55(4): 244-265.
- Alfonsi GA, Datta SD, Mickiewicz T, Koutsky LA, Ghanem K, et al. (2011) Prevalence of high-risk HPV types and abnormal cervical cytology in American Indian/Alaska Native women, 2003-2005. Public Health Rep 126(3): 330-337.
- Poljak M (2011) Review of 20 years of HPV research in Slovenia. Acta dermatovenerol Alp Pannonica Adriat 20(3): 99-112.
- Burk RD, Harari A, Chen Z (2013) Human papillomavirus genome variants. Virology 445(1-2): 232-243.
- Doorbar J, Quint W, Banks L, Bravo IG, Stoler M, et al. (2012) The biology and life-cycle of human papillomaviruses. Vaccine 30 Suppl 5: F55-F70.
- Liu X, Clements A, Zhao K, Marmorstein R (2006) Structure of the human Papillomavirus E7 oncoprotein and its mechanism for inactivation of the retinoblastoma tumor suppressor. Journal of Biological Chemistry 281(1): 578-586.
- Zur Hausen H (2002) Papillomaviruses and cancer: from basic studies to clinical application. Nat Rev Cancer 2(5): 342-350.
- Ly LV, Sluijter M, Versluis M, Luyten GP, van Stipdonk MJ, et al. (2010) Peptide vaccination after T-cell transfer causes massive clonal expansion, tumor eradication, and manageable cytokine storm. Cancer Res 70(21): 8339-8346.
- Singh M, O'Hagan DT (2002) Recent advances in vaccine adjuvants. Pharm Res 19(6): 715-728.
- Ashrafi S, Shapouri R, Mahdavi M (2017) Immunological consequences of immunization with tumor lysate vaccine and propranolol as an adjuvant: A study on cytokine profiles in breast tumor microenvironment. Immunol Lett 181: 63-70.
- Binnewies M, Roberts EW, Kersten K, Chan V, Fearon DF, et al. (2018) Understanding the tumor immune microenvironment (TIME) for effective therapy. Nat Med 24(5): 541-550.
- Bachmann M, Horn K, Poleganov MA, Paulukat J, Nold M, et al. (2006) Interleukin‐18 secretion and Th1‐like cytokine responses in human peripheral blood mononuclear cells under the influence of the toll‐like receptor‐5 ligand flagellin. Cell Microbiol 8(2): 289-300.
- Barbosa MS, Lowy DR, Schiller JT (1989) Papillomavirus polypeptides E6 and E7 are zinc-binding proteins. J Virol 63(3): 1404-1407.
- Carter JR, Ding Z, Rose BR (2011) HPV infection and cervical disease: a review. Aust N Z J Obstet Gynaecol 51(2): 103-108.
- El Bakkouri K, Descamps F, De Filette M, Smet A, Festjens E, et al. (2011) Universal vaccine based on ectodomain of matrix protein 2 of influenza A: Fc receptors and alveolar macrophages mediate protection. J Immunol 186(2): 1022-1031.
- Ashrafi S, Shapouri R, Shirkhani A, Mahdavi M (2018) Anti-tumor effects of propranolol: Adjuvant activity on a transplanted murine breast cancer model. Biomed Pharmacother 104: 45-51.
- Chen DS, Mellman I (2017) Elements of cancer immunity and the cancer-immune set point. Nature 541(7637): 321-330.
- Juneja VR, McGuire KA, Manguso RT, LaFleur MW, Collins N, et al. (2017) PD-L1 on tumor cells is sufficient for immune evasion in immunogenic tumors and inhibits CD8 T cell cytotoxicity. J Exp Med 214(4): 895-904.
- Nagarsheth N, Wicha MS, Zou W (2017) Chemokines in the cancer microenvironment and their relevance in cancer immunotherapy. Nat Rev Immunol 17(9): 559-572.






























