Bortezomib as a Salvage Therapy for Severe Refractory Thrombotic Thrombocytopenic Purpura
Snigdha Nutalapati1, Syed Qudsiyah Rufai1, Shahla Bari1 and Sanjay Jain2*
1Department of Internal Medicine, Morehouse School Of Medicine, USA
2Division of Hematology and Oncology, Morehouse School Of Medicine, USA
Submission: August 11, 2017; Published: August 28, 2017
*Corresponding author: Sanjay Jain, Division of Hematology and Oncology, Morehouse School of Medicine, USA, Tel: 404-756-1366; Email: sjain@msm.edu
How to cite this article: Snigdha N, Syed Qudsiyah R, Shahla B, Sanjay J. Bortezomib as a Salvage Therapy for Severe Refractory Thrombotic Thrombocytopenic Purpura. J Tumor Med Prev. 2017; 1(5): 555573. DOI: 10.19080/JTMP.2017.01.555573
Abstract
Thrombotic thrombocytopenic purpura (TTP) is considered a medical emergency requiring early identification and administration of prompt therapy to reduce mortality. We hereby report a case of TTP refractory to conventional treatment modalities, later responded to Bortezomib. A 19 year old female presented to the emergency department with altered mental status, thrombocytopenia with a platelet count of 13,000 (13K) microangiopathic hemolytic anemia and normal coagulation studies. Peripheral blood smear revealed numerous schistocytes, normal platelet morphology and nucleated red blood cells. TTP was suspected and daily plasma exchange was initiated along with systemic methyl prednisone. Serum ADAMTS-13 activity and ADAMTS-13 inhibitor later resulted as <3% and 8.7 BEU respectively conforming severe TTP. Platelet counts improved only to 16,000 by Day 5 and so PEX and steroid dose was increased along with Rituximab initiation. No significant improvement was noted with counts of 32,000 on day 13, Bortezomib at dose of 1.3 mg/m2 was then initiated. Platelet counts improved dramatically to 171,000 by day 20. Repeat ADAMTS-13 activity levels were reported to be 91% on day 23. Subsequently with improving platelet counts and patient’s mentation returning to baseline PEX therapy was discontinued on day 42 and patient was discharged to subacute rehabilitation on oral steroid taper.
Keywords: Bortezomib; Refractory; TTP
Introduction
Thrombotic thrombocytopenic purpura (TTP) is a hematological disorder characterized by microangiopathic hemolytic anemia and thrombocytopenia secondary to reduced activity of Von Willebrand factor (vWF) -cleaving protease ADAMTS-13. It is considered as a medical emergency requiring early identification and administration of prompt therapy to prevent mortality. The corner stone therapy for TTP include daily plasma exchange (PEX) aiming at clearing the circulating anti ADAMTS 13 antibodies and repletion of ADAMTS-13 levels. PEX has dramatically improved the survival rates to 80-85% [1]. Immunomodulatory therapy with Rituximab was shown to beneficial as an adjunctive therapy in cases with suboptimal response to PEX [2-7]. Cases of refractory TTP responding to Bortezomib were rarely reported. Adding to the current literature we hereby report a case of TTP refractory to daily plasma exchange, high dose systemic steroids and Rituximab who responded to Bortezomib.
Case Description
A 19 year old female with no significant medical history presented to the emergency department with altered mental status of one day duration. Vitals on presentation were body temperature 101.4F, heart rate of 105 beats/minute, blood pressure of 110/70mm Hg, respiratory rate of 21 per minute and oxygen saturation of 92% on room air. Physical examination revealed a confused young female with conjunctival icterus, skin pallor and petechiae noted on bilateral upper and lower extremities. The rest of the physical examination was unremarkable except for sinus tachycardia.
Laboratory findings on presentation revealed thrombocytopenia with hemolytic anemia. The serum hemoglobin level was 4.5g/dL, hematocrit is 13.5%, MCV 77.0 fL, absolute reticulocyte count 344, platelet count was 13,000/ uL and white blood cell (WBC) count 9,700/mm3. Peripheral blood smear revealed numerous schistocytes, normal platelet morphology and nucleated red blood cells. The serum level of lactate dehydrogenase (LDH) was elevated at 3253U/L, and total bilirubin level was 3.2mg/dL which is predominantly indirect hyper bilirubinemia of 2.7mg/dL. Serum D-dimer was elevated at 28,895ng/mL and fibrinogen level was low at 198mg/dl concerning for disseminated intravascular coagulation (DIC). However, no significant elevation was noted with respect to prothrombin time (PT) and activated partial thromboplastin times (aPTT) which were 13.7 seconds and 26.7 seconds respectively. The blood urea nitrogen (BUN) and creatinine levels were within the normal limits at 24mg/dL and 1.0mg/dL respectively
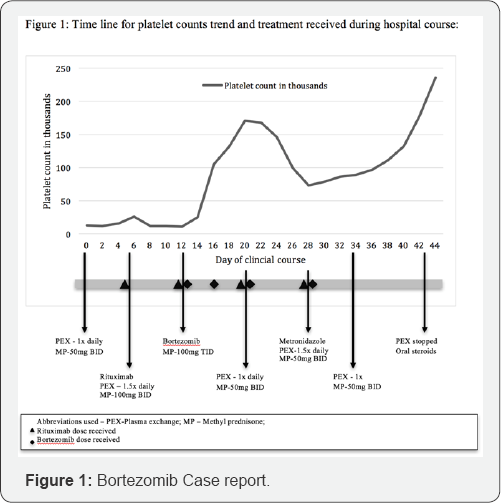
Thrombotic thrombocytopenic purpura was suspected given the evidence of severe thrombocytopenia with microangiopathic hemolytic anemia and normal coagulation studies. Serum samples for ADAMTS-13 activity and ADAMTS-13 inhibitor later resulted as <3% and 8.7 Bethesda units BEU respectively conforming severe TTP. She was admitted to the intensive care unit and daily plasma exchange was initiated along with intravenous methyl prednisone 50mg twice daily However no significant improvement was noted with platelet counts improving only to 16,000 by Day 5 (Figure 1). Rituximab at 375mg/m2 every 7 days was initiated on day 6 as an adjunctive treatment along with increasing PEX therapy to 1.5 times the plasma volume (1.5x) and systemic methyl prednisone dose to 100 mg twice daily. Platelets count improved only to 32,000 by day 13 and neurological status deteriorated with magnetic resonance imaging of brain showing multifocal areas of punctate abnormal diffusion restriction concerning for acute or subacute infraction. Patient was deemed as slow responder and Bortezomib at dose of 1.3mg/m2 was initiated. She received Bortezomib on days 13 and 16 after which platelet counts dramatically improved to 171,0 by day 20. Given improving platelet count PEX holiday was given on day 22 and resumed on day 23 at a reduced rate of 1x the plasma. Decline in platelet count was noted around day 24 which was likely attributed to multitude of reasons including decreased intensity of therapy and newly diagnosed Clostridium difficle colitis for which metronidazole was initiated.
PEX therapy was resumed to 1.5x on day 25. Patient in total received 4 doses of Bortezomib on days 13, 16, 20 and 28. Repeat ADAMTS-13 activity levels were reported to be 91% on day 25 and 151% on day 31. Platelet counts gradually improved to 23,0 by day 36 and PEX was transitioned to 1x every other day. Subsequently with improving platelet count and patient's mentation returning to baseline PEX therapy was discontinued on day 42 and patient was discharged to subacute rehabilitation on oral steroid taper.
Discussion
TTP is a medical emergency which is almost always fatal without prompt clinical suspicion and treatment initiation. Wide spread hyaline thrombi in capillaries and arterioles now considered pathological hallmark of TTP was first described by Moschcowitz in 1925 [8]. The estimated annual incidence of TTP in the United states was reported to be 1 in 50,000 hospital admissions. Reported prevalence rates from Canada and UK were 2.2 and 3.2 cases per million population respectively [9]. Initial diagnostic suspicion is largely based on the physical examination and laboratory investigations consistent with microvascular thrombi and hemolytic anemia along with the presence of schistocytes in the peripheral smear with no other alternative diagnosis. ADAMTS-13 activity of <10%, drawn before the treatment initiation strongly supports the diagnosis [10]. Plasma exchange (PEX) remains the corner stone therapy for TTP, which works by removing the inhibitor antibodies and replacing ADAMTS13 [11]. PEX should be continued daily until treatment response is seen, defined by a platelet count of 150,000 for 2 days with normal/near normal LDH and stable/improving neurological function is achieved [12]. Steroids were also shown too beneficial in management of TTP likely by suppressing his production of ADAMTS-13 antibodies. PEX along with steroids was shown to improve survivals to 91% [13].
Management of TTP is largely based on clearing inhibitory autoantibodies against ADAMTS-13 by plasmapheresis along with suppression of antibody production using immunomodulatory and immunosuppressive agents. Refractory TTP is defined as failure of platelets to respond after 4 to 7 days of therapy or development of new neurological symptoms. 10 to 40% of TTP cases do not respond of conventional PEX-Corticosteroid therapy [14-17]. Treatment options for refractory TTP include twice daily PEX, pulse dose corticosteroids and Rituximab [6,18-21]. Alternative therapies shown to be beneficial in cases of persistent refractory TTP include splenectomy, Cyclosporine, Cyclophosphamide and Vincristine [22-25].
Bortezomib is first in class of proteasome inhibitors used in treating plasma cell disorders, lymphoid malignancies and antibody-mediated rejection of solid organs [26,27]. The mechanism of action is by blocking 26S proteasome thereby altering the ubiquitin-proteasome pathway of cellular protein homeostasis [28]. Role of Bortezomib in treating refractory TTP was described by Shortt et al in a case of TTP refractory to Cyclophosphamide and Rituximab. They noticed the absence of CD19 B cells and presence of scattered CD 138+ B cells in bone marrow cytometry and postulated that Bortezomib induces remission by targeting autoreactive B-cells and plasma cells [29]. Bortezomib was also found to induce apoptosis in immature dendritic cells necessary for CD4 cell activation there by leading to decreased production of inhibitory ADAMTS 13 antibodies from CD4 cells [30,31]. In our case, initiation of Bortezomib led to improved platelet count by more than 50% as reported in previous studies. Though Bortezomib seems promising in the setting refractory TTP, cases of Bortezomib induced TTP were also reported [32,33]. More prospective trails should be done to determine the safety, efficacy and the timing of initiation of Bortezomib in refractory TTP cases.
References
- Rock GA, Shumak KH, Buskard NA, Blanchette VS, Kelton JG, et al. (1991) Comparison of plasma exchange with plasma infusion in the treatment of thrombotic thrombocytopenic purpura. N Engl J Med 325(6): 393-397.
- Froissart A, Buffet M, Veyradier A, Poullin P, Provôt F, et al. (2012) Efficacy and safety of first-line rituximab in severe, acquired thrombotic thrombocytopenic purpura with a suboptimal response to plasma exchange. Experience of the French Thrombotic Microangiopathies Reference Center. Criti Care Med 40(1): 104-111.
- Scully M, McDonald V, Cavenagh J, Hunt BJ, Longair I, et al. (2011) A phase 2 study of the safety and efficacy of rituximab with plasma exchange in acute acquired thrombotic thrombocytopenic purpura. Blood 118(7): 1746-1753.
- Jasti S, Coyle T, Gentile T, Rosales L, Poiesz B (2008) Rituximab as an adjunct to plasma exchange in TTP: a report of 12 cases and review of literature. J Clin Apher 23(5): 151-156.
- Ling HT, Field JJ, Blinder MA (2009) Sustained response with rituximab in patients with thrombotic thrombocytopenic purpura: a report of 13 cases and review of the literature. Am J Hematol 84(7): 418-421.
- Scully M, Cohen H, Cavenagh J, Benjamin S, Starke R, et al. (2007) Remission in acute refractory and relapsing thrombotic thrombocytopenic purpura following rituximab is associated with a reduction in IgG antibodies to ADAMTS-13. Br J Haematol 136(3): 451461.
- De la Rubia J, Moscardo F, Gomez MJ, Guardia R, Rodriguez P, et al. (2010) Efficacy and safety of rituximab in adult patients with idiopathic relapsing or refractory thrombotic thrombocytopenic purpura: results of a Spanish multicenter study. Transfus Apher Sci 43(3): 299-303.
- Moschcowitz E (2003) An acute febrile pleiochromic anemia with hyaline thrombosis of the terminal arterioles and capillaries: an undescribed disease. 1925. Mt Sinai J Med 70(5): 352-355.
- Miller DP, Kaye JA, Shea K, Ziyadeh N, Cali C, et al. (2004) Incidence of thrombotic thrombocytopenic purpura/hemolytic uremic syndrome. Epidemiology 15(2): 208-215.
- Bianchi V, Robles R, Alberio L, Furlan M, Lammle B (2002) Von Willebrand factor-cleaving protease (ADAMTS13) in thrombocytopenic disorders: a severely deficient activity is specific for thrombotic thrombocytopenic purpura. Blood 100(2): 710-713.
- Zheng XL, Kaufman RM, Goodnough LT, Sadler JE (2004) Effect of plasma exchange on plasma ADAMTS13 metalloprotease activity, inhibitor level, and clinical outcome in patients with idiopathic and nonidiopathic thrombotic thrombocytopenic purpura. Blood 103(11): 4043-4049.
- Sarode R, Bandarenko N, Brecher ME, Kiss JE, Marques MB, et al. (2014) Thrombotic thrombocytopenic purpura: 2012 American Society for Apheresis (ASFA) consensus conference on classification, diagnosis, management, and future research. J Clin Apher 29(3): 148-167.
- Bell WR, Braine HG, Ness PM, Kickler TS (1991) Improved survival in thrombotic thrombocytopenic purpura-hemolytic uremic syndrome: clinical experience in 108 patients. N Engl J Med 325(6): 398-403.
- Blitzer JB, Granfortuna JM, Gottlieb AJ, Smith JR, Theodorakis ME, et al. (1987) Thrombotic thrombocytopenic purpura: treatment with plasmapheresis. Am J Hematol 24(4): 329-339.
- Roberts AW, Gillett E, Fleming SJ (1991) Hemolytic uremic syndrome/ thrombotic thrombocytopenic purpura: outcome with plasma exchange. J Clin Apher 6(3): 150-154.
- Onundarson PT, Rowe JM, Heal JM, Francis CW (1992) Response to plasma exchange and splenectomy in thrombotic thrombocytopenic purpura: a 10-year experience at a single institution. Arch Inter Med 152(4): 791-796.
- Shah N, Rutherford C, Matevosyan K, Shen YM, Sarode R (2013) Role of ADAMTS13 in the management of thrombotic microangiopathies including thrombotic thrombocytopenic purpura (TTP). Br J Haematol 163(4): 514-519.
- Nguyen L, Li X, Duvall D, Terrell DR, Vesely SK, et al. (2008) Twice- daily plasma exchange for patients with refractory thrombotic thrombocytopenic purpura: the experience of the Oklahoma Registry, 1989 through 2006. Transfusion 48(2): 349-357.
- Fakhouri F, Vernant JP, Veyradier A, Wolf M, Kaplanski G, et al. (2005) Efficiency of curative and prophylactic treatment with rituximab in ADAMTS13-deficient thrombotic thrombocytopenic purpura: a study of 11 cases. Blood 106(6): 1932-1937.
- Lim W, Vesely SK, George JN (2015) The role of rituximab in the management of patients with acquired thrombotic thrombocytopenic purpura. Blood 125(10): 1526-1531.
- Balduini CL, Gugliotta L, Luppi M, Laurenti L, Klersy C, et al. (2010) High versus standard dose methylprednisolone in the acute phase of idiopathic thrombotic thrombocytopenic purpura: a randomized study. Ann Hematol 89(6): 591-596.
- Dubois L, Gray DK (2010) Splenectomy: Does it still play a role in the management of thrombotic thrombocytopenic purpura? Can J Surg 53(5): 349-355.
- Cataland SR, Jin M, Lin S, Kennedy MS, Kraut EH, et al. (2007) Ciclosporin and plasma exchange in thrombotic thrombocytopenic purpura: long-term follow-up with serial analysis of ADAMTS13 activity. Br J Haematol 139(3): 486-493.
- Kierdorf H, Maurin N, Heintz B (1993) Cyclosporine for thrombotic thrombocytopenic purpura. Ann Intern Med 118(12): 987-988.
- Beloncle F, Buffet M, Coindre JP, Munoz-Bongrand N, Malot S, et al. (2012) Splenectomy and/or cyclophosphamide as salvage therapies in thrombotic thrombocytopenic purpura: the French TMA Reference Center experience. Transfusion 52(11): 2436-2444.
- Kouroukis T, Baldassarre F, Haynes A, Imrie K, Reece D, et al. (2014) Bortezomib in multiple myeloma: systematic review and clinical considerations. Curr Oncol 21(4): e573-603.
- Everly M, Everly J, Susskind B, Brailey P, Arend L, et al. (2009) Proteasome inhibition reduces donor-specific antibody levels. Transplant Proc 41(1): 105-107.
- Yang H, Zonder JA, Dou QP (2009) Clinical development of novel proteasome inhibitors for cancer treatment. Expert Opin Investig Drugs 18(7): 957-971.
- Shortt J, Oh DH, Opat SS (2013) ADAMTS13 antibody depletion by bortezomib in thrombotic thrombocytopenic purpura. N Engl J Med 368(1): 90-92.
- Sorvillo N, Pos W, Van den Berg LM, Fijnheer R, Martinez-Pomares L, et al. (2012) The macrophage mannose receptor promotes uptake of ADAMTS13 by dendritic cells. Blood 119(16): 3828-3835.
- Subklewe M, Sebelin-Wulf K, Beier C, Lietz A, Mathas S, et al. (2007) Dendritic cell maturation stage determines susceptibility to the proteasome inhibitor bortezomib. Hum Immunol 68(3): 147-155.
- Salmenniemi U, Remes K (2012) Thrombotic microangiopathy associated with bortezomib treatment in a patient with relapsed multiple myeloma. Hematol Rep 4(2): e13.
- Morita R, Hashino S, Shirai S, Fujita N, Onozawa M, et al. (2008) Thrombotic microangiopathy after treatment with bortezomib and dexamethasone in a patient with multiple myeloma. Int J Hematol 88(2): 248-250.






























