CAR-T Based Immuno-Therapy: New Hope for Cancer Patients
Tapashi Mandal, Rupesh Tyagi and Gunjan Bhardwaj*
Innoplexus AG, Germany
Submission: July 11, 2017; Published: July 26, 2017
*Corresponding author: Gunjan Bhardwaj*, Innoplexus AG, Frankfurter Strasse 63, 65760 Eschborn, Germany, Email: gunjan@innoplexus.com
How to cite this article: Tapashi M, Rupesh T, Gunjan B. CAR-T Based Immuno-Therapy: New Hope for Cancer Patients. J Tumor Med Prev. 2017; 1(4): 555566. DOI: 10.19080/JTMP.2017.01.555566
Abstract
Challenges like limited efficacy and numerous side-effects of standard chemotherapy and small molecule based cancer therapies have compelled researchers to look for more viable and effective treatment options. Harnessing and enhancing the body's innate immune system to fight cancer is proving to be the next hope for cancer patients with advanced tumors which are difficult to treat. Though chimeric antigen-receptor T cells (CAR-T) based therapies have yielded unprecedented efficacy in B cell malignancies, they are also responsible for eliciting expected and unexpected toxicities. This mini-review enumerates the efforts of various pharmaceutical companies in developing CAR-T based treatments and the toxicities associated with them. In addition, some novel CAR based designs which are being considered to reduce levels of toxicities have also been discussed.
Introduction
Major new approvals of drugs for cancer in the last few years have been in the immuno-oncology field where the body's own defense systems have been empowered to combat cancer [1]. Drugs which target the immune check-point protein like Keytruda, Opdivo, Yervoy, Bavencio and others have been launched into the market based on their unique treatment paradigms. Other immunotherapies which are also being tried are cancer vaccines [2] and oncolytic viruses [3].
Another type which is the most promising in 2017 is immunotherapy based cancer treatment in the form of CAR-T based therapies [4]. CAR-T cells (or) (chimeric antigen-receptor T cells) are genetically engineered from patient's immune cells and express artificial receptors on their surfaces, which then binds to specific antigens on the surface of cancer cells and directs the body's own immune system to recognize and attack tumors. Also, known as adoptive cell transfer (ACT) therapy, currently this type of treatment is only available to patients registered for clinical trials. Promising results have been shown in these studies in patients with various form of hematological malignancies especially advanced acute lymphoblastic leukemia and diffuse large B-cell lymphoma. Some of the studies, which mostly recruit patients not responding to other available treatment options for their form of cancer, showed huge remission rates, of up to 94% in severe forms of cancer [5].
Discussion
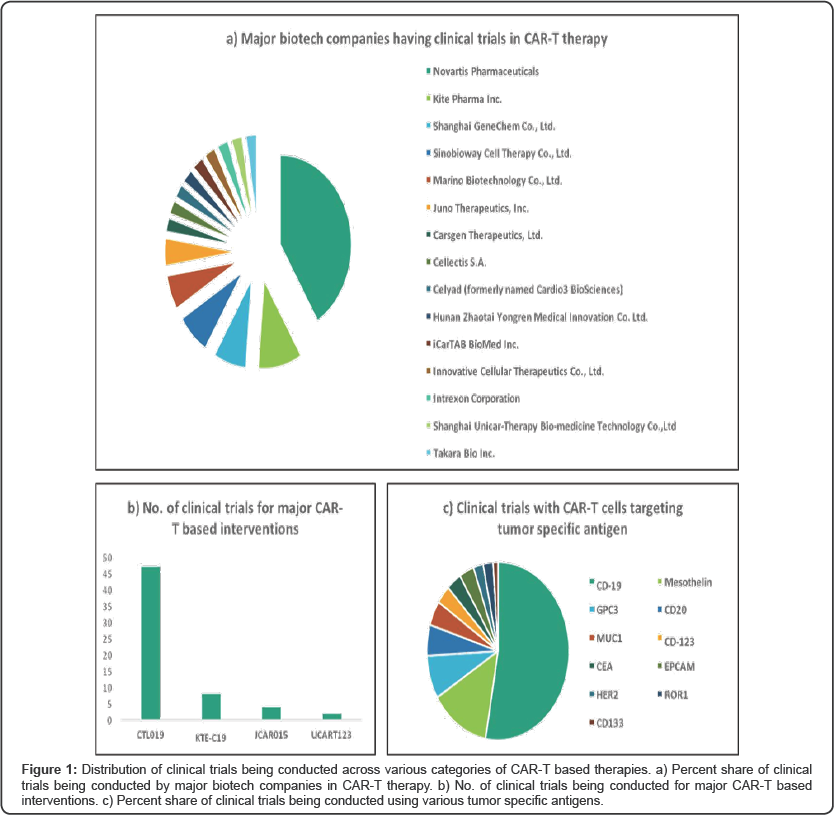
Many biotech companies are testing their CAR-T based therapies in clinical trials with Novartis and Kite Pharma leading the race. Novartis has recently received breakthrough status from FDA for CTL019 (tisagenlecleucel-T), for relapsed/ refractory (r/r) diffuse large B-cell lymphoma (DLBCL) [6]. The drug has also received a breakthrough status for r/r B-cell acute lymphoblastic leukemia (ALL) in pediatric and young adult patients, and was also given a priority review at the end of March 2017. This helps Novartis to be in a competitive position to launch their product when compared to its closest competitor Kite Pharma. Following the breakthrough labels for DLBCL, transformed follicular lymphoma TFL and primary mediastinal B-cell lymphoma PMBCL, Kite Pharma has done its rolling submission to the FDA this March, for a BLA of axicabtagene ciloleucel (KTE-C19) as a treatment for patients with r/r B-cell non-Hodgkin lymphoma who are ineligible for autologous stem cell transplant [7]. (Figure 1a &1b) shows the distribution of clinical trials in different categories and companies conducting them in CAR-T based therapy [Source: iPlexus™].
Until recently, Juno Therapeutics was also developing drugs to treat adults with desperate cases of acute lymphocytic leukemia (ALL) with JCAR015 but the trials were terminated after 5 deaths due to cerebral edema was reported during their execution [8]. CAR-T based therapies are also associated with other serious side-effects like cytokine release-syndrome, B-cell aplasia and tumor lysis syndrome which occurs mainly as CAR-T cells target both normal and cancerous B-cells. To avoid such side- effects and make the target more specific to tumor cells, protein antigens such as MUC1 and mesothelin are being investigated in numerous other clinical trials. Use of CART-meso, in addition to targeting tumor cells which overexpress mesothelin, would also help in better infiltration of advanced solid tumors like ovarian cancer, epithelial mesothelioma and pancreatic cancer [9]. (Figure 1c) shows the distribution of clinical trials that are being conducted using various tumor specific antigens [Source: iPlexus™].
Other upcoming companies which are also developing therapeutics using this technology are Sorrento Therapeutics (developing CAR.TNK™ Chimeric Antigen Receptor Tumor- attacking NK cells in collaboration with NantKwest) [10], Vor BioPharma (a unit of PureTech Health plc) [11] and BioAtla in collaboration with F1 Oncology [12]. Researchers are also trying to develop "off-the-shelf CART-therapy” which involves establishing a bio-bank of CAR modified immune cells from healthy donors and distribute them to required points of care when needed. As FDA has approved the IND application of Cellectis for an off-the-shelf CAR-T candidate, UCART123, phase 1 trials would start in patients with acute myeloid leukemia (AML)and blastic plasmacytoid dendritic cell neoplasm (BPDCN) [13]. Cell Medica and Fate Therapeutics are two other companies which has teamed up with investigators at Baylor College of Medicine in Texas and the Memorial Sloan Kettering Cancer Center respectively to develop off-the-shelf CAR-T therapies.
Conclusion
These recent advancements in the field of CAR-T therapy raises a beacon of hope for patients with advanced cancers for whom there are no current available treatments. Yet more studies would be required to correctly assess their full potential as well as the limitations due to known side-effects. Irrespective of which company leads the race and comes up with the first CAR-T based therapy, others would surely benefit from the collective shared insights and clinical trial studies.
Acknowledgement
iPlexus™ (https://iplexus.ai/), the The Intelligence Machine™ developed by Innoplexus having terabytes of data from clinical trials, publications, scientific conferences, news and press releases from pharma companies and much more, was used to collect information for this article.
References
- Yang Y (2015) Cancer immunotherapy: harnessing the immune system to battle cancer. J Clin Invest 125(9): 3335-3337.
- Guo C, Manjili MH, Subjeck JR, Sarkar D, Fisher PB, et al. (2013) Therapeutic Cancer Vaccines: Past, Present and Future. Adv Cancer Res 119: 421-475.
- Vacchelli E, Eggermont A, Sautès-Fridman C, Galon J, Zitvogel L, et al. (2013) Trial watch: Oncolytic viruses for cancer therapy. Oncoimmunology 2(6): e24612.
- Guo Y, Wang Y, Han W (2016) Chimeric Antigen Receptor-Modified T Cells for Solid Tumors: Challenges and Prospects. J Immunol Res 2016: 3850839.
- Wang Z, Wu Z, Liu Y, Weidong Han (2017) New development in CAR-T cell therapy. J Hematol Oncol 10(1): 53.
- (2017) Novartis CAR-T cell therapy CTL019 receives FDA Breakthrough Therapy designation for treatment of adult patients with r/r DLBCL. Novartis
- (2017)Kite Completes Submission of U.S. Biologics License Application (BLA) for Axicabtagene Ciloleucel as the First CAR-T Therapy for the Treatment of Patients With Aggressive Non-Hodgkin Lymphoma (NHL). Kite Pharma.
- Derek Lowe (2016) More Juno CAR-T Deaths. Science Translational Medicine.
- Bonifant CL, Jackson HJ, Brentjens RJ, Curran KJ (2016) Toxicity and management in CAR T-cell therapy. Mol Ther Oncolytics 3: 16011.
- Cellular Therapy. Sorrento Therapeutics.
- Vor Biopharma.
- CAR-T Science and Technology. F1 Oncology.
- (2017)Cellectis is preparing to start the first clinical trial ever using an allogeneic CAR-T therapy that could reduce time and costs over its competitors. Labiotech.eu.






























