- Review Article
- Abstract
- Introduction and Background
- Aldred Scott Warthin
- An Inconvenient Truth
- Hodgkin's Cancer Comes Under Attack
- Dr. Virginia Livingston
- Momentum Builds
- Recognition
- Focus on Breast Cancer
- The Single Most Convincing Study of How Bacteria Causes Cancer
- The Politics of Cancer
- Parallels with Plant Cancer
- Seibert Rules Out Contaminants in the Cancer Germ
- Experimental Medicine for the Masses
- BCG
- Livingston's Legacy
- Conclusion
- Mycobacterium-Induced Malignant-like Multinucleated Giant Cells
- Cancer Observations in Old England
- Long Island Breast Cancer
- Long island New York, 1886
- The Malignant Threat of the Mycobacteria
- Gland Seeking Mycobacteria
- Viralizing Cancer
- Bacterial Phage Viruses
- References
Cancer and the Science of Denial –with Breast Cancer/Long Island Breast Cancer
Dr. Lawrence Broxmeyer, MD*
New York Institute of Medical Research, USA
Submission: May 24, 2017; Published: July 14, 2017
*Corresponding author: Dr. Lawrence Broxmeyer, MD New York Institute of Medical Research, New York, USA, Tel: (718) 229-3694; Email: drlawrencebroxmeyermd@alumni.usc.edu
How to cite this article: Broxmeyer L. Cancer and the Science of Denial. J Tumor Med Prev. 2017; 1(3): 555563. DOI: 10.19080/JTMP.2017.01.555563
- Review Article
- Abstract
- Introduction and Background
- Aldred Scott Warthin
- An Inconvenient Truth
- Hodgkin's Cancer Comes Under Attack
- Dr. Virginia Livingston
- Momentum Builds
- Recognition
- Focus on Breast Cancer
- The Single Most Convincing Study of How Bacteria Causes Cancer
- The Politics of Cancer
- Parallels with Plant Cancer
- Seibert Rules Out Contaminants in the Cancer Germ
- Experimental Medicine for the Masses
- BCG
- Livingston's Legacy
- Conclusion
- Mycobacterium-Induced Malignant-like Multinucleated Giant Cells
- Cancer Observations in Old England
- Long Island Breast Cancer
- Long island New York, 1886
- The Malignant Threat of the Mycobacteria
- Gland Seeking Mycobacteria
- Viralizing Cancer
- Bacterial Phage Viruses
- References
Abstract
The word ‘cancer’ is of Latin derivation and means crab. By the turn of the 20th Century organized medicine had come to the conclusion that it was not a matter of whether infectious disease caused cancer, but which one. Then, in 1910, certain American medical powers did a 180-degree rotation –abruptly deciding that cancer was not caused by a microbe. This flew in the face of over two hundred years of research in which a cancer germ had been discovered and rediscovered. Of all the infectious possibilities for cancer, unquestionably the one class of microbes that has been long recognized to most consistently mimic and imitate ‘cancer’ at both clinical and tissue levels are the mycobacteria of the family Actinomycetales of which tuberculosis and leprosy are premier examples. The association of TB with carcinoma was initially described about 200 years ago by Bayle who considered the lung malignancy ‘cavitation cancereuse’ to merely be one of the various types of tuberculosis. Ever since, almost as if in reflex to the obvious –the potential association between TB and subsequent development of cancer has drawn active investigation. In a combined 2017 Cleveland Clinic/Case Western probe, Wang et al compared the microorganisms in breast tissue of 57 patients with breast cancer (the most common cancer in women worldwide), and 21 healthy individuals undergoing breast surgery for cosmetic purposes. Three out of the four pathogens they found in breast cancer tissue belonged to the order Actinomycetales.
- Review Article
- Abstract
- Introduction and Background
- Aldred Scott Warthin
- An Inconvenient Truth
- Hodgkin's Cancer Comes Under Attack
- Dr. Virginia Livingston
- Momentum Builds
- Recognition
- Focus on Breast Cancer
- The Single Most Convincing Study of How Bacteria Causes Cancer
- The Politics of Cancer
- Parallels with Plant Cancer
- Seibert Rules Out Contaminants in the Cancer Germ
- Experimental Medicine for the Masses
- BCG
- Livingston's Legacy
- Conclusion
- Mycobacterium-Induced Malignant-like Multinucleated Giant Cells
- Cancer Observations in Old England
- Long Island Breast Cancer
- Long island New York, 1886
- The Malignant Threat of the Mycobacteria
- Gland Seeking Mycobacteria
- Viralizing Cancer
- Bacterial Phage Viruses
- References
Introduction and Background
Falagas's et al. [1] Tuberculosis & Malignancy review alone, with 211 references, cites 125 cases in which TB seemed to masquerade as malignant tumors. In all of these, clinical and/ or radiology findings indicated malignant tumor, but tissue pathology came back as mycobacterial tubercular infection. In this same report, an additional 52 cases and two retrospective studies focused on the co-existence of malignant tumors with TB at the same site. Finally, 14 additional case reports evaluated a history of TB as a risk factor for the development of a malignant tumor. A major confounding factor -that the medium time it took between the initial appearance of TB and the detection of malignancy was greater than 20 years. Yet the review's main findings were that first TB infection could be associated with the subsequent development of cancer; second that TB and malignancy may co-exist in some cases and third that similarities in the presentation of both diseases could easily lead to misdiagnosis.
Although Yu et al. [2] in 2011, documented that tubercular lung infection caused 11 times the incidence of lung cancer as normal control subjects, it is its “cell-wall-deficient” (CWD) forms (also called “L-forms”) that have recently repeatedly been found through genetic analysis and appropriate stains in such cancer tissue -suggesting to Zang et al. [3] that CWD tuberculosis or atypical tuberculosis “is likely to be involved in the occurrence or development of lung carcinoma”
Cell-Wall-Deficient (CWD) tuberculosis is so named because the cell-wall, which usually surrounds the deadly pathogen, is not intact. This allows the germ to have many forms, some of which are viral-sized. Such cell-wall-deficient tuberculosis is by far the favored form of the microbe, a survival strategy through which TB survives the inclement and harsh conditions of the cells and tissues of our body -particularly our immune system. CWD mycobacterial forms are almost indestructible and at the same time co-exist inside certain white blood cells (macrophages) of the body comfortably, as Chauhan et al. [4] documented in all intracellular macrophage-grown M. tuberculosis observed. Through eons of experience the pathogen has mastered this survival strategy which has allowed it to once again be the number one infectious killer on the planet [5].
Both lung and breast cancer and lung and breast tuberculosis might seem to be unrelated, but they are not as the two organs can be interconnected through the lymphatic system and can become infected through these lymphatics or by direct extension. Recently Yang et al. [6], using intensified Kinyoun acid-fast staining and in situ hybridization techniques for the detection of MPB64 gene expression in the nucleus of breast cancer cells, concluded that cell-wall-deficient (CWD) or “L-forms” of Mycobacterium tuberculosis exist in breast cancer tissue as well, and that the MPB64 tubercular gene was highly expressed in the all-important nucleus of breast cancer cells, where premalignant and malignant change occurs.
It had already been suggested that M. tuberculosis was closely linked to the occurrence of lung cancer [7]; and that in particular its L-forms, with breeched cell walls could act in a manner similar to oncogenic viruses to induce all types of cancer by DNA or in some cases RNA integration [8].
A prime example of why latent cell-wall-deficient, L-form breast tuberculosis has been grossly and consistently been underestimated as “rare” lies in part in the archaic techniques presently being used to detect it. Kakkar's et al. [9] study, showed the 'hit or miss' technology of stains and cultures presently being used to detect breast tuberculosis worldwide -most of which fail to use specific tubercular L-form stains such as Yang's intensified Kinyoun acid-fast staining and in situ hybridization techniques for sorting out breast tuberculosis from breast cancer. Kakkar [9], reported a study of one hundred sixty cases of breast TB in Acta Cytologica, all clinically suspected to have breast carcinoma, but on fine needle aspiration (FNA) they proved to have breast TB. Of the 160 cases, 118 (73.75%) had cytomorphology diagnostic of tuberculosis -epithelioid cell granulomas with caseous necrosis. Whereas only eleven of the remaining 42 cases were positive for acid-fast bacilli (AFB) using the traditional Ziehl-Neelsen (ZN) stain used in most labs today to determine TB.
Pioneer investigator Warthin originally stated that breast tuberculosis is anything but uncommon, as attested to recently on a world-wide scale by Puneet et al. [10] and Vagholkar et al. [11] -who again found that despite flawed technique -on a world-wide scale it is again anything but uncommon. By 2014 Vagholkar [11] observed, “Tuberculosis of the breast, which was once upon a time a rare disease, has become quite common especially in the developing world, where tuberculosis is still a major health care problem.” Obviously nothing in this statement implies that the developed world is immune to tuberculosis of the breast in this time of international commerce and migration.
Under the best conditions it is extremely difficult to find and identify cell-wall-deficient tubercular L-forms without using hybridization techniques and PCR as a back-up. Not only does it take special stains and cultures to detect CWD mycobacteria, but even in the case of the sensitive PCR used to detect the DNA of the organism -if DNA is extracted from stable tubercular L-forms in the breast or elsewhere, it is often negative. This is because, with the loss or disruption of tubercular cell-walls, their cell membrane may become greatly thickened. Therefore it is difficult to break the membrane in cell-wall-deficient (CWD) tuberculosis to release the DNA. Liu showed that under electron- micrographic analysis the thickness of cell membrane in CWD M. tuberculosis could be as thick as 40.54nm, whereas the thickness of the cell membrane plus cell wall in classical TB forms is only 34.84nm [12]. It is for exactly this reason that Dai determined that the use of physical grinding with glass sand gave results superior to traditional methods and should be used where all other methods of DNA extraction fail [13]. But this is not being routinely practiced.
In addition, dormant tubercular cell-wall-deficient or “L-forms” are among the most difficult microbes to cultivate and identify, especially in their early non-cultivable or so-called “invisible” stage [14]. Therefore to find them in the living or dead organism takes mandatory novel strategies including special growth techniques to enrich and revive them to an actively growing, colony-forming state, such as the use of growth stimulants which create nutrient starvation or hypoxic conditions for M. tuberculosis in vitro [15]. But beyond all of this, when most laboratories refuse to routinely perform these specialized L-form assays and most clinicians refuse to order them, their diagnosis becomes an impossibility.
- Review Article
- Abstract
- Introduction and Background
- Aldred Scott Warthin
- An Inconvenient Truth
- Hodgkin's Cancer Comes Under Attack
- Dr. Virginia Livingston
- Momentum Builds
- Recognition
- Focus on Breast Cancer
- The Single Most Convincing Study of How Bacteria Causes Cancer
- The Politics of Cancer
- Parallels with Plant Cancer
- Seibert Rules Out Contaminants in the Cancer Germ
- Experimental Medicine for the Masses
- BCG
- Livingston's Legacy
- Conclusion
- Mycobacterium-Induced Malignant-like Multinucleated Giant Cells
- Cancer Observations in Old England
- Long Island Breast Cancer
- Long island New York, 1886
- The Malignant Threat of the Mycobacteria
- Gland Seeking Mycobacteria
- Viralizing Cancer
- Bacterial Phage Viruses
- References
Aldred Scott Warthin
Although it has been commonly documented that the coexistence of carcinoma and tuberculosis in the breast and axillary lymph nodes was originally described by Warthin [16] at the University of Michigan -ignored are Warthin's thoughts and findings in that paper. When Aldred Scott Warthin became Head of Pathology at Ann Arbor, his pathology textbooks were already being widely used in medical colleges, and he was at one point President of The American Association for Cancer Research. Few American pathologists were more respected and his paper “The Coexistence of Carcinoma and Tuberculosis of the Mammary Gland" is a good example as to why. Warthin realized that in America tuberculosis of the mammary gland, as elsewhere, was looked for by pathologists with the blinders of seeking out only its bacillary form to the exclusion of all other tubercular cell- wall-deficient forms. Thus, against the many voices (up to the present day) that claimed otherwise, Warthin concluded: “It is, however, probably that the disease (breast TB) occurs with relative frequency, but the possibilities of its escaping observation are very great"and Warthin added to this: “In spite of the probable relative frequency of this condition (mammary tuberculosis) and the fact that carcinoma of the breast is one of our most common clinical conditions, the coexistence of the two processes in the same gland is apparently rare." But he then went on to discuss a case where breast TB actually turned into breast carcinoma right under his microscope.
Dr. Carl Warden of Ishpeming, Michigan, sent Warthin portions of a breast tumor, withholding the history of the patient. Only after Warthin returned a diagnosis of tuberculosis of the breast did Warden supply the following history. The specimens he sent Warthin were from a forty-year-old female -a Scandinavian, who gave birth to her 4th and last child at the age of thirty-nine. Previously, at the time of birth of her last child, the patient noticed a general tenderness of her right breast with a “sore” nipple that gradually became retracted. So for this last child, the patient only used her left breast to feed her newborn infant. Shortly after weaning this last child, however, her right breast for some reason again became sore and this time there was a lump, about the size of a hazel-nut in the upper outer portion of her right breast. A month later she went back to Dr. Warden who this time palpated a hard, irregular mass -moveable and not attached to the overlying skin. But still a week later from that point the mass had increased very rapidly, now involving the lower segment of her right breast. In addition enlarged lymph nodes were now found in the right armpit (axilla), and there were sharp pains radiating up to her right shoulder. Because Warden understood that this could also have been a tubercular involvement of the right axilla and breast, after sending Warthin the first specimen which came back with the pathologic diagnosis of TB of the breast, Warden held off another week -at which time the entire organ showed involvement and the overlying skin had a red mottled appearance which seemed glued to the growth below it. Warden had seen enough. A clinical diagnosis of malignancy was made and an operation was advised -and performed two days later. The growth in the meantime had softened and fluctuation was evident. At operation, an exploratory incision was made below the right nipple and about an ounce of creamy pus was evacuated.
The mammary gland now had a stony character and a portion of its substance was once again taken and sent to Warthin. Although Warthin had previously returned a diagnosis of tuberculosis, Warden now suspected more and so sent these additional tissue specimens back to Warthin. At operation, Warden removed the diseased portions of the gland and after the procedure there was a marked improvement. The pain had almost entirely disappeared, although for a time there was moderate purulent discharge. Sometime after, Dr Warden informed Warthin that the patient had recovered and was now in good health - for the time being.
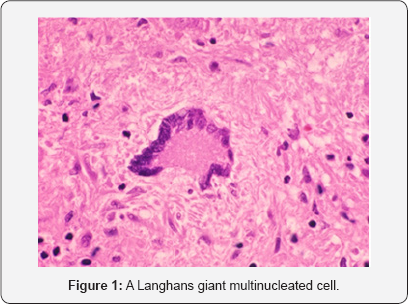
Warthin carefully reviewed his microscopic findings of this most recent tissue Warden had sent him. Classic tubercular "giant cells” were numerous with evidence of their phagocytic action as they tried to contain the infection. The name of these large, multinucleated cells found in a patient with tuberculosis were Langhans giant cells. The cell was named after Theodor Langhans, a German pathologist. It is a specific type of giant cell in which several epithelioid (meaning the cells are large and pink, like the cells of the skin) macrophages fuse together, the nuclei forming a puzzling horse-shoe shape around the periphery of the cell. In the past, Langhans cells were said to be specific for tuberculosis, but they are now known to occur in other types of granulomatous diseases, many of which have since been implicated in mycobacterial disease as well (Figure 1).
Such multinucleated cells are and have always been a mystery. Eighty years after Warthin -Rosen [17], at Memorial Sloan-Kettering would note that although these giant cells were considered benign, and all too common in the widespread benign lumps in women's breasts, that such tubercular multinucleated cells could easily simulate and were also found in various types of invasive carcinoma. The purpose of Rosen's investigation was "to call attention to and describe this relatively frequent but often unnoticed change.” Rosen [17] might have been concerned how such benign giant cells of "undetermined significance” could be misinterpreted as invasive cancer, but what really bothered him was that the median age span of those who developed them were women between 40 and 50, whether they occurred in benign or malignant breast disease. This was in the perimenopausal age bracket. And so, after speculating as to whether the appearance of such giant cells were hormonally related, Rosen ended his paper this way: "It remains to be determined whether the lesion [multinucleated giant cells in the stroma of breast cancer victims] is more frequent in the breasts of women who have carcinoma or if it has any significance as an indicator of risk for the future development of cancer."
Staring at the large multinuclear cells before him Warthin couldn't help but think of the pioneer work of Ludvig Hektoen [18] on such tubercular giant cells. Hektoen [18] said that the nuclei in these were frequently atypical; and could be dumbbell shaped, flask-shaped, or very long and drawn out. Budding processes connected with the main nucleus by a slender thread or stem were also present, with irregular, bizarre shapes not infrequent. Occasionally, Hektoen mentioned, fairly well- preserved karyokinetic figures of division were also found in a single nucleus of a giant cell.
Warthin noticed that throughout his fields of tissue investigation there was the appearance of an invasion ofmammary glandular structure by infected tubercular epithelioid tissue arising in the connective tissue. In several places the mammary gland tissue entirely disappeared, replaced by large tubercular areas of caseating epithelioid tissue, abundantly infiltrated by lymphocytes. Invasive tuberculosis was wreaking havoc with the epithelial cells of the mammary gland and it was in the case of epithelial cells loosened from their basement membrane by this tubercular invasion that there appeared a marked tendency to proliferate towards malignancy Warthin noticed abundant cell- division forms, both mitotic and amitotic, but mostly the tell-tale pathological amitotic forms of a developing cancer, forming cells with large, irregular branching nuclei. Warthin knew that as a general rule, the more bizarre the nuclei, the more aggressive the cancer, but the grotesque nuclei he spotted in the giant cells of these latest tissue specimens especially, seemed more than half-way towards such malignancy. But what he could not know was how other studies, performed decades later would prove just how devastating TB could be to the chromosomal apparatus of cell cultures of human tissue. In such studies, an increase in pathological mitoses, arrest of cell division in metaphase, and the actual appearance of chromosomal adhesions absent in control cultures appeared. Indeed, early tubercular involvement was not only destructive against chromosomes but the very spindles that separated them [19-21].
Since it was in the case of epithelial cells loosened from their basement membrane by this tubercular invasion that there appeared in the field a marked tendency to proliferate towards malignancy -Warthin now traced the stage-by-stage evolution of tuberculous-incited mammary gland epithelial cells on their relentless progression towards full-blown carcinoma. He concluded that in this particular case, it was the tubercular epithelial cells, bearing no resemblance to normal epithelial cells that were behind the genesis of this patient's breast cancer
And it was just such carcinomatous proliferation that Warthin also found in several breast ducts. Staining for tubercle bacilli was successful but in characteristic scanty numbers. He had just witnessed the changes in the genetic structure of human cells from tubercular attack. But in the TB turned to breast cancer case before him, the pus obtained from the surgical wound had been stained by Dr. Warden without success. Shortly later, as a testimony to the many (pleomorphic) forms of the inevitable cell-wall-deficient tuberculosis in all tubercular infections, Warthin did detect in the pus a few tubercular forms, but these bacilli were "beaded.” Koch, the discoverer of tuberculosis also commonly noticed these non-acid-fast beaded forms, in older cultures and infected tissues. Somewhat granular and protruding from stalks, Koch thought they were potential "spores” through which infection could be propagated. But Koch was unable to observe the granules break off into separate segments. Hans Much [22], on the other hand, for decades, not only watched the granules break off (Much's granules) but regenerate into classical TB bacilli. Later M.C. Kahn [23] confirmed this.
A guinea pig had been inoculated with the pus sample sent to him by Warthin, but its death and post-mortem were carried out in Warthin's absence and so nothing definite was gleaned. Nevertheless, Warthin, like Warden, had seen enough. His cytologic diagnosis was changed from tuberculosis of the breast to tuberculosis of the breast leading to epithelioid growth and secondary breast cancer.
Ribbert [24], a contemporary of Warthin, believed that whenever the processes existed together, no matter the organ, that the carcinomatous growth was as a result of the tuberculous process. Ribbert [24] believed that he had seen the histological structure of tuberculosis in eleven cases of carcinoma: six of the lip, and one each of the mouth, tongue, gum, eyelid, and penis.
- Review Article
- Abstract
- Introduction and Background
- Aldred Scott Warthin
- An Inconvenient Truth
- Hodgkin's Cancer Comes Under Attack
- Dr. Virginia Livingston
- Momentum Builds
- Recognition
- Focus on Breast Cancer
- The Single Most Convincing Study of How Bacteria Causes Cancer
- The Politics of Cancer
- Parallels with Plant Cancer
- Seibert Rules Out Contaminants in the Cancer Germ
- Experimental Medicine for the Masses
- BCG
- Livingston's Legacy
- Conclusion
- Mycobacterium-Induced Malignant-like Multinucleated Giant Cells
- Cancer Observations in Old England
- Long Island Breast Cancer
- Long island New York, 1886
- The Malignant Threat of the Mycobacteria
- Gland Seeking Mycobacteria
- Viralizing Cancer
- Bacterial Phage Viruses
- References
An Inconvenient Truth
One cannot review the pharmaceutical and biologic treatments for cancer today without being struck with their activity against tuberculosis and the mycobacteria as well. Time and again, this proves to be the case. It is said that Kaposi's sarcoma can be cured by rapamycin, an antibiotic with strong activity against TB. Such an antibiotic could not possibly be working against human herpes virus 8, thought to be associated with and a cause of Kaposi's since 1994 [25,26]. Rapamycin, on the other hand, enhances the killing of mycobacteria like tuberculosis by human macrophages [27]. But the phenomenon, of curing cancers like Kaposi's with anti-tubercular antibiotics and agents is certainly not limited to Kaposi's alone.
Tamoxifen (Nolvadex) has been used for over 40 years to treat hormone-receptor positive breast cancer. But it also has anti-tuberculosis activity against drug-sensitive strains (MIC, 3.125-6.25μg/ml) as well as drug resistant strains (MIC, 6.25 to 12.5μg/ml). In addition, tamoxifen profoundly decreases the number of intracellular TB in macrophages in a dose-dependent manner [28].
The anti-tuberculous effect of the cancer agent bleomycin, a potent inhibitor of tuberculosis is already on record [29]. Similarly, the cancer drug Doxorubicin (Adriamycin®) has strong anti-TB activity. Gajadeera et al. [30] reported not only the anti- mycobacterial, anti-tubercular activity for doxorubicin, but for daunorubicin and idarubicin. Consequently, Forbes et al. [31] showed that not only was the anti-cancer anthracycline class mycobactericidal and anti-tubercular -but that three other anti-cancer agents also identified: 6-mercapto-purine, 5-fluoro-uracil and teniposide, had similar activity. Teniposide (trade name Vumon®) is a chemotherapeutic medication used in the treatment of certain brain tumors, childhood acute lymphocytic leukemia (ALL), Hodgkin's lymphoma, and other types of cancer
Disseminated tuberculosis, of course, has been associated with a variety of hematological abnormalities -and in a substantial proportion of the reported cases, the underlying tuberculous process was diagnosed only at autopsy. Tubercular leukemoid reaction, which closely simulates blastic leukemia and in some cases is impossible to differentiate from true leukemia, have been reported in patients suffering from disseminated tuberculosis. TB can easily simulate the blast findings in acute leukemia [32].
Two of those agents mentioned in the Forbes study, 5-fluoro- uracil and teniposide, were previously identified in a high throughput screen for anti-TB agents [33]. And the activity of 6-mercaptopurine against Mtb has also been recently reported [34].
One of the leading antitumor drugs, the toxic cisplatin, which has been used for more than three decades on a variety of cancers is also toxic toward Mycobacterium tuberculosis with a minimum inhibitory concentration of ~40|iM [35]. Other tumor agents such as certain thiosemicarbazone derivatives, which show cytotoxicity against breast cancer cells, actually have activity equivalent or greater than those of some commercial anti-M. tuberculosis drugs now being used [36].
Further parallels exist even in the same newer anti- angiogenesis cancer drugs. The blood vessels supplying the dense masses of the immune cells in pulmonary granulomas in TB have the same sort of structural and functional abnormalities seen in solid tumors. So treatment with, for example, the cancer anti-angiogenesis drug bevacizumab (Avastin) to normalize granuloma vasculature could also enhance the delivery of anti-TB drugs and potentially reduce the growing problem of antibiotic resistance [37].
All members of the genus Mycobacterium with the exception of Mycobacterium tuberculosis and M. leprae are considered non- tuberculous mycobacteria (NTM). This includes Mycobacterium avium (fowl tuberculosis), which pioneer cancer investigator Dr Virginia Livingston considered extremely important in the genesis of cancer, and for which there is no definitive antibiotic cure. More than 160 species of NTM exist. There is widespread belief that NTM infections are increasingly common, particularly among women. Among the NTM, rapidly growing mycobacteria (RGM) have recently gained increasing attention because they are associated with specific diseases and are characterized by extensive resistance to antimicrobial drugs. RGMs are diverse and include Mycobacterium abscessus, M. chelonae, M. fortuitum, M. immunogenum, and M. smegmatis.
Recent attempts to treat cancer like an infectious disease by using antibiotics and other prescription drugs have already been performed. But again, many of these agents have anti- mycobacterial or anti-tubercular activities, which have not been taken into consideration. Such was certainly the case in the recent collaborative study by the UK's University of Manchester, the Kimmel Cancer Center in Philadelphia and the Albert Einstein College of Medicine in the Bronx, New York [38].
Among the antibiotics chosen for the study was azithromycin, a potent commonly prescribed macrolide antibiotic, which this study found to inhibit many different tumor types, including ER- (Estrogen Receptor negative) breast cancer, ovarian, lung, pancreatic and prostate cancer, as well as melanoma. But azithromycin is also a first-line drug against a frequent visitor to the human cancer map, fowl tuberculosis (Mycobacterium avium). Indeed in a study previous to the Einstein/UK study, azithromycin exhibited in-vitro activity against 20 clinical isolates of Mycobacterium avium complex for which the MIG» was 32mg/L and 22 clinical isolates of other mycobacteria [39].
Also chosen in the Manchester/Einstein study was the tetracycline-based antibiotic doxycycline, which showed varying anti-tumor inhibition against all 10 cell lines tested across 6 different cancer types- including two commonly used ER+ breast cancer cell lines in which it was most effectively at concentration range of from 50-to-100|iM. Although doxycycline shows bacteriostatic action against nearly all aerobic and anaerobic bacteria, whether gram negative or gram positive, it also has role as an anti-mycobacterial. Besides suppressing the mycobacterial growth of TB itself in vitro and in guinea pigs [40], doxycycline displays significant in vitro anti-mycobacterial activity against the rapidly growing mycobacteria (RGM) with only a handful of exceptions.
But such action of doxycycline against RGM mycobacteria was nothing compared to the 3rd choice for the Einstein study - tigecycline, which is a glycylcycline derivative of the tetracycline minocycline. In the Manchester/Einstein study, tigecycline inhibited tumor formation across all 10 cell lines tested in a similar manner to doxycycline, however tigecycline was much more effective in the inhibition of cell lines for breast ER-, ovarian, pancreatic and to a more limited extent melanoma then was doxycycline. Tigecycline is a strong, strong inhibitor of the rapid-growing mycobacteria, displaying 100% activity in one study in which it successfully inhibited all 40 RGM strains [41].
The next antibiotic tested against tumor cells was chloramphenicol: As in the case of azithromycin, doxycycline and tigecycline, chloramphenicol's anti-tumor action was again attributed to being "an inhibitor of mitochondrial biogenesis”, but apparently not a very good one and therefore "the least potent of the mitochondrial inhibitors tested.” Chloramphenicol, with its specter of bone marrow shutdown (aplastic anemia), especially as an oral antibiotic, was an odd choice to begin with. Although this side effect is said to be rare, it can be fatal, and no oral formulation of chloramphenicol is now available in the U.S. and if it were, doctors would think ten times before using it. In addition chloramphenicol itself can increase the risk of childhood leukemia, as demonstrated in a Chinese case- controlled study -and the risk increases with length of treatment [42]. Another study pointed to the potential for chloramphenicol to induce leukemogenesis possibly leading towards leukemia regardless of age [43]. If chloramphenicol was 'the least potent' antibiotic in the panel for anti-tumor suppression, then it finds a parallel in Smith's study which reported that chloramphenicol was only moderately active against Gram-positive bacteria and Mycobacterium tuberculosis [44]. And Youmans et al. [45], also testing strains of virulent human-type M. tuberculosis in vitro concluded that chloramphenicol was again, only moderately active when compared with streptomycin or para-amino salicylic acid.
In addition, the authors of the Manchester/Einstein study cite the anti-diabetic drug metformin, the safety of which they were concerned with, yet which definitely had anti-cancer properties as shown in two other studies [46,47]. But, again, in 2014, metformin was shown to control the growth of drug- resistant tubercular strains, ameliorate its lung pathology, reduce its chronic inflammation, and enhance the specific immune response and the efficacy of conventional TB drugs. In two separate human cohorts, metformin treatment was therefore associated with improved control of Mtb tubercular infection and decreased disease severity. Collectively, these data indicated that metformin was a promising candidate host-adjunctive therapy for improving the effective treatment of TB [48].
Finally in the Manchester/Einstein study, the agent Pyrvinium pamoate was explored. But besides its long-held reputation for antitumor activity [49], the anti-helminthic agent pyrvinium pamoate again possesses robust anti-tubercular activity as a strong inhibitor of M. tuberculosis [50,51].
When the powers in medicine that be arbitrarily decided in 1910 that cancer could not be caused by a microbe, they also decided that anyone who thought otherwise was a heretic, a charlatan or a quack. But Dr. Virginia Livingston [52] and her network were none of the above, their meticulous peer-reviewed research and publications, done at the height of US post World War II technology. Researcher, MD Alan Cantwell [78] grew up thinking that all germs responsible for the important diseases were supposed to have already been discovered. But much to his dismay, he found one that was left out: the cancer germ. Cantwell knew that for finding this, Livingston had already been branded by traditional medicine, leaving what he thought to be perhaps the major discovery of the 20th century largely discredited.
The striking analogy between cancer and tuberculosis was noticed long before the tubercle bacillus was discovered. In 1877, Sir John Simon clearly pointed out the similarity and in fact argued very strongly in favor of a microbial origin for cancer But Simon's vindication would have to wait for Livingston's germ, which although tuberculosis-like, was not tuberculosis but an atypical form of this mycobacterium, melded from the mycobacterium and other related Actinomycetales. Had medical science and the powers that be spent as much time in investigating Virginia Livingston's cancer germ as they did in attacking her and those around her, cancer might be curable today.
- Review Article
- Abstract
- Introduction and Background
- Aldred Scott Warthin
- An Inconvenient Truth
- Hodgkin's Cancer Comes Under Attack
- Dr. Virginia Livingston
- Momentum Builds
- Recognition
- Focus on Breast Cancer
- The Single Most Convincing Study of How Bacteria Causes Cancer
- The Politics of Cancer
- Parallels with Plant Cancer
- Seibert Rules Out Contaminants in the Cancer Germ
- Experimental Medicine for the Masses
- BCG
- Livingston's Legacy
- Conclusion
- Mycobacterium-Induced Malignant-like Multinucleated Giant Cells
- Cancer Observations in Old England
- Long Island Breast Cancer
- Long island New York, 1886
- The Malignant Threat of the Mycobacteria
- Gland Seeking Mycobacteria
- Viralizing Cancer
- Bacterial Phage Viruses
- References
Hodgkin's Cancer Comes Under Attack
When Virginia Livingston was a student at Bellevue Medical College her pathology teacher mentioned, rather disparagingly, that there was a woman pathologist at Cornell who thought Hodgkin's disease (a form of glandular cancer) was caused by avian (fowl) tuberculosis [52]. This lady had published, but no one had confirmed her findings. Afterwards, Livingston compared slides of both. In Hodgkin's, the large multinucleated giant cells were called Reed-Sternberg cells. They were similar to the giant cells of tuberculosis, which formed to engulf the tubercle bacilli. Livingston stored away in her memory that the lady pathologist was probably right but she would have a difficult time in gaining acceptance.
By 1931, Pathologist Elise L'Esperance was seeing 'acid fast' tuberculosis-like bacteria riddling her Hodgkin's tissue samples. And that germ, once injected into guinea pigs, caused them to come down with Hodgkin's too, fulfilling Koch's postulates. L'Esperance brought her stained slides to former teacher and prominent Cornell cancer pathologist James Ewing [52]. Ewing initially confirmed that her tissue slides were indeed Hodgkin's disease. But when he found out that her slides came through guinea pig inoculation of the human avian (fowl) tuberculosis she had found in Hodgkin's patients, Ewing, visibly upset that he had been upstaged, said that the slides then could not be cancer. It betrayed his checkered history. In 1907, you could have approached Dr. James Ewing [54] about a cancer germ, and he would have embraced you over it. At that time, both for him and the rest of the nation's medical authorities, it was not a question of whether cancer was caused by a germ, but which one. Was not it Ewing, at onetime, who had proclaimed that tuberculosis followed Hodgkin's disease "like a shadow”? And did not Ewing say that the evidence for tuberculosis or atypical tuberculosis (such as fowl or Avium tuberculosis) as being behind Hodgkin's was "somewhat formidable”. He also saw tubercular links to lymphoma, leukemia, sarcoma and carcinoma. But the technology to prove this was not present in his day. Nevertheless with regard to Hodgkin's disease, Ewing said this about German scientist Hans Much: "Much's claim that a granular (CWD) form of the tubercle bacillus exists in many lesions [of Hodgkin’s] demands attention in this field.” Ewing was referring to the granules that Fraenkel & Much [56] consistently isolated in the sediment of Hodgkin's nodes. Prior to staining, Hans Much [22], who found these granules by digesting Hodgkin's lymph node material with antiformin, spent over 27 years watching such granules spring back to TB's classic bacillus. Much's granules themselves did not stain with classic tubercular "acid-fast” stain, but the TB bacilli they eventually evolved into did. Ewing [53] said that such tubercular granules "demands attention” because Much's Hodgkin's results were being validated by others, including Meyer and Sticker Such CWD granular forms in Hodgkin's' bothered Ewing, who was aware that Much's granules, although present rather consistently in Hodgkin's, were found only in small numbers and to investigators unfamiliar with them were difficult to recognize. Furthermore Much & Fraenkel's [56] findings indicated that it was not the tubercle bacilli per se that was behind Hodgkin's disease, but its granular cell-wall-deficient forms -then and now known as Much's granules.
Although certain historians relate that shortly after, James Ewing [54 ], "the Father of Oncology”, sent a sword thru the heart of an infectious cause of cancer with his book "Neoplastic Diseases", this was certainly not for lack of the documentation of the potential importance of TB and atypical TB in the formation of cancer. But soon Ewing would become an ambitious zealot for radiation therapy with the directorship of what would one day be called Sloan-Kettering squarely on his mind. His entry lay in prominent philanthropist James Douglas. A vote for Ewing, Douglas knew, was a vote for continued radiation and James Douglas began sizeable uranium extraction operations from Colorado mines thru his company, Phelps Dodge, Inc. [54].
Shortly Sloan became known as a radium hospital and went from an institution with a census of less than 15% cancer patients, separated by partition, lest their disease spread to others, to a veritable cancer center. But the very history of radiation revealed its flaws, and by the early 1900s nearly 100 cases of leukemia were documented in radium recipients and not long thereafter it was determined that approximately 100 radiologists had contracted that cancer in the same way [55]. Still, Ewing, by now an Honorary Member of the American Radium Society, persisted.
Elise L'Esperance [54] was anything but alone in linking Hodgkin's to a tuberculosis-like germ called Avium or fowl tuberculosis. Historically Sternberg himself, namesake of Hodgkin's trade-mark Reed-Sternberg cell, believed initially that Hodgkin's was caused by tuberculosis, which he spotted regularly in Hodgkin's tissue. Both Fraenkel & Much [56] held, as L'Esperance, that it was caused by a peculiar form of tuberculosis, and of all the cancers, debate over the infectious cause of Hodgkin's waxed the hottest. L'Esperance's [57] own studies had shown that unlike animals injected with human tuberculosis, which did not have the same preference for the lymphatic nodes as seen in Hodgkin's disease, that avian tuberculosis had precisely this penchant. Into this arena L'Esperance [57] stepped in 1931, working with primitive tools by today's standards and with few listening. She would publish Studies in Hodgkin's Disease in an issue of the Annals of Surgery. It proved to be the one legacy that no one, not even Ewing, who would soon die from a self-diagnosed cancer, could take away from her.
- Review Article
- Abstract
- Introduction and Background
- Aldred Scott Warthin
- An Inconvenient Truth
- Hodgkin's Cancer Comes Under Attack
- Dr. Virginia Livingston
- Momentum Builds
- Recognition
- Focus on Breast Cancer
- The Single Most Convincing Study of How Bacteria Causes Cancer
- The Politics of Cancer
- Parallels with Plant Cancer
- Seibert Rules Out Contaminants in the Cancer Germ
- Experimental Medicine for the Masses
- BCG
- Livingston's Legacy
- Conclusion
- Mycobacterium-Induced Malignant-like Multinucleated Giant Cells
- Cancer Observations in Old England
- Long Island Breast Cancer
- Long island New York, 1886
- The Malignant Threat of the Mycobacteria
- Gland Seeking Mycobacteria
- Viralizing Cancer
- Bacterial Phage Viruses
- References
Dr. Virginia Livingston
"Our (cancer) cultures were scrutinized over and over again. Strains were sent to many laboratories for identification. None could really classify them. They were something unknown. They had many forms but they always grew up again to be the same thing no matter how they were cultured. They resembled the mycobacteria more than anything else. The tubercle bacillus is a mycobacterium or fungoid bacillus.”-Virginia Livingston [52].
Virginia Wuerthele-Caspe Livingston [52] received her M.D. from N.Y.U., the first female medical resident ever in New York City. With time Livingston became a Newark school physician where one day a staff nurse asked medical assistance. Already diagnosed with Raynaud's syndrome, the tips of this nurse's fingers were ulcerated and bled intermittently. Livingston also diagnosed scleroderma. But upon further examination there was a hole in the nasal septa, something that Livingston had previous seen in the mycobacterial diseases TB and Leprosy. Livingston approached dermatologist Eva Brodkin and a pathologist for confirmation, all the while convinced that mycobacterial infection was causing the scleroderma. She performed cultures from a sterile nasal swab -mycobacteria appeared, everywhere [52]. Injected into experimental chicks and guinea pigs, all but a couple died. Upon autopsy, the guinea pigs had indeed developed the hardened skin patches of scleroderma -some of which were cancerous.
- Review Article
- Abstract
- Introduction and Background
- Aldred Scott Warthin
- An Inconvenient Truth
- Hodgkin's Cancer Comes Under Attack
- Dr. Virginia Livingston
- Momentum Builds
- Recognition
- Focus on Breast Cancer
- The Single Most Convincing Study of How Bacteria Causes Cancer
- The Politics of Cancer
- Parallels with Plant Cancer
- Seibert Rules Out Contaminants in the Cancer Germ
- Experimental Medicine for the Masses
- BCG
- Livingston's Legacy
- Conclusion
- Mycobacterium-Induced Malignant-like Multinucleated Giant Cells
- Cancer Observations in Old England
- Long Island Breast Cancer
- Long island New York, 1886
- The Malignant Threat of the Mycobacteria
- Gland Seeking Mycobacteria
- Viralizing Cancer
- Bacterial Phage Viruses
- References
Momentum Builds
Livingston, now possessed, solicited fresh sterile specimens of cancer from any operating room that would give them to her. All cancer tissues yielded the same acid-fast mycobacteria. New Jersey Pathologist Roy Allen confirmed her findings. Livingston & Allen [58] then found that they could actually differentiate malignant from benign tissue by their mycobacterial content. But still the explanation for why the cancer germ showed so many different forms was elusive. Try as she might, part of Virginia Livingston's problems in an American validation of her multi-shaped cancer germ lay firmly entrenched in the history of medicine, especially in the constantly changing field of microbiology. Louis Pasteur could handle being quickly rushed off a Paris Academy of Sciences podium to escape harsh reaction to his suggestion that children's milk be boiled first, but he could not tolerate his rival Pierre Bechamp's statement that a single bacteria could assume many, many forms. On his deathbed, Pasteur was said to have changed his mind when he said: "The terrain is everything”, meaning the culture or milieu that bacteria grew on or in could change their shape or characteristics. But it was too late and even today, most conventional microbiologists deny the existence of such form changing (or pleomorphic) germs. Robert Koch, Father of Bacteriology and discoverer of tuberculosis, could have helped. When he first worked with the bacteria anthrax, Koch noticed that anthrax's classical rod shape became thread-like inside the blood of laboratory mice. And then, after multiplying, they assumed spore-like forms.
Aware of what she faced, Livingston methodically went about proving cancer's true cause. First in her line of attack were the long suspected and well-publicized tumor agents of Rous, Bittner and Shope. By photomicrographs, Livingston and her group demonstrated acid-fast mycobacterial/tubercular forms in each of these so-called "viral” cancers. This included the famed Rous chicken sarcoma.
Early on, Virginia Livingston [52] had decided that she needed help in validating her cancer germ and nobody knew the shapes and staining capacities of mycobacterial-related germs better than Dr. Eleanor Alexander-Jackson of Cornell. As far back as 1928, Eleanor Alexander-Jackson, bacteriologist, had discovered unusual and to that point unrecognized forms of the TB bacillus, including its filterable forms. By 1951, Alexander-Jackson was considered the expert TB microbiologist at Cornell. In the same year, another American, HC Sweany [59] proposed that both the granular and other forms of tuberculosis that passed thru a filter caused Hodgkin's disease. This was subsequently supported by studies by Mellon, Beinhauser & Fisher [60,61]. Mellon prophetically warned that tuberculosis could assume both classical red acid-fast forms as well as blue nonacid-fast forms indistinguishable from common germs such as Staphylococci, fungi and the corynebacteria and that this would surely perplex microbiologists.
When organized medicine choose to ignore these studies, Jackson cautioned that a so-called cure for TB could be as short-lived as it took classical TB rods, for the moment gone underground as a nonacid-fast form, to resurface one day and spring back towards destruction. Although American medicine had no serious time for Alexander-Jackson or her discoveries, it would not disturb her for as long as she focused on tuberculosis and its cousin leprosy. But when her focus shifted towards Livingston's cancer germ, it would move to destroy her. She simply posed too great a threat.
- Review Article
- Abstract
- Introduction and Background
- Aldred Scott Warthin
- An Inconvenient Truth
- Hodgkin's Cancer Comes Under Attack
- Dr. Virginia Livingston
- Momentum Builds
- Recognition
- Focus on Breast Cancer
- The Single Most Convincing Study of How Bacteria Causes Cancer
- The Politics of Cancer
- Parallels with Plant Cancer
- Seibert Rules Out Contaminants in the Cancer Germ
- Experimental Medicine for the Masses
- BCG
- Livingston's Legacy
- Conclusion
- Mycobacterium-Induced Malignant-like Multinucleated Giant Cells
- Cancer Observations in Old England
- Long Island Breast Cancer
- Long island New York, 1886
- The Malignant Threat of the Mycobacteria
- Gland Seeking Mycobacteria
- Viralizing Cancer
- Bacterial Phage Viruses
- References
Recognition
By December of 1950 Livingston [52], who had written over 17 peer reviewed articles by the end of her career, wrote together with Jackson and four other prominent researchers, what still stands as a milestone on the infectious nature of cancer [62]. At the AMA's 1953 New York exhibit, participants interest was particularly riveted towards an exhibit of Livingston's cancer germ, live. The press, muzzled by Sloan Kettering's head, Cornelius Rhodes, was not allowed to interview or report on this exhibit. Projected on a screen above, the cancer germs seemed indestructible, surviving a five-day experience in the intolerable heat of closed-circuit microscopy [52].
As Livingston & Jackson's [63] work on the cancer germ became more and more convincing, her opponents surfaced and became more and more vocal. Also with recognition, came visitors. One a pathologist from Scranton, Dr. George Clark, told Livingston he had cultured Dr. Thomas Glover's [63] famed cancer germ from human cancer and developed metastasizing tumors in animals from it [64]. Clark assured her that Glover was on to the same bacterial pathogen as she was. For more than two hundred years, the same organism had been discovered and rediscovered, named and renamed, each discoverer adding to what was known about the cancer germ, but thus far to no avail.
- Review Article
- Abstract
- Introduction and Background
- Aldred Scott Warthin
- An Inconvenient Truth
- Hodgkin's Cancer Comes Under Attack
- Dr. Virginia Livingston
- Momentum Builds
- Recognition
- Focus on Breast Cancer
- The Single Most Convincing Study of How Bacteria Causes Cancer
- The Politics of Cancer
- Parallels with Plant Cancer
- Seibert Rules Out Contaminants in the Cancer Germ
- Experimental Medicine for the Masses
- BCG
- Livingston's Legacy
- Conclusion
- Mycobacterium-Induced Malignant-like Multinucleated Giant Cells
- Cancer Observations in Old England
- Long Island Breast Cancer
- Long island New York, 1886
- The Malignant Threat of the Mycobacteria
- Gland Seeking Mycobacteria
- Viralizing Cancer
- Bacterial Phage Viruses
- References
Focus on Breast Cancer
Virginia Livingston went specifically after breast cancer Thirty different sterile cancerous breast specimens were transported directly from operating room to lab. Cancers were isolated from each breast and when axillary tissue from under the arm was supplied, the cancerous portion was cut from this too. Livingston and Jackson found the cancer germ everywhere, and in the case of underarm glands, even when the pathology report was negative, the cancer microorganism surfaced [52].
Champion of toxic chemotherapy, Cornelius Rhoads replaced Ewing at Sloan. Rhoads, head of chemical warfare during the Korean War, was deeply committed to chemotherapy and the huge grants it brought from the pharmaceutical industry. It is poorly recognized that the chemotherapy or "chemo” used against cancer began as a weapon of mass destruction par excellence [65]. When the Axis folded, nitrogen mustard, declassified, first came under real medical scrutiny for cancer. Initially evaluated for lymphosarcoma in mice, human studies soon followed as more and more variants of nitrogen mustard were concocted and tried [65]. Other related classes of chemotherapeutic agents followed and so did their repercussions. Most had the potential to cause a second entirely different cancer [66]. Even tamoxifen for breast cancer was associated with a two to three-fold increased risk of cancers of the lining of the uterus (endometrial), some of which were high grade with a poor forecast [67]. Nevertheless, Cornelius Rhoads remained committed to the treatment, and at the same time prepared a series of major roadblocks to stop Livingston.
- Review Article
- Abstract
- Introduction and Background
- Aldred Scott Warthin
- An Inconvenient Truth
- Hodgkin's Cancer Comes Under Attack
- Dr. Virginia Livingston
- Momentum Builds
- Recognition
- Focus on Breast Cancer
- The Single Most Convincing Study of How Bacteria Causes Cancer
- The Politics of Cancer
- Parallels with Plant Cancer
- Seibert Rules Out Contaminants in the Cancer Germ
- Experimental Medicine for the Masses
- BCG
- Livingston's Legacy
- Conclusion
- Mycobacterium-Induced Malignant-like Multinucleated Giant Cells
- Cancer Observations in Old England
- Long Island Breast Cancer
- Long island New York, 1886
- The Malignant Threat of the Mycobacteria
- Gland Seeking Mycobacteria
- Viralizing Cancer
- Bacterial Phage Viruses
- References
The Single Most Convincing Study of How Bacteria Causes Cancer
By 1965, Edith Mankiewicz [68], Director of labs at Montreal's Royal Edward Chest Hospital and assistant professor of bacteriology at McGill, by examining human cancer tissue, established mycobacterial-like germs inside cancer. In the bibliography of her landmark paper is reference to personal communication with Dr. Eleanor Alexander-Jackson. One of the cancers under Mankiewicz's trained eye was lung cancer Lung cancer, or bronchogenic cancer, was first reported in the nineteenth century at a time when it was practically unknown- while mycobacterial disease of the lung, primarily tuberculosis, was so rampant as to be called 'white plague' or in certain circles: 'captain of the men of death.' By the middle of the seventeenth century, one in five deaths was due to tuberculosis and at the end of the nineteenth century, there was fear that it would destroy the very civilization of Europe. So difficult was it to differentiate tuberculosis from the newly discovered bronchogenic cancer that it was only after cases first mistakenly diagnosed as lung cancer were operated on that the benefits of surgical resection of tuberculosis were recognized [69].
Mankiewicz [68] not only showed the cancer germ in malignant tissue but significantly demonstrated how it probably evolved from tuberculosis and related microorganisms when some of the viral phages that lived in them jumped germs, bringing genetic materials which altered the target germs virulence. In fact beneath Mankiewicz's microscope lay a pictorial of how the cancer germ emerged from TB-like bacilli to create pre-malignant change in mammalian tissue [68].
By 1970, Sakai Inoue [71], a PhD from Maebashi, Japan; and Marcus Singer, a doctor at Case Western's Developmental biology, completed the single most convincing study of how bacteria cause cancer altogether -with mycobacteria. Supported by grants from the American Cancer Society and the National Institutes of Health (NIH), their study used cold-blooded animals, namely the newt or salamander and the frog. But similar studies showed its applicability to mice [70] and humans [71,72]. Inoue: "An organism similar to the mycobacterium described here has been isolated and cultured from tumors and blood of tumorous mammals, including man, and when injected into mice and guinea pigs, has been reported to yield a chronic granulomatous disease, neoplasm (cancer), or some intergrade."- Inoue & Singer [73].
Back in the spring of 1953, Sakai Inoue noticed an adult salamander with a hard mass on its stomach. He removed the mass, which turned out to be malignant. Then when he injected tissue from the mass into healthy animals again, cancer developed. In the work that followed, Inoue and Singer, from electron micro pictographs, knew that bacteria were involved, bacteria which stained acid-fast: mycobacteria [73]. Inoue inoculated three other types of mycobacteria into healthy animals. All came down with cancer, something that did not happen when other germs such as staphylococcus or streptococcus were used. Amazingly Inoue and Singer even noted regressions in some of the cancers, especially if very dilute solutions of the germs were used to initiate them. Furthermore, since cancers stemming from 'carcinogens' were structurally identical to mycobacterial induced cancers, the investigators' results suggested that such 'carcinogens' might merely be factors that activate pre-existing infection. The phages inside mycobacteria are viruses known to be activated by carcinogens such as UV light and chemicals [74]. Mankiewicz [68], five years previously, had shown that these phages, once activated, could cause pre-malignant changes in mammalian tissue. Sakai Inoue and Marcus Singer's study should have once and for all convinced Virginia Livingston's opponents of the veracity of her results, and that she was not mistaking common contaminants such as staphylococci or streptococci for the cancer germ -but it did not.
- Review Article
- Abstract
- Introduction and Background
- Aldred Scott Warthin
- An Inconvenient Truth
- Hodgkin's Cancer Comes Under Attack
- Dr. Virginia Livingston
- Momentum Builds
- Recognition
- Focus on Breast Cancer
- The Single Most Convincing Study of How Bacteria Causes Cancer
- The Politics of Cancer
- Parallels with Plant Cancer
- Seibert Rules Out Contaminants in the Cancer Germ
- Experimental Medicine for the Masses
- BCG
- Livingston's Legacy
- Conclusion
- Mycobacterium-Induced Malignant-like Multinucleated Giant Cells
- Cancer Observations in Old England
- Long Island Breast Cancer
- Long island New York, 1886
- The Malignant Threat of the Mycobacteria
- Gland Seeking Mycobacteria
- Viralizing Cancer
- Bacterial Phage Viruses
- References
The Politics of Cancer
It was public knowledge in early 1951 that the Black-Stevenson Cancer Foundation intended to give two huge Black grants of $750,000 each towards cancer research and that the first would go to Livingston's group at Newark's Presbyterian; with an equivalent amount to go to The Memorial Center for Cancer (now Sloan-Kettering), which Rhoads headed. The trustees having already decided this, the actual allocation was left in the hands of Newark lawyer Charles R. Hardin. But fate intervened.
Livingston: "Hardin, the lawyer in charge of allocation, soon would lie dying of cancer at Memorial and while still alive was prevailed upon by design of Rhoads to sign a paper giving Rhoads power over how Presbyterian's grant was to be spent. And that wasn't going to include further research towards an infectious cause for cancer”-Livingston [52].
Still Rhoads was not finished. Livingston, already world- recognized, took her cancer microbe and a guest named George Clark to Rome's Sixth International Congress for Microbiology, a trip paid for by her husband's firm as a consultant to British industry. In Rome, Livingston met Emmy Klieneberger-Nobel at the Lister institute. Klieneberger-Nobel [75] was a pioneer in uncovering bacteria without cell walls which led them to assume many forms. She called them 'L-forms' in deference to the Institute at which she worked. Her exploration also covered bacteria with cell-wall breeches. In either case, the resulting germs, later called 'cell-wall-deficient' by Lida Mattman [79], assumed many forms.
Livingston immediately saw Klieneberger's work as clearing a large part of the confusion over her many-formed cancer germ.
By the time Virginia Livingston returned to the States, the Rome conference had been highlighted by several news services. Beginning with the New York Times and The Washington Post, other papers quickly followed suite: the cancer germ had been found. Reaction quickly followed. At The New York Academy of Medicine, spokesman Iago Gladston, fresh from executive session, held his own sort of news conference: "This is an old story and it has not stood up under investigation. Microorganisms found in malignant tumors have been found to be secondary invaders and not the primary cause of malignancy.'-Livingston [52].
Livingston returned to Newark. Barely unpacked from Europe, Livingston's husband would now be hounded by the IRS regarding where they got the funds for the European trip. Someone had implied the money came from his wife's grants. This did not bear out and the couple demanded to know who had instigated the inquiry. "Someone high up in New York in cancer.” The IRS agent replied [52].
- Review Article
- Abstract
- Introduction and Background
- Aldred Scott Warthin
- An Inconvenient Truth
- Hodgkin's Cancer Comes Under Attack
- Dr. Virginia Livingston
- Momentum Builds
- Recognition
- Focus on Breast Cancer
- The Single Most Convincing Study of How Bacteria Causes Cancer
- The Politics of Cancer
- Parallels with Plant Cancer
- Seibert Rules Out Contaminants in the Cancer Germ
- Experimental Medicine for the Masses
- BCG
- Livingston's Legacy
- Conclusion
- Mycobacterium-Induced Malignant-like Multinucleated Giant Cells
- Cancer Observations in Old England
- Long Island Breast Cancer
- Long island New York, 1886
- The Malignant Threat of the Mycobacteria
- Gland Seeking Mycobacteria
- Viralizing Cancer
- Bacterial Phage Viruses
- References
Parallels with Plant Cancer
By 1925 Mayo's Charles Mayo became interested in Erwin Smith's discovery of cancer in plants, called crown gall. Livingston and Jackson, sensing a possible link between Smith's work and their own, went to the Bronx Botanical Garden to request cultures of Bacterium tumefaciens, the plant cancer germ he had discovered. No mere accident led Virginia Livingston towards Smith's work. Smith stained his plant cancer germ with Fuchsin, long used to spot tuberculosis. And Smith's bacteria, like Livingston's, had many shapes. He had stumbled across B. tumefaciens in 1904, when he received some New Jersey daisies with overgrowths superficially resembling olive tuberculosis, a known disease of plants, but which proved to be plant cancer.
Smith had long suspected a bacterial cause for human cancer and criticized pathologists for drawing: "Too sharp a demarcation between malignant tumors, on the one hand, where the cells of the animal or human host, acting under some unknown stimulus are responsible for the tumerous growth, and granulomata (benign tumors) on the other hand, such as tuberculosis and actinomycosis, where a visible microbe is responsible for the primary tumor, and the direct migration of this microbe for any secondary tumors that may appear." -Rogers [76].
Smith's conclusion: "At the bottom, I think the distinction between such a disease, for example, as tuberculosis or leprosy and malignant tumors is not as sharp as some histologists have been inclined to believe." -Rogers [76].
It could be said that at one time the entire medical and scientific community was set on fire by Erwin Frink Smith's discovery of the bacteria that caused plant cancer. Twice honorably mentioned in The Journal of the American Medical Association, their Editorial "Is Cancer of Infectious Nature?" mentioned how Smith's work made "a very strong case in favor of his view of the infectious cause of cancer in general.’’ (JAMA, 1912). Even James Ewing devoted a chapter to Smith's discovery in his authoritive Neoplastic Diseases [53].
- Review Article
- Abstract
- Introduction and Background
- Aldred Scott Warthin
- An Inconvenient Truth
- Hodgkin's Cancer Comes Under Attack
- Dr. Virginia Livingston
- Momentum Builds
- Recognition
- Focus on Breast Cancer
- The Single Most Convincing Study of How Bacteria Causes Cancer
- The Politics of Cancer
- Parallels with Plant Cancer
- Seibert Rules Out Contaminants in the Cancer Germ
- Experimental Medicine for the Masses
- BCG
- Livingston's Legacy
- Conclusion
- Mycobacterium-Induced Malignant-like Multinucleated Giant Cells
- Cancer Observations in Old England
- Long Island Breast Cancer
- Long island New York, 1886
- The Malignant Threat of the Mycobacteria
- Gland Seeking Mycobacteria
- Viralizing Cancer
- Bacterial Phage Viruses
- References
Seibert Rules Out Contaminants in the Cancer Germ
The only time that Dr. Florence Seibert [77], long part of established medicine, ran into resistance and suppression, was when she decided to have a closer look at Livingston's cancer germ. One of America's finest PhD/Biochemist's, while still at Yale she resolved the mystery of the many fevers coming from distilled water for injection and thought to be caused by fever-producing 'pyrogens', quickly proving that these were in fact bacterial contaminants. Having solved the mystery of pyrogens, Seibert was asked by Dr. Esmond Long to stay on at the University of Chicago to develop the Tuberculin skin test. Long suggested a European trip to learn techniques practiced on the continent [77]. At the Pasteur Institute of Paris, she exchanged ideas with Boquet, Calmette and Guerin: the three investigators who presented to the world the only recognized vaccine for tuberculosis, called BCG [77]. Seibert returned to the US and when Long left Chicago to head laboratory operations at the Henry Phipps Institute in Philadelphia, she accompanied him.
By 1903, Henry Phipps, wealthy partner of Andrew Carnegie, sought a charitable outlet for his wealth. He then joined Lawrence F. Flick, a doctor with a vision to open a center solely dedicated to the study, treatment and prevention of tuberculosis. Still working off grants from the National Tuberculosis Association, Seibert was asked at Phipps to continue her work for a skin test using Koch's original Old Tuberculin (OT). Seibert refined and purified the protein in her TB skin test. She named it PPD-S, both because it was a purified protein derivative and was intended to serve as a standard (S) for the US Government, which it eventually became.
Then, after 30 years in tuberculosis research, Seibert turned towards cancer. In 1948, Margaret Lewis of Philadelphia's Wistar Institute asked Seibert to do a nucleic acid analysis on Wistar rat tumor extracts, to which Seibert agreed. Next, Irene Diller [80], who networked extensively with Livingston, asked Seibert to look at her slides of the cancer microbe. Seibert relates what she saw: "I saw tiny, round, coccoid organisms, many of which were magenta in color. The slides had been stained with Ziehl-Neelsen reagent, which we regularly used to stain our tubercle bacilli red. When I learned that she had isolated them from a rat tumor and could do so regularly from tumors in general, as well as from the blood of leukemic patients, I asked, Could you find them in the rat sarcoma tumor I am studying?" -Seibert [79].
Diller agreed to try. Lewis allowed Seibert to forward the tissue sections. The results came back. The same cancer germ appeared. Seibert immediately saw the implications: "This looked terribly important to me, and I was thenceforth willing to do whatever I could to help in this promising field. We did help by studying the immunological relationship to our tubercle bacilli, as well as to the "atypical" bacteria closely related to our tubercle bacilli." -Seibert, [79].
Seibert was even more impressed with how Diller, following the footsteps of Livingston and Jackson, proved, thru Koch's postulates, that her germ was the cancer germ. "It is based on her (Diller's) work that I am willing to say I believe she has found the cause of cancer, which I think no one can refute, and this work should be welcomed and confirmed by other cancer researchers, and not be ignored, even in view of the great stir at present about viruses."-Seibert, [79].
Florence Seibert joined Livingston's crusade in earnest in the 1960s, turning her cancer organism over to Frank Dunbar, chief of laboratories at The Southwest Tuberculosis Hospital in Tampa. Dunbar's conclusion: the multi-formed germ did not belong to his groups of known "atypical" mycobacteria, even though it did have some of the properties of the mycobacteria [79].
- Review Article
- Abstract
- Introduction and Background
- Aldred Scott Warthin
- An Inconvenient Truth
- Hodgkin's Cancer Comes Under Attack
- Dr. Virginia Livingston
- Momentum Builds
- Recognition
- Focus on Breast Cancer
- The Single Most Convincing Study of How Bacteria Causes Cancer
- The Politics of Cancer
- Parallels with Plant Cancer
- Seibert Rules Out Contaminants in the Cancer Germ
- Experimental Medicine for the Masses
- BCG
- Livingston's Legacy
- Conclusion
- Mycobacterium-Induced Malignant-like Multinucleated Giant Cells
- Cancer Observations in Old England
- Long Island Breast Cancer
- Long island New York, 1886
- The Malignant Threat of the Mycobacteria
- Gland Seeking Mycobacteria
- Viralizing Cancer
- Bacterial Phage Viruses
- References
Experimental Medicine for the Masses
In The Cancer Microbe, Alan Cantwell acknowledged the invaluable help of four women who pioneered the early microbiology of cancer: Virginia Livingston, M.D.; Eleanor Alexander-Jackson, PhD; Florence Seibert PhD and Dr. Irene Diller [78].
Eventually Virginia Livingston gained university affiliations in San Diego working out of the University of San Diego with Dr Gerhard Wolter of nearby San Diego State. In 1970, Wolter and Livingston discovered actinomycin-like compounds produced by the cancer germ, one of which, Actinomycin D or Dactinomycin, despite its toxicity, was being used in cancer. In 1966, Charles Huggins of the University of Chicago went to Stockholm and received a Nobel Prize for determining the effects of sex hormones, particularly estrogen, on cancer that had spread. Following this, the practice of castrating cancer victims came into vogue. Consequently, someone came to the conclusion that if castration helped initially, any recurrence would better be treated by cutting out the adrenal glands, housed on top of each kidney. And since this never produced earth-shaking results, to further cut estrogen production, a new procedure was devised to cut through the nose and remove the pituitary – the master gland of the body, lodged near the brain. Virginia Livingston had established that abnormal hormonal stimulation was coming from the toxic materials and hormonal derangers manufactured by her germ. In response America was chopping out the glands of its cancer patients.
- Review Article
- Abstract
- Introduction and Background
- Aldred Scott Warthin
- An Inconvenient Truth
- Hodgkin's Cancer Comes Under Attack
- Dr. Virginia Livingston
- Momentum Builds
- Recognition
- Focus on Breast Cancer
- The Single Most Convincing Study of How Bacteria Causes Cancer
- The Politics of Cancer
- Parallels with Plant Cancer
- Seibert Rules Out Contaminants in the Cancer Germ
- Experimental Medicine for the Masses
- BCG
- Livingston's Legacy
- Conclusion
- Mycobacterium-Induced Malignant-like Multinucleated Giant Cells
- Cancer Observations in Old England
- Long Island Breast Cancer
- Long island New York, 1886
- The Malignant Threat of the Mycobacteria
- Gland Seeking Mycobacteria
- Viralizing Cancer
- Bacterial Phage Viruses
- References
BCG
"It seems to me that it is entirely rational to state that the reason the BCG vaccine is effective not only against tuberculosis, but leprosy as well as cancer is because of the fact that the cancer germ is closely related to the BCG since it is in the same family, the Actinomycetales." - Livingston, [52].
When Florence Seibert met Boquet, Calmette and Guerin in Paris to discuss their BCG, the only recognized vaccine for tuberculosis in the world, made from cow or bovine tuberculosis, none of them had any idea that it would one day be used against cancer. But in fact, currently, this dilute vaccination of Mycobacterium bovis or cow tuberculosis is the most effective treatment for transitional cell carcinoma, a cancer of the urinary bladder. Moreover, BCG is the most successful therapy of its kind, called 'immunotherapy' [80]. Within the circles of 'immunotherapy', it soon became fashionable to suppose that BCG or cow tuberculosis somehow 'bolstered' the immune system, but noted immunologist Steven Rosenberg held that the immune system was highly specific. One immune stimulant such as the BCG against tuberculosis should not stimulate a response from another immune stimulant to cancer, said Rosenberg [81]. Unless, of course, they were related diseases to begin with. The precise mechanism as seen by a 1993 University of Illinois study was that initially cancer cells seemed to eat (or phagocytize) and kill the Mycobacteria bovis in BCG. But then, suddenly, the cancer cells too died. Although investigators in the study admitted the relationship wasn't clear, a strong 'tumoricidal agent', inside the Mycobacteria was postulated [82]. Livingston felt that investigators were probably unwittingly looking at was common phenomena in nature known as 'lysogeny'. Lysogeny is what happens when one colony of similar bacteria kills another by hurling their viral phage weaponry towards it, without itself being harmed.
By the late 1970s Virginia Livingston could no longer ignore Chisato Maruyama of Japan and sent John Majnarich of Seattle's BioMed Laboratories to Japan to have a closer look. In 1935, Maruyama, of the Nippon Medical School began to develop a vaccination against tuberculosis which turned out to be good against cancer. The Maruyama vaccine was similar to BCG, but instead of using cow tuberculosis as its base, the Japanese version used human tuberculosis. Chisato Maruyama had long noted that patients with either Mycobacterium tuberculosis or leprosy seldom had cancer [83]. By the 1970s Maruyama's vaccine was proving quite successful in that he claimed that half of the 8,000 cancer patients he had treated had benefited [84].
- Review Article
- Abstract
- Introduction and Background
- Aldred Scott Warthin
- An Inconvenient Truth
- Hodgkin's Cancer Comes Under Attack
- Dr. Virginia Livingston
- Momentum Builds
- Recognition
- Focus on Breast Cancer
- The Single Most Convincing Study of How Bacteria Causes Cancer
- The Politics of Cancer
- Parallels with Plant Cancer
- Seibert Rules Out Contaminants in the Cancer Germ
- Experimental Medicine for the Masses
- BCG
- Livingston's Legacy
- Conclusion
- Mycobacterium-Induced Malignant-like Multinucleated Giant Cells
- Cancer Observations in Old England
- Long Island Breast Cancer
- Long island New York, 1886
- The Malignant Threat of the Mycobacteria
- Gland Seeking Mycobacteria
- Viralizing Cancer
- Bacterial Phage Viruses
- References
Livingston's Legacy
By the early 1970s Virginia Livingston, badly beaten by the medical establishment, was ready to launch a counterattack in the form of a study which showed that her cancer microbe secreted human choriogonadotropic hormone (HCG) -a growth hormone long associated with cancer. Initially, despite laboratory evidence to the contrary, her contention that a bacteria could produce a human hormone was not believed. But then reports from traditional bastions such as Allegheny General, Princeton and Rockefeller University confirmed her findings.
Livingston believed that this growth hormone, secreted by her cancer germ built up uncontrollably to stimulate tumor growth, turning normal cells into malignant ones when either the body's immune system was weak or essential nutrients were deficient. Dr. Hernan Acevedo of Allegheny, in fact, showed that all cancer cells had the hormone [85]. Livingston's discovery, a medical milestone, gave further impetus to a microbial theory of cancer with well over a century of research behind it. Yet despite this, the premise behind an infectious cause was stubbornly refused by orthodox medicine.
Virginia Livingston was past 80 when she died on June 30th, 1990. Just months before, a subpoena was issued to her prohibiting her vaccinations, made from the patient's own cancer germ (autogenous), with which she had had great success. Following this, her vaccine was stigmatized as an "unproven method” in the March-April 1990 issue of CA -The Journal of the American Cancer Society [86] with references to her mistaking several different types of bacteria, rare and common, for a unique microbe. This despite droves of research papers establishing mycobacteria as either coming before or coexisting with cancer. Ironically, Acevedo, who had lauded her discovery that the cancer germ could manufacture human growth hormone was key and instrumental to the Society's conclusion. Yet when questioned by this author approximately a decade later, Acevedo admitted that he had ignored acid-fast forms which were indeed present in the cancer preparations Livingston sent to him. He felt these irrelevant, and mentioned that besides, the technology was not available at the time to pursue these acid-fast forms further On such fuzzy logic, it seemed that perhaps the most important scientific cancer lead in this or any other century was buried.
- Review Article
- Abstract
- Introduction and Background
- Aldred Scott Warthin
- An Inconvenient Truth
- Hodgkin's Cancer Comes Under Attack
- Dr. Virginia Livingston
- Momentum Builds
- Recognition
- Focus on Breast Cancer
- The Single Most Convincing Study of How Bacteria Causes Cancer
- The Politics of Cancer
- Parallels with Plant Cancer
- Seibert Rules Out Contaminants in the Cancer Germ
- Experimental Medicine for the Masses
- BCG
- Livingston's Legacy
- Conclusion
- Mycobacterium-Induced Malignant-like Multinucleated Giant Cells
- Cancer Observations in Old England
- Long Island Breast Cancer
- Long island New York, 1886
- The Malignant Threat of the Mycobacteria
- Gland Seeking Mycobacteria
- Viralizing Cancer
- Bacterial Phage Viruses
- References
Conclusion
By the twentieth century, polymorphic, partially acid- fast (AF), cell-wall-deficient (CWD), filter passing forms of tuberculosis were being isolated from tumor homogenates and the blood of leukemic patients [87-94]. And research in the last two decades did nothing but confirm this. Repeatedly, recent studies have shown atypical tubercular cell-wall- deficient forms (also known as "L-forms”), and tubercular DNA integrated into the genome of malignant tissue [95-102]. This is not a coincidence. L-forms (CWD forms) predominate and are crucial to the survival of mycobacteria in vivo and they have been documented by fluorescence microscopy in all intracellular macrophage-grown M. tuberculosis observed [4].
Recently Yang and others (2013), using intensified Kinyoun acid-fast staining and in situ hybridization techniques for the detection of MPB64 gene expression in the nucleus of breast cancer cells, concluded that cell-wall-deficient (CWD) or "L-forms” of Mycobacterium tuberculosis exist in breast cancer tissue, and that the MPB64 tubercular gene was highly expressed in the nucleus of breast cancer cells [6].
The extent to which tuberculosis of the breast can masquerade as carcinoma was amply demonstrated by Shinde's study, in which 100 patients clinically diagnosed as having breast malignancy were found to have tuberculous mastitis instead [103] . A lump in the breast with or without ulceration was the commonest presentation, the others being diffuse nodularity and multiple sinuses. Breast TB can mimic breast carcinoma clinically and radiologically. Similar to breast cancer, concomitant axillary lymph nodes are found in one-third of the patients with breast TB. Similar to breast cancer the upper outer quadrant of the breast is commonly affected, and also similar to breast cancer, breast TB can show multinucleate giant cells easily mistaken for highly miotic breast malignancies. At every twist and turn the two conditions mimic one another. BRCA Gene Mutation Testing, also known as BRCA or Breast Cancer Susceptibility Genes 1 and 2. Standard BRCA1 and BRCA2 breast cancer susceptibility gene tests are used to detect mutations that are known to increase the risk of breast and ovarian cancer development. The presence of a BRCA1 or BRCA2 mutation means that the person tested is at an increased risk for developing hereditary breast and/or ovarian cancer syndrome. However "hereditary” can also connote a chronic perinatal infectious process and BRCA1 was also found in the fibroblasts of 41 out of 48 cases of pulmonary tuberculosis as well -yet altogether absent in lung inflammation without TB[104] .
The lump is the most common presentation in breast tuberculosis. These breast lumps are mostly misdiagnosed as fibroadenoma, fibroadenosis, malignancy or breast abscess. In Puneet's series, 12 patients with breast TB were clinically misdiagnosed as fibroadenoma, 17 as fibroadenosis and 8 as carcinoma [10].
Although TB of the Breast is said to be "rare” or "very rare”, the difficulty in differentiating culture negative tuberculosis from other causes of chronic granulomatous mastitis is common as is the difficulty in finding tubercular microorganisms altogether in this paucibacillary disease. Certainly Puneet did not find the condition rare in his study, and there are other investigators that report breast tuberculosis as common, depending upon where the study is done [105]. As Warthin [16] made clear, just because tuberculous mastitis, especially cell-wall-deficient tuberculous mastitis is rarely reported, or for that matter grossly under reported - does not mean that this is not because of the clinical and laboratory difficulties involved in its diagnosis.
- Review Article
- Abstract
- Introduction and Background
- Aldred Scott Warthin
- An Inconvenient Truth
- Hodgkin's Cancer Comes Under Attack
- Dr. Virginia Livingston
- Momentum Builds
- Recognition
- Focus on Breast Cancer
- The Single Most Convincing Study of How Bacteria Causes Cancer
- The Politics of Cancer
- Parallels with Plant Cancer
- Seibert Rules Out Contaminants in the Cancer Germ
- Experimental Medicine for the Masses
- BCG
- Livingston's Legacy
- Conclusion
- Mycobacterium-Induced Malignant-like Multinucleated Giant Cells
- Cancer Observations in Old England
- Long Island Breast Cancer
- Long island New York, 1886
- The Malignant Threat of the Mycobacteria
- Gland Seeking Mycobacteria
- Viralizing Cancer
- Bacterial Phage Viruses
- References
Mycobacterium-Induced Malignant-like Multinucleated Giant Cells
Rosen [17] was never able to settle with certainty whether the multinucleated giant cells (MGCs) that he found in both benign and malignant breast disease were instrumental in carcinogenesis, but he certainly was considering this carefully The female patients with no demonstrable breast cancer that had MGCs within benign masses were of the same premenopausal to postmenopausal age range as those with carcinoma and MGCs –with a tendency to cluster in the 40 to 50-year age group.
Such multinucleated giant cells (MGCs), which can simulate malignancies closely are known to be generated from mycobacterial-induced granulomas -both from human Mycobacterium tuberculosis and fowl-derived Mycobacterium avium, but in different ways. The highly virulent M. tuberculosis induces large multinucleated giant cells with greater than 15 nuclei per cell, whereas the lower virulence M. avium generally have less than 7 nuclei per multinucleated giant cell. In addition it is highly virulent TB that results in granulomas in which it's MGCs lose their phagocytic capacity entirely; while in the case of M. avium, its fewer nuclei per cell still retain phagocytic activity. So it becomes a case of the more virulent the organism, the larger the number of it’s MGCs and the less ability of such MGCs to mediate any new mycobacterial uptake through phagocytosis [106]. Therefore, in M. TB -induced granulomas, MGCs seemed to have all that they can handle in just further attempting to destroy those bacilli that had already been previously ingested by other macrophages and mononuclear cells.
As it turns out, Paul Peter Rosen's quest for a hormonal common denominator for the appearance of the bizarre mitotic forms of multinucleated giant cells in both malignant and non-malignant breast disease (during the premenopausal to postmenopausal age range) has a basis in mycobacterial disease that he probably was not even aware of. Although with human tuberculosis the progression-to-disease and mortality rates are higher in females during their reproductive years, after this period such rates turn again to be higher in men [107,108]. However, this is not true for Mycobacterium avium (fowl tuberculosis, also called MAC or Mycobacterium avium complex) which is now appreciated to be a significant clinical global problem characterized by among other features, nodules on a CT scan. Furthermore, Avium's great preponderance is in postmenopausal women and as much as 80% to 90% of the cases occur in women, most of whom are older. Menopause normally occurs between the ages of 42 and 60 years, accompanied by the decline in the production of estrogen and progesterone. This suggests the involvement of sex hormone fluctuations in the establishment of this type of MAC disease -a relationship which Tsuyuguchi et al. [109] proved in 2001. As estrogen levels dipped, fowl tuberculosis grew.
The incidence of Mycobacterium avium complex (MAC) disease is steadily increasing globally [110,111]. In addition to disseminated cases, which often occur in AIDS patients, pulmonary disease in patients without obvious predisposing conditions is becoming an important clinical problem. This type of disease is characterized by nodules and/or bronchiectasis on CT scans, and is also characterized by its preponderance in postmenopausal women [112,113].
The incidence of breast cancer rises after 40, but the highest incidence (approximately 80% of invasive cases) also occurs in women over the age of 50, an age pattern not unlike what Rosen [17] detected in finding MGCs in benign as well as malignant breast tissue [114]. Moreover, coincidental or not, there is an increasing rate of NTM (Non-Tubercular Mycobacterial) disease, mostly from Mycobacterium avium complex (MAC) infection in Caucasian women with a current diagnosis or history of breast cancer [115].
NTM infections such as fowl tuberculosis (M. avium) are acquired directly from the environment, where they are often present in soil and various water sources [116]. Fecal contamination of the environment by infected birds or fowl shedders is the major means of mycobacterial and Mycobacterium avium disease dissemination [117,118]. Water obtained from municipal treatment facilities, hospitals, and homes grew NTM such as Avium in 10%-95% of samples in European and American surveys [119].
- Review Article
- Abstract
- Introduction and Background
- Aldred Scott Warthin
- An Inconvenient Truth
- Hodgkin's Cancer Comes Under Attack
- Dr. Virginia Livingston
- Momentum Builds
- Recognition
- Focus on Breast Cancer
- The Single Most Convincing Study of How Bacteria Causes Cancer
- The Politics of Cancer
- Parallels with Plant Cancer
- Seibert Rules Out Contaminants in the Cancer Germ
- Experimental Medicine for the Masses
- BCG
- Livingston's Legacy
- Conclusion
- Mycobacterium-Induced Malignant-like Multinucleated Giant Cells
- Cancer Observations in Old England
- Long Island Breast Cancer
- Long island New York, 1886
- The Malignant Threat of the Mycobacteria
- Gland Seeking Mycobacteria
- Viralizing Cancer
- Bacterial Phage Viruses
- References
Cancer Observations from Old England
By the turn of the 20th century, various UK investigators began appearing in the British Medical Journal tracing cancer to polluted soil and waters. E. N. Nason, through a detailed study of cancer incidence in the Nuneaton, a town in Warwickshire, and Stratford-on-Avon districts, concluded that cancer cases tended to group themselves chiefly in the vicinity of sewage polluted areas, either drained into nearby bodies of water such as sluggish streams, bays, estuaries -or water drained off through contaminated subsoil [120].
For nineteen years Lloyd Jones [121] worked on the local distribution of malignant disease in Cambridge and nearby vicinities. Jones couldn't help but notice that some parts of the towns under his investigation were comparatively free from cancer, while other parts, often adjoining suffered severely. Also it did not escape his attentions that elevated sites were less liable than low-lying ones and that areas of chalk subsoil were less liable as opposed to that subsoil contaminated with waste. In fact it was decaying vegetation, filth and collections of manure that were often found in cancer districts. In like manner, Mason examined the problem of cancer distribution for the Lamington district of Warwick [122]. Harold Mason [122] also believed that whatever the ultimate cause of cancer might be, that it would be found to be associated with sewage-contaminated subsoil, and that it was the end houses of houses in a row, and corner houses of streets that were often "cancer houses” owing to drain leakages with subsequent sewage contamination being most frequent at those spots.
Below is a map for Female Breast Cancer U.S. Mortality Rates for 1970-1994. Its distribution seems a bit strange. Without question, the highest death rates were in the Northeast and a small portion of the Midwest. What could have happened that was responsible for this? (Figure 2).
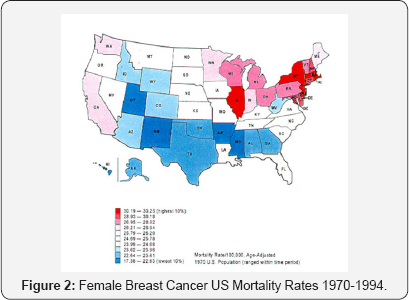
- Review Article
- Abstract
- Introduction and Background
- Aldred Scott Warthin
- An Inconvenient Truth
- Hodgkin's Cancer Comes Under Attack
- Dr. Virginia Livingston
- Momentum Builds
- Recognition
- Focus on Breast Cancer
- The Single Most Convincing Study of How Bacteria Causes Cancer
- The Politics of Cancer
- Parallels with Plant Cancer
- Seibert Rules Out Contaminants in the Cancer Germ
- Experimental Medicine for the Masses
- BCG
- Livingston's Legacy
- Conclusion
- Mycobacterium-Induced Malignant-like Multinucleated Giant Cells
- Cancer Observations in Old England
- Long Island Breast Cancer
- Long island New York, 1886
- The Malignant Threat of the Mycobacteria
- Gland Seeking Mycobacteria
- Viralizing Cancer
- Bacterial Phage Viruses
- References
Long Island Breast Cancer
In the early nineteenth century in the United States, cancer of the breast was a rare disease. Yet as this disease took off, and for some time thereafter it was well known that breast cancer mortality was higher in the northeastern part of the United States compared with the rest of the country [123]. Within this concentration there was a statistically significant and geographically broad cluster of breast cancer deaths in the New York City -Philadelphia, Pennsylvania, metropolitan area, which had a 7.4% higher mortality rate than the rest of the Northeast [124]. Yet within that cluster, the high mortality rates from breast cancer on Long Island could not be ignored [125-127]. Even when compared to New York State as a whole, the cancer rates on Long Island seemed elevated [128] (Figure 3).
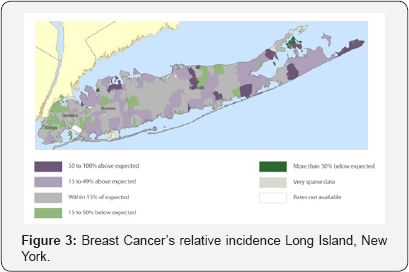
Many were convinced environmental factors were at work on Long Island, and through Senators Alfonse D'Amato of New York and Tom Harkin of Iowa, advocates secured nearly $30 million in funding for a massive, long-term study of Long Island breast cancer cases. For nearly a decade, researchers studied thousands of Long Island women, looking for causes which ranged from exposure to dangerous chemical pollutants to electromagnetic fields. The result, a massive library of data gathered from over ten studies, known as the Long Island Breast Cancer Study Project (LIBCSP) [129]. At one point, follow-up studies pointed to such possible links as that between diabetes and higher risk of death from breast cancer, but no one at any time seemed to have considered the massive amounts of duck and fowl excrement with its attendant Mycobacterium avium complex (MAC) that had found its ways into Long Island and its waterways since the early 1900s.
The first probable description of MAC came with the finding of “tuberculosis” in chickens (avian) that mimicked disease seen in humans, described in England in 1868. By 1890, it was recognized that this avian bacteria (now known as Mycobacterium avium) was distinct in the laboratory from the human variety of M. tuberculosis. Human disease due to MAC was not recognized until almost a half century later. In 1933, human- derived disease causing (pathogenic) strains were reported. Later studies revealed the organisms to be M. avium complex. In 1943, one of the first human cases of MAC was described when a mycobacterial species later identified as Mycobacterium avium was recovered from the sputum of a patient suffering from chronic lung disease with an associated underlying lung illness called silicosis (related to silica inhalation). By 1953 (10 years later), more cases of M. avium like organisms in humans were described by other investigators along with four cases that later were identified as M. intracellulare. At that time, these and other scientists thought that these organisms probably had little or no ability to cause disease (virulence) and that these strains were actually avian tubercle organisms that had lost their ability to cause disease in chickens.
- Review Article
- Abstract
- Introduction and Background
- Aldred Scott Warthin
- An Inconvenient Truth
- Hodgkin's Cancer Comes Under Attack
- Dr. Virginia Livingston
- Momentum Builds
- Recognition
- Focus on Breast Cancer
- The Single Most Convincing Study of How Bacteria Causes Cancer
- The Politics of Cancer
- Parallels with Plant Cancer
- Seibert Rules Out Contaminants in the Cancer Germ
- Experimental Medicine for the Masses
- BCG
- Livingston's Legacy
- Conclusion
- Mycobacterium-Induced Malignant-like Multinucleated Giant Cells
- Cancer Observations in Old England
- Long Island Breast Cancer
- Long island New York, 1886
- The Malignant Threat of the Mycobacteria
- Gland Seeking Mycobacteria
- Viralizing Cancer
- Bacterial Phage Viruses
- References
Long island New York, 1886
Long Island is about one hundred and twenty miles in length from which it draws its name. It also ranges from fifteen to thirty miles in width. One end is within half a mile of New York City and the other end stretches northeast, directly south of the western line of Rhode Island. The Long Island Sound is north of the island and the open Atlantic is to the south.

The shoreline of Long Island, north and south, are indented with many bays, coves, small rivers and creeks. And by 1900, a large number of wealthy New Yorkers built summer homes on the island. This led to a ritual that continues to this day wherein each season thousands of city dwellers spend a few weeks to several months at Long Island seaside resorts. Some became famous, as when Oyster Bay, twenty miles from New York City on the north shore became President Teddy Roosevelt's summer home.
The raising of ducks did not become a full-time industry on Long Island until sometime between 1880 and 1885; prior to that time the raising of ducks was a supplemental activity to farming and fishing. What we know is that growing Pekin Ducks on Long Island began by about 1877, approximately at the same time as James Rankin, known as the father of the Pekin Duck industry in America began operations in a small way in South Easton, Massachusetts. Other historians insist that Pekin Ducks were first imported in 1873 by James Palmer of Stonington, Connecticut, who also made the trip to China and returned with ducks. At any rate the duck business grew steadily on Long Island until by 1907 there were between 35 and 40 duck farms and ranches on the island with an annual output in excess of three hundred and fifty ducklings, more than ninety per cent of which were marketed in New York City(Figure 4). The largest of these was owned by AJ Hallock, owner of the Atlantic Duck Farm in Speonk, Long Island. Other Eastern States had large duck plants, notably Pennsylvania (Philadelphia area) and Massachusetts, but Long Island produced more than any area in the world. Eventually however, as a result of the continuing costs of upgrading their treatment plants to comply with the ever more stringent pollution control requirements, many of the duck farmers folded or relocated to the Midwest, -Wisconsin, Indiana, and Illinois, where costs were cheaper and environmental regulations less rigorous (Figure 2). In addition Illinois was the leading state for chicken “broilers” before that industry moved south and east to Kentucky and Arkansas because of stricter environmental laws.

The American Pekin duck, Pekin duck, or Long Island duck is a breed of domestic duck used primarily for egg and meat production. It was bred from the mallard in China. The ancestors of those ducks originated from the canals which linked waterways in Nanjing, the capital of Jiangsu Province and originally had small bodies and black feathers. Over time, the ducks slowly increased in size and grew white feathers. By the Five Dynasties, the new breed of duck had been domesticated by Chinese farmers [130]. The Pekin duck is the most popular commercial duck breed in the United States. Nanjing is the capital city of Jiangsu Province (Figure 5).
But Jiangsu province itself has one of the highest mortality rates for breast cancer in China [131]. The annual mortality rate per 100,000 people from breast cancer in Jiangsu has increased by 30.7% since 1990, an average of 1.3% a year.
During the peak production years of the Long Island duck industry, which spanned the 1940s, 1950s and early 1960s, duck farms could be found on almost all the freshwater streams in the Riverhead, Eastport and Moriches areas. By the end of the 1930s, about six million ducks were produced on approximately 90 farms located in Suffolk County and towards the late 1940s and early 1950s, the approximately 70 duck farms located in Suffolk County produced about two-thirds of all the ducks eaten in the United States. But this entire peak of duck farming on Long Island predated the laws and regulations that were meant to control runoff and pollution, and much of the effluent ran untreated into streams that then discharged into Long Island's bays.
To better characterize the impact the duck farm industry has had on regional ecology, the amount of effluent produced by duck farms was computed [132]. The pollution load from the 34 duck farms operating in Suffolk County during 1968, which raised about 7 million ducks that year, included [133] 70 tons total solid excremental waste/day (43 tons suspended solids/ day).
And this is not even to mention the waste from other poultry By 1998, Cornell undertook a survey of NYS poultry producers. New York State poultry producers for the most part housed their flocks in high-rise caged-layer houses with concrete floors. The tractor was the primary method of manure removal from such poultry facilities. Manure removal was done semi-annually or annually [134]. Fecal bacteria enter surface water by direct deposit of feces and by overland runoff. The movement of animal wastes into surface waters can be a major factor contributing to the pollution of available water in a region. Further aggravating this, many farmers and poultry interests use cellars, tanks or landfills to store manure. Water leaching from these storage sites may also contaminate groundwater, especially during periods of rainfall. The application of animal manure to agricultural lands as fertilizer is common practice throughout the world. Bacteria present in the manure may leach into the groundwater
In general terms, the greatest microbial risks are associated with ingestion of water that is contaminated with human or animal feces. Wastewater discharges in fresh waters and costal seawaters are the major source of fecal microorganisms, pathogens included [135-137]. Not that the EPA (Environmental Protection Agency) had, for some time been aware of this serious problem, but simply not to the extent that it should have been.
Although Mycobacterium avium is an important pathogenic and contagious bacterium in most birds and fowl [138], its residence in domestic Pekin ducks was only recently (in September 2016) shown, and then only with the help of PacBio single-molecule real-time technology, which yielded extremely drug resistant, virulent strains of M. avium [139].
As early as 2003, the EPA was on to the importance of the threat and dangers of having fowl tuberculosis (Mycobacterium avium complex or MAC) harbored in drinking water. But their actual assessment of just how dangerous the organism was, even in immunocompetent individuals, was flawed. And as a result MAC in drinking water is still basically being ignored -while a host of other "carcinogens” and other less-dangerous bacteria are circled in on.
By 2003 the EPA said this: "After evaluating the available information, the infection risk from MAC-contaminated tap water appears to be limited to a few populations including people with AIDS, transplant recipients and others patients receiving immunosuppressive therapies, individuals with compromised pulmonary systems, and children" [140].
What is wrong with this statement? First of all, at the time of this 2003 statement there were over 73 million "children" below the age of 18 in the United States [141]. This was in itself 25% of the entire US population at the time, and certainly not being portrayed properly in the context of MAC-contaminated tap water's infectious risk as being "limited to a few populations".
Second, for quite some time it had been obvious that fowl tuberculosis (MAC) did not need AIDS immunosuppression or immunosuppressive therapies to be dangerous. Depite Rosenzweig and Wolinsky's [142,143] back-to-back studies that found that with MAC one needs not be immunosuppressed (nearly one-third of Rosenzweig's 100-patient-series were entirely free from coexisting disease), the mantra has persisted that MAC needs some special prior immunosuppressive disease to infect humans. This, again was inaccurate as merely the immunosuppression from a childhood infection with tuberculosis, which is not uncommon, will provide more than enough immunosuppression to later on acquire a non-tubercular infection from the Mycobacterium avium complex [142-144]. Human tuberculosis itself, often acquired very early in life is even more immunosuppressive than the AIDS virus and could easily create "compromised pulmonary systems" on a fairly regular basis. Although previously demonstrated [145,146], the actual ferocity of CD4 immune cells from tubercular attack was amply shown in papers such as Hirsch's [147] 1999 Ohio study, which showed that not only 30% of CD4 but also non-CD4 immune cells were slaughtered within 98 hours of co-culture with TB, a 20-fold increase. Hirsch's expose was published by the Journal of Infectious Diseases. But it didn't stop there. The immune system's B-cells and macrophages were also decimated by tuberculosis [148-151]. And in a follow-up study, Hirsch found that destruction through apoptosis of immune cells was increased at the site of active MTB infection in patients with pleural TB, regardless of whether the patient had HIV or did not have HIV. This included macrophages [152]. And it was the annihilation of just such infection-swallowing macrophages, critical to reticuloendothelial immune ultrastructure, that M. tuberculosis joined later by M. avium, can work in concert to further devastate the human immune system [153].
Nevertheless, and to this day, there is no treatment technique listed in the EPA's drinking water standards and health advisories for the Mycobacterium avium complex [154]. Mycobacteria such as Mycobacterium avium are resistant to both chemical and ultraviolet disinfection [155]. They are 700 to 3000 times as resistant to chlorine as Escherichia coli and 50 times as resistant to ozone. Among waterborne pathogens, the dose of ultraviolet C (UVC) needed to inactivate mycobacteria was much higher than the levels required for all other pathogenic bacteria studied.
- Review Article
- Abstract
- Introduction and Background
- Aldred Scott Warthin
- An Inconvenient Truth
- Hodgkin's Cancer Comes Under Attack
- Dr. Virginia Livingston
- Momentum Builds
- Recognition
- Focus on Breast Cancer
- The Single Most Convincing Study of How Bacteria Causes Cancer
- The Politics of Cancer
- Parallels with Plant Cancer
- Seibert Rules Out Contaminants in the Cancer Germ
- Experimental Medicine for the Masses
- BCG
- Livingston's Legacy
- Conclusion
- Mycobacterium-Induced Malignant-like Multinucleated Giant Cells
- Cancer Observations in Old England
- Long Island Breast Cancer
- Long island New York, 1886
- The Malignant Threat of the Mycobacteria
- Gland Seeking Mycobacteria
- Viralizing Cancer
- Bacterial Phage Viruses
- References
The Malignant Threat of the Mycobacteria
Although association of chronic inflammation and cancer has been well-documented, causal relationship between tubercular infection and cancers, including lung cancer, are not understood. Yet by 2009 Nalbandian et al. [157] had already documented experimental evidence showing a causal relation between chronic tubercular infection and the formation of squamous cell dysplasia leading towards squamous cell carcinoma (SCC) in the lung. And when such squamous cell aggregates appeared in TB infected lung tissue, this tissue was transplantable -causing malignant tumors in other laboratory animals. Microscopically, it was evident that TB-infected macrophages played a pivotal role in causing cancer through local DNA damage, the production of a potent epidermal growth factor, and the massive local production of reactive species -all felt responsible for both the squamous metaplasia and tumor formation that followed. The more severe the prior tubercular-mediated tissue damage, the more pronounced the carcinogenic changes. Together these findings showed a causal link not only between pulmonary tuberculosis and lung cancer, but established a genetic model for the mechanism activated by TB in cancers elsewhere [157].
It has not been emphasized enough how destructive mycobacteria such as tuberculosis can be with regard to DNA damage and its interference with normal cellular mitosis, leading towards premalignant change. In a recent study comparing DNA damage and cellular abnormalities in tuberculosis, lung cancer and chronic obstructive pulmonary disease (COPD), it was the TB group that showed the highest frequency of DNA damage, defect in cytokinesis, apoptotic and necrotic cells [158]. Many of these changes could in turn cause pre-malignancy leading towards malignancy. TB had already shown how damaging it could be to the chromosomal apparatus of cell cultures of human tissue. In such studies, an increase in pathological mitoses, arrest of cell division in metaphase, and the actual appearance of chromosomal adhesions absent in control cultures appeared. Indeed, early tubercular involvement was not only destructive against chromosomes but the very spindles that separated them [19-21].
By 2012 Daley & Iseman [159], in response to a study done by Lande [160], asked regarding fowl tuberculosis and lung cancer: "Mycobacterium Avium Complex and Lung Cancer -Chicken or Egg? Both?" Lande had reported unexpectedly high rates of lung cancer in individuals with prior or concurrent Mycobacterium avium Complex (MAC), even in non-smoking women. And by 2014 Hosoda et al. [161] added that in their study lung cancer "was frequent among patients with pulmonary MAC infections", with both diseases tending to be in their early stage
For some time, even in the case of human tuberculosis, the lines between TB and cancer had been blurring. In the prechemotherapy era before 1950, clinicians felt that because of the high early adulthood mortality of tuberculosis and the fact that most lung cancer occurred after 50 -that lung cancer was uncommon among those with TB. But with the advent of curative TB therapy, lung cancer began appearing among TB survivors. At this point it was maintained that tuberculosis was causing an inflammatory dysplasia. In dysplasia, the cells look abnormal under a microscope but are not cancer. Yet subsequently, dysplasia may or may not become cancer, although such tubercular inflammatory dysplasias seemed to be moving in a cancerous direction.
But unlike untreated TB, fowl derived MAC is unlikely to cause rapidly progressive disease and premature death and is rather a chronic disease extending over decades -a scenario which fits the mold in studies like Falagas's extended time-frame from the onset of TB to the onset of cancer. One of the striking oddities regarding MAC lung disease over the past few decades is its increasing prevalence rates among women [162]. But along with this has been demonstrated an association between such MAC-positive bronchial washings and the presence of lung cancer [115].
Yet the struggle to prove typical and atypical mycobacterial/ tubercular forms as causative for cancer has been a long one. By 1952 Bender [163] pondered this problem as best he could with the available technology of his time. With certainty it could be stated that primary pulmonary carcinoma which was considered a rarity two decades ago, in the early 1930s had become by the time of Bender's publication one of the most frequent and important primary malignant lesions the incidence of which was undoubtedly increasing. He knew through other studies that the incidence of the association of carcinoma of the lung with pulmonary tuberculosis was approximately eight to 10 percent [164] (Figure 6).
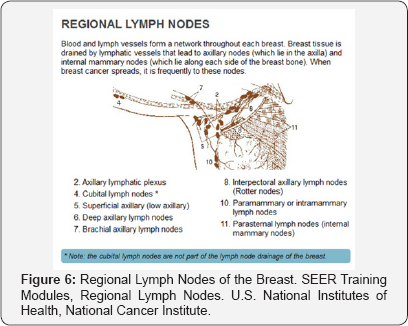
Some of Bender's contemporaries believed that tuberculosis played no significant role in the production of pulmonary carcinoma. Others said that although the association of active pulmonary tuberculosis and carcinoma of the lung might only be coincidental, that the same did not hold with regard to “healed” tuberculosis. The reason for this was that several cases of carcinoma of the lung arising in tuberculous cavities or scar tissue or calcified foci in the lung or bronchial lymph nodes had already been reported. Therefore it was believed by practically all that a calcified focus or healed tuberculous scar might mark an area of the lung particularly susceptible to the development of carcinoma. At that point, the principal factor responsible for the cancer was said, again, to be metaplasia of the bronchial mucosa as a result of chronic irritation [165].
- Review Article
- Abstract
- Introduction and Background
- Aldred Scott Warthin
- An Inconvenient Truth
- Hodgkin's Cancer Comes Under Attack
- Dr. Virginia Livingston
- Momentum Builds
- Recognition
- Focus on Breast Cancer
- The Single Most Convincing Study of How Bacteria Causes Cancer
- The Politics of Cancer
- Parallels with Plant Cancer
- Seibert Rules Out Contaminants in the Cancer Germ
- Experimental Medicine for the Masses
- BCG
- Livingston's Legacy
- Conclusion
- Mycobacterium-Induced Malignant-like Multinucleated Giant Cells
- Cancer Observations in Old England
- Long Island Breast Cancer
- Long island New York, 1886
- The Malignant Threat of the Mycobacteria
- Gland Seeking Mycobacteria
- Viralizing Cancer
- Bacterial Phage Viruses
- References
Gland Seeking Mycobacteria
The breasts are essentially milk-producing, tear shaped glands. 90% of breast cancer are adenocarcinomas, which also arise from glandular tissue. Furthermore the upper, outer quadrant of the breast contains a large amount of glandular tissue and is the site of 60 percent of carcinomas of the breast, as well as being a frequent site of TB of the breast. Both the clinical and radiological features of tuberculous mastitis are not diagnostic and easily can be confused with either breast cancer or pyogenic breast abscess by clinicians.
That the mycobacteria in general, and human and fowl tuberculosis in particular, seek out the lymphatics and lymph nodes (lymph glands) in our body has been long known, and certainly in most studies lymph nodes are far and away the most common site of extra-pulmonary tuberculosis (EPTB) [167-170]. In 1829, Astley Cooper [171] spoke of Breast TB as “The Scrofulous Swelling of the Breast”, exactly because he occasionally saw a breast lump or breast lumps form in young woman with enlarged or inflamed lymph nodes in their neck from TB or M. avium infection [called Scrofula].
Such tuberculous lymphadenitis is the most common form of tuberculosis outside the chest cavity. Cervical lymphadenitis due to mycobacteria in adults (scrofula) is caused by M. tuberculosis in about 90% of adult patients and non-tuberculous mycobacteria in about 10% of patients (the reverse is true in children; see below). Cervical lymphadenitis is the usual expression of non-tuberculous mycobacterial infection in children, who, in contrast to adults, seldom develop pulmonary infection from these microorganisms. In children M. avium is by far the most common isolate (80% of cases) in neck nodes, followed by M. scrofulaceum.
Tuberculosis, the sovereign disease of the 19th century, was the leading cause of death, as feared as it was widespread. In Europe, the work of the German physician Rudolph Virchow (1821-1902), the father of “cellular pathology,” advanced medical knowledge. His contributions were substantial; for example, he identified leukemia in 1845. Virchow proclaimed the tissue changes characteristic of tuberculosis as emblematic for disease processes in general, and cancer in particular. According to Virchow, malignancy, like the tubercle bacillus, traveled through the lymph channels and proceeded in an orderly, mechanical fashion from the local site as in the breast -progressing to the glands under the arms, and from there migrating to distant sites. A tacit reverence for and acceptance of Virchow's theory that the lymph is the highway of the cancerous process persists today, though we know that metastases requires blood supply (angiogenesis), and also travel through the circulatory system to distant (metastatic) sites as well.
Elise L'Esperance [54] discovered in her animal trials on Hodgkin's disease that M. avium in particular, has a tendency to seek out glandular tissue inside the body [57]. In her guinea pig experiments, although these animals had a natural resistance to avian infection demonstrated by the six to eighteen months before generalization of the disease -the lesions that developed differed from those seen in animals inoculated with human tuberculosis in that they seemed to have a predilection for the lymphatic system, shown by the extensive involvement of all nodes; cervical, axillary, mediastinal and bronchial (Figure 6) –with less significant although sometimes extensive lesions of the liver, lungs and spleen. This was similar to malignant metastases. Although L'Esperance [54] had fowl tuberculosis (M. Avium) in her sights as being responsible for Hodgkin's disease because TB could not always be isolated from Hodgkin's tissue, it soon became obvious that previous lymph node human TB could also be linked to Hodgkin's, although again not always immediately after Hodgkin's disease appeared. Recent publications from Poland and Germany confirmed that TB bacilli could not always be visualized, complicated by the fact that many of the anticancer drugs to which the ensuing Hodgkin's actually responded also have impressive activity against tuberculosis itself. Another confounding finding was that sometimes just acid-fast bacilli were found, leaving the question open as to whether these bacilli were from fowl or human tuberculosis. Finally, none of these studies addressed the far more prevalent tubercular "L-form" or "Cell-Wall-Deficient" forms which would have required special stains, cultures and assays [172,173].
In general lymphadenopathy of neck nodes from either TB or Avium is a mystery. But what we know is the causative organisms usually differ in adults and children. Disseminated infection occurs with Mycobacterium tuberculosis (a pathogenic bacterium) in adults and nontuberculous mycobacterium, which includes mainly Mycobacterium avium occurs commonly in children. The rate of mycobacterial infections from atypical tuberculosis such as from birds or fowl in immune competent individuals has been noticed to be increasing independently for some time now [174]. Indeed, M. avium is a common cause of chronic cervical lymphadenopathy, or enlarged lymph nodes of the neck, in children –the great majority of which are supposedly not immunologically impaired. The epidemiological evidence suggests that these infections are acquired from environmental sources, including soil, water, dust, and aerosols [175]. The predilection for younger children is probably related to their tendency to put objects contaminated with soil, dust or standing water in their mouths.
The greatest difficulty usually is in distinguishing between adenitis caused by atypical mycobacteria and adenitis due to M. tuberculosis. Cultures for atypical mycobacteria may be negative even when infection is present. Only about 50% of excised diseased lymph nodes will be culture positive [176]. And with a mean time of about eight weeks for culture and twelve weeks for sensitivity results, initial diagnosis depends greatly on the clinical features [177].
Regarding fowl tuberculosis there can be no longer be any doubt that poultry workers are subject to increased risks of deaths from liver, pancreatic and other cancers. Some researchers hypothesized that this was possibly due to exposure to oncogenic poultry "viruses". It was not the first, nor would it be the last time that "viruses" would be blamed on cancer [178,179].
- Review Article
- Abstract
- Introduction and Background
- Aldred Scott Warthin
- An Inconvenient Truth
- Hodgkin's Cancer Comes Under Attack
- Dr. Virginia Livingston
- Momentum Builds
- Recognition
- Focus on Breast Cancer
- The Single Most Convincing Study of How Bacteria Causes Cancer
- The Politics of Cancer
- Parallels with Plant Cancer
- Seibert Rules Out Contaminants in the Cancer Germ
- Experimental Medicine for the Masses
- BCG
- Livingston's Legacy
- Conclusion
- Mycobacterium-Induced Malignant-like Multinucleated Giant Cells
- Cancer Observations in Old England
- Long Island Breast Cancer
- Long island New York, 1886
- The Malignant Threat of the Mycobacteria
- Gland Seeking Mycobacteria
- Viralizing Cancer
- Bacterial Phage Viruses
- References
Viralizing Cancer
Actually virology was saved from probable extinction by Andre Lwoff at the Pasteur Institute, when he incorporated and generalized the mechanism of the bacterial phage virus (bacteriophage, mycobacteriophage) into the field of virology in the early 1950s. It was this tactical explanation that brought us the modern concept of the virus.
To a limited extent viruses and retroviruses are still being implied as causing cancer. ultra-structure, never believed in the cancer-causing potential for retroviruses like the Bovine Leukemic Virus (BLV). Rather he mentions that retroviruses are rather widespread in healthy animals and humans where they typically cause latent infections and antiviral immunity and that "The leukemia risk of such [retroviral] infections is less than 0.1% and thus about as low as that of virus-free controls [179]." And even as far back as 1957, Dmochowski & Grey [181], virologists at The University of Texas M.D. Anderson Hospital warned about attributing "virus-like particles" such as found in the Miller's original 'discovery' of the Bovine Leukemic Virus (BLV) as the direct cause of cancer. From its origin, the very concept of the "BLV leukemic virus" has been on shaky, unstable ground. In 1969, veterinarians Janice and Lyle Miller from the University of Wisconsin-Madison spotted C-shaped "virus-like" particles in cattle lymphosarcoma insisting that these were similar to other C-type viruses "regarded as the cause of leukemia in other species" [182] (Figure 7).
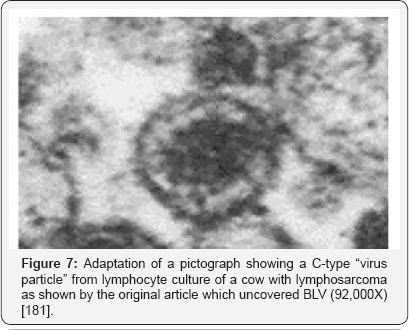
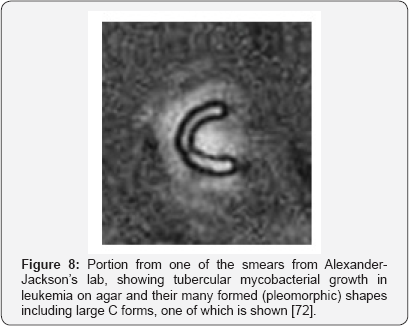
But by 1978, Alexander-Jackson [92] at Downstate also found atypical mycobacterial forms, including its preferred filterable virus-sized "L" or cell-wall-deficient (CWD) forms in not only leukemia but all other malignancies -all having, as their common denominator the continuous presence of mycobacterial C-shaped forms [72] (Figure 8).
Furthermore Seibert [183] showed these TB-like cancer microbes were not laboratory contaminants because her team was able to isolate such mycobacteria from every malignant tumor and leukemic blood studied. When Seibert made "immediate imprints" of fresh cancerous breast tissue smeared onto slides and stained these, cell-wall-deficient coccal forms of the atypical mycobacteria infection were easily identifiable.
By 2014, Buehring [184] reported that Bovine Leukemic Virus (BLV), a common oncogenic retrovirus of cattle, was present in some humans, primarily localized to the breast epithelium -the very cell type from which most breast malignancies arise. By 2015, Buehring [185] revisited this subject. This study found that 59 percent of breast cancer samples had evidence of exposure to BLV, as determined by the presence of viral DNA. By contrast, 29 percent of the tissue samples from women who never had breast cancer showed exposure to BLV. The investigators carefully noted that their results did not prove that the virus causes cancer, but that the study was a most important first step in an ongoing investigation that needed to confirm that the infection with the virus happened before, not after, breast cancer developed - and if so, how. Another area to be addressed was how the virus infected the breast tissue samples in their study. The virus could have come through the consumption of unpasteurized milk or undercooked meat, or it could have been transmitted by other humans.
However our own evidence showed standard BLV preparations to be laced with cell-wall-deficient tubercular forms [186]. At one point Bittner reported on a milk-borne mouse breast cancer attributed to still another virus [187]. But Post World War II investigators demonstrated that the retroviruses in the Rous, Bittner, and Shope tumors were actually filterable forms of mycobacteria [52]. Tuberculosis-like, such “viruses” could be variably stained with acid-fast dyes and readily passed through a filter, but were found to be mycobacterial forms of the Actinomycetales having many of the characteristics of mycobacteria such as tuberculosis.
If recent estimates suggest over 83% of U.S. dairy operations are currently positive for BLV, they also show that approximately 68% are positive for cell-wall-deficient Mycobacterium avium subspecies paratuberculosis (MAP) -which, as all of the virulent mycobacteria, has its own share of viral-like CWD forms. Long ago, by 1868 Jean-Baptiste Auguste Chauveau [188] in France and Edwin Klebs [189] in Prague, proposed that eating infected meat could transmit bovine tuberculosis. Subsequently Andreas Gerlach's [190] experiments confirmed that either milk or flesh from tubercular cows could transmit tuberculosis to other animals and humans to which they were fed. Schliesser also saw meat from tuberculous animals as constituting a significant risk to humans if available for consumption [191]. With regard to bovine tuberculosis, anyone handling or consuming meat from an infected animal is at risk of contracting this disease. Early twentieth century recognition of the spread of cow tuberculosis was obvious and at one time American milk contained the words: “tuberculin tested,” an epitaph to the up to 30% of human cases of pre-pasteurization tuberculosis that occurred in this way. Mycobacteria, particularly Mycobacterium avium paratuberculosis (MAP), are among the most resistant organisms to pasteurization.
- Review Article
- Abstract
- Introduction and Background
- Aldred Scott Warthin
- An Inconvenient Truth
- Hodgkin's Cancer Comes Under Attack
- Dr. Virginia Livingston
- Momentum Builds
- Recognition
- Focus on Breast Cancer
- The Single Most Convincing Study of How Bacteria Causes Cancer
- The Politics of Cancer
- Parallels with Plant Cancer
- Seibert Rules Out Contaminants in the Cancer Germ
- Experimental Medicine for the Masses
- BCG
- Livingston's Legacy
- Conclusion
- Mycobacterium-Induced Malignant-like Multinucleated Giant Cells
- Cancer Observations in Old England
- Long Island Breast Cancer
- Long island New York, 1886
- The Malignant Threat of the Mycobacteria
- Gland Seeking Mycobacteria
- Viralizing Cancer
- Bacterial Phage Viruses
- References
Bacterial Phage Viruses
But there is another “virus” which deserved serious attention with regards to carcinogenesis and that is the virus which lives inside all virulent mycobacteria (mycobacteriophages). In her own review on this subject Seibert mentioned that the 'viral' cancer findings of Demochowski [192] and Grey as well as those of Dameshek could be taken in an altogether different light [107]. These viral studies, pointed out Seibert [182], found the usual inclusion bodies measuring from about 0.25-1.7|i in the tissues and blood cells of patients or animals with leukemia or other malignancies. But these inclusion bodies in turn had much smaller “virus-like” inclusions of about 0.03|i. Furthermore, not only was it striking to Seibert how closely the larger inclusion bodies measurements approximated the sizes of the cell- wall-deficient, variably acid-fast mycobacterial forms that she had isolated in all cancers [0.26-1.7|i] -but also that the tiny inclusions that she likewise saw within these forms -measuring about 0.01-0.05|i were not only similar in size to those found by the virus hunters, but to the mycobacteriophage -the same mycobacteriophage that Mankiewicz [69] not only found in isolates of mammalian tissue, but that were responsible for premalignant change in those mammalian tissues [94].
So Seibert’s cancer attentions were therefore not only riveted on pleomorphic, variable acid-fast, cell-wall-deficient (CWD) tubercular-like forms within cancer tissue, but the minute inclusions representative of mycobacteriophage (Figure 9).
When this bacterial virus, also called a “phage” or in this case a mycobacteriophage infects a mycobacterium such as tuberculosis, the germ itself can become the phage's workshop, the phage hijacking the machinery of the mycobacterial cell, turning it from its usual purposes to the sole task of replicating the virus's genetic material and protein coat. So effectively can this happen, with so many copies of the mycobacteriophage being produced -that the germ can eventually, in effect, commit suicide, bursting under the pressure of phage offspring. If this happens then multiplying bacteriophages are set free -to infect other germs, continuing the cycle. In addition mammalian and tissue cells, as innocent bystanders will be subject to certain changes by the mycobacteriophages thrown into their midst. But in other cases, the germ lives on, with the phage DNA (in the form of a prophage) just being merged with the germ it has attacked, creating changes in staining and pleomorphic architecture which become a diagnostic nightmare, as well as the potential of increasing the attacked microbe's virulence or pathogenicity. Also, it has been postulated that such mycobacteriophages from pathogens such as fowl tuberculosis (M. avium) and Mycobacterium kansasii drastically increase the virulence of existing human tuberculosis often acquired early in life.
Mycobacteriophages are the viruses within all virulent infections of the Actinomycetales, whether tubercular, mycobacterial or related variable acid-fast species. And it was at this point that Florence Seibert speculated as to just how the nuclear material of mammalian cells and tissues could become vulnerable to malignant transformation during their prolonged and apparently symbiotic residence with mycobacterial/ tubercular-like pathogens in the body. Specifically, on the subject of such mycobacteriophage inspired carcinogenesis, Seibert modeled what she felt was happening along the lines of "prophage activation" (Figure 10).

Seibert's "prophage activation" hypothesis can occur when an insult or stress, such as with Ultra-Violet (UV) light (or any other human carcinogen) causes its bacterial, or in this case mycobacterial host damage. When this happens, the prophage of phage genetic material, already lodged in bacterial chromosomes, is then excised from them and can go into a reproductive cycle in which the viral phage commandeers the bacterial cell's reproductive machinery. Seibert and Mankiewicz, therefore, were merely pointing out what should have been an obvious and universal mechanism behind "cancer" to begin with. If the mycobacteriophage could tamper with a germ's reproductive mechanism then why could it not also eventually do mayhem with mammalian and human tissue reproductive mechanisms? Obviously Mankiewicz had already proven that it could.
The role of phages, the viruses which lives in all virulent tubercular and CWD mycobacteria has always been underestimated in the genesis of cancer. Livingston [52] noted that there was no viral agent that was equivalent to the phage which has been proven to be causative in cancer
Seibert's thoughts were not without precedent. By 1948, Raymond Latarjet [193], disciple of d'Herelle, working out of the Pasteur Institute and fascinated by the link between irradiation and cancer ran some studies. Specifically he looked at how the effects of radiation, namely on phages, might cause cancers in animals. By 1962, Latarjet, now working with J.F. Duplan, confirmed that viruses found in tissue of mice who had acquired leukemia thru radiation could be injected into healthy animals never exposed to radiation and that they too would contract leukemia, provided that the radioactive viral injections were given once weekly over a 3 week period [193]. Latarjet determined that irradiated mice increased their viral concentration and concluded that although all mice harbored the leukemia "virus", it was irradiation that broke a natural equilibrium to allow them out. For his model, he used the presence of cancer causing phage genes inside microbes, spurred into activity by radiation, first killing the bacteria that harbored them and then going on to cause the tumor in the animal.
In Biological Order, early phage worker Andre Lwoff agreed. Lwoff[75] thought it more than a coincidence that all of the agents that activated bacteriophages, including radiation -causing phage viruses to spring to life, multiply, and kill the bacteria they were in, were considered carcinogens. His list included radiation, U.V. light, X-rays, gamma rays; certain chemicals, nitrogen mustard, organic peroxides, epoxides and the ethyleneamines. In fact to Lwoff, the bacteriophage virus which didn't immediately kill its bacteria, but whose DNA joined to and multiplied as the bacteria's (in what is called a prophage) represented a true Sword of Damocles with regards to cancer. In Greek legend, a courtier of ancient Syracuse, known as Damocles, talked so much about the happiness of being king that his own king, Dionysius, decided to demonstrate the dangers in a ruler's life by seating Damocles at a banquet table just below a sword hanging by a hair. Thus became immortalized in Greek Mythology the Sword of Damocles -which Lwoff now saw poised over bacterial heads, loosened with the release of deadly phage by carcinogens such as radiation or toxic chemicals. Unfortunately though, Damocles sword in this case could not only kill the bacteria the viral phage was housed and activated in, but in so doing spray activated phages with their virulent DNA over other nearby irradiated tissue. So in truth the Sword was also pointed at human life. Lwoff: "The fact that inducing agents (agents which cause phages to spring to life or be activated) are mutagenic (cancer causing) is not a great help: induction of a lysogenic bacterium (so that its viral phages become activated) doesn't mean mutation. But the fact that inducing agents (that activate phages) are able to initiate malignant growth is rather exiting" [75].
As mentioned, much of the recent work regarding TB and its related microorganisms as causative of cancer has dealt with the "L" or "cell-wall-deficient" forms of typical or atypical Mycobacterium tuberculosis. It is known that such cell-wall- deficient variants can produce and eject as much as 12 to 23 times the amount of phage units into mammalian tissue as can generate from their parent microbes, which in this case is the tubercle bacilli and related mycobacteria [194,195].
Then what is the nature and origin of these L-forms or cell-wall-deficient forms of the tubercular-like mycobacteria that have captured the majority of attention in recent cancer studies? How do cell-wall-deficient forms themselves form?
In a study in the The Journal of Infectious Diseases, Nelson & Pickett [196] maintained that such cell-wall-deficient (CWD) states were largely the result of bacteriophage or in the case of the mycobacteria, mycobacteriophage attack with the other causes that have been proposed for cell-wall-deficiency simply enhancing phage activity to throw the cell into a cell-wall deficient state. According to Nelson, why bacteria or mycobacteria became 'cell-wall-deficient' and therefore assumed many forms was simply the battering that their bacterial cell walls took from phage attack (Figure 11).
Although Nelson & Pickett [196] acknowledged that other agents could contribute to cell-wall deficiency, they saw these as simply activating such phage intrusion. For example, they saw Kruger's et al. [197] work with penicillin, a known cause of cell- wall-deficient forms, as just another substance that enhanced phage activity. Moreover Nelson and Pickett showed that such phage attack could, in certain instances, keep a microorganism locked in a cell-wall-deficient state indefinitely, a finding which fit together with Takahashi's [198] Japanese study of the different cell-wall-deficient forms of tuberculosis.
Further indirect evidence for the role of CWD (cell-wall- deficient) mycobacteria and their mycobacteriophages in cancer lies in the ability of one mycobacteria, such as BCG (dilute Mycobacterium bovis) to combat cancer in the same manner that one mycobacteria (M. smegmatis), transfected with the appropriate mycobacteriophage can kill other mycobacteria, such as virulent strains of M. avium and M. tuberculosis [199]. Currently, bacillus Calmette-Guérin (BCG), a live attenuated strain of BCG treatment can eradicate this cancer in 70% of patients with CIS (Carcinoma-in-situ) of the bladder when used correctly, though to prevent cancer recurrence, long-term maintenance therapy following the induction phase is necessary.
The striking analogy between cancer and tuberculosis was noticed long before the tubercle bacillus was discovered. In a letter dated November 20, 1912 Kansas City surgeon Dr. W. W. Duke wrote to famed surgeon William S. Halsted of radical mastectomy fame regarding Duke's patient Mrs. J. L. Buxton. Buxton first came to Duke in August of 1912 and was definitively diagnosed with breast cancer and operated on that November Then Buxton died in April of 1913. When she first saw Dr. Duke, the patient had pain in her left breast. But no mass was at that time present where the cancer would soon develop. Rather there was a small moveable mass in another location in the left breast which Duke believed was an enlarged subpectoral lymph gland. But even when it soon became obvious to Duke that another mass, a probable breast cancer was growing Duke wrote Halsted that he "told her husband that it was carcinoma but said I hoped it would prove tuberculosis or an infection of some kind” [200]. It was in this fashion that surgeons and medical doctors discussed differential diagnoses of masses or lumps at that time. And they still do.
- Review Article
- Abstract
- Introduction and Background
- Aldred Scott Warthin
- An Inconvenient Truth
- Hodgkin's Cancer Comes Under Attack
- Dr. Virginia Livingston
- Momentum Builds
- Recognition
- Focus on Breast Cancer
- The Single Most Convincing Study of How Bacteria Causes Cancer
- The Politics of Cancer
- Parallels with Plant Cancer
- Seibert Rules Out Contaminants in the Cancer Germ
- Experimental Medicine for the Masses
- BCG
- Livingston's Legacy
- Conclusion
- Mycobacterium-Induced Malignant-like Multinucleated Giant Cells
- Cancer Observations in Old England
- Long Island Breast Cancer
- Long island New York, 1886
- The Malignant Threat of the Mycobacteria
- Gland Seeking Mycobacteria
- Viralizing Cancer
- Bacterial Phage Viruses
- References
References
- Falagas ME, Kouranos VD, Athanassa Z, Kopterides P (2010) Tuberculosis and malignancy. Q J M 103(7): 461-487.
- Yu YH, Liao CC, Hsu WH, Chen HJ, Liao WC, et al. (2011) Increased lung cancer risk among patients with pulmonary tuberculosis: a population cohort study. J Thorac Oncol 6(1): 32-37.
- Zhang S, Guang-ling Z, Yan-sheng T (2009) Detection of Mycobacterium tuberculosis L-form infection in tissues of lung carcinoma. Chin J Public Health 25: 1317-1318.
- Chauhan A, Madiraju MV, Fol M, Lofton H, Maloney E, et al. (2006) Mycobacterium tuberculosis Cells Growing in Macrophages Are Filamentous and Deficient in FtsZ Rings. J Bacteriol 188(5): 1856-1865.
- World Health Organization, Global Tuberculosis Report (2016) Geneva: World Health Organization, USA.
- Yang B, Tian Y, Cui X, Zhang W, Ma Y, et al. (2013) Detection of Mycobacterium tuberculosis L-forms and MPB64 in breast cancer tissues. The Journal of Practical Medicine 29(15): 2552-2555.
- KJ Ryanand, CG Ray (2004) Sherris Medical Microbiology, 4th (edn), McGraw-Hill, USA.
- Guliang H, Tefu L (1999) Mycobacterium tuberculosis L-forms. Microbial Ecology in Health and Disease 10: 129-133.
- Kakkar S, Kapila K, Singh MK, Verma K (2000) Tuberculosis of the breast. A cytomorphologic study. Acta Cytol 44(3): 292-296.
- Puneet, Satyendra KT, Ragini R, Sanjay Singh, Guptav SK, et al. (2005) Breast Tuberculosis: Still Common In India. The Internet Journal of Tropical Medicine 2(2).
- Vagholkar K, Gopinathan I, Pandey S, Maurya I (2014) Tuberculosis of the Breast (Case Report and Review of Literature). The Internet Journal of Surgery 31(1).
- Liu Y, Lin TF (1996) Studies on morphology and electron-micrographic analysis of M tuberculosis filamentous L-forms. Chinese J Micro Boil Immunobiol 16(Suppl 2): 49.
- Dai YH, Lin TF, Huang GL (1996) A serial study on mycobacteria L-forms. Zhongguo Fanglao Zazhi 45-46.
- Shleeva MO, Salina EG, Kaprel'iants AS (2010) Dormant form of Mycobacterium tuberculosis. Mikrobiologiia 79(1): 3-15.
- Marcova N, Slavchev G, Michailova L (2012) Unique biological properties of Mycobacterium tuberculosis L-form variants: impact for survival under stress. Int Microbiol 15(2): 61-68.
- Warthin AS (1899) The Coexistence of Carcinoma and Tuberculosis of the Mammary Gland. Am J M Sci 118: 25-35.
- Rosen PP (1979) Multinucleated mammary stromal giant cells-a benign lesion that simulates invasive carcinoma. Cancer 44(4): 13051308.
- Hektoen L (1898) The fate of the giant cells in healing tuberculous tissue, as observed in a case of healing tuberculous meningitis. J Exp Med 3(1): 21-52.
- Iakimenko LN (1976) Changes in the Mitotic Regime of a Cell Culture under the Influence of Sensitins. Biull Eksp Biol Med 81(2): 237-239.
- Golubchik IS, Iakimenko LN, Lazovskaya AL (1972) Effect of Tuberculin on the Mitotic Regime in Cell Cultures. Biull Eksp Biol Med 73(5): 105.107.
- Rao VV, Gupta EV, Thomas IM (1990) Chromosome Damage in Untreated Tuberculosis Patients. Tubercle 71(3): 169-172.
- Much H, Uber Die Granuläre, Nach Ziehl Nicht Färbbare Form des Tuber kulosevirus. Beit Z Klin Tuberk 8th (edn), 85(1907): 85-97.
- Kahn MC (1929) The Developmental Cycle of the Tubercle Bacillus as Revealed by Single Cell Cultures. Am Rev Tuberc 20(1929): 150.
- Ribbert H (1894) Beitrage zur Histogenese des Carcinoms. Arch Pathol. Anat U Physiol Virchow's 135: 433-469.
- Chang Y, Cesarman E, Pessin MS, Lee F, Culpepper J, et al. (1994) Identification of herpesvirus-like DNA sequences in AIDS-associated Kaposi's sarcoma. Science 266(5192): 1865-1869.
- Huang YQ, Li JJ, Kaplan MH, Poiesz B, Katabira E, et al. (1995) Human herpesvirus-like nucleic acid in various forms of Kaposi's sarcoma. Lancet 345(8952): 759-761.
- Floto RA, Sarkar S, Perlstein EO, Kampmann B, Schreiber SL, et al. (2007) Small Molecule Enhancers of Rapamycin-Induced TOR Inhibition Promote Autophagy, Reduce Toxicity in Huntington's Disease Models and Enhance Killing of Mycobacteria by Macrophages. Autophagy 3(6): 620-622.
- Jang WS, Kim S, Podder B, Jyoti A, Nam KW, et al. (2015) Anti- Mycobacterial Activity of Tamoxifen against Drug-Resistant and IntraMacrophage Mycobacterium tuberculosis. J Microbiol Biotechnol 25(6): 946-950.
- Ertem E, Yüce K, Karakartal G, Onal O, Yüce G (1990) The antituberculous effect of bleomycin. J Antimicrob Chemother 26(6): 862-863.
- Gajadeera C, Willby MJ, Green KD, Shaul P, Fridman M, et al. (2015) Antimycobacterial activity of DNA intercalator inhibitors of Mycobacterium tuberculosis primase DnaG. J Antibiot (Tokyo) 68(3): 153-157.
- Forbes L, Ebsworth-Mojica K, Di Done L, Li S-G, Freundlich JS, et al. (2015) A High Throughput Screening Assay for Anti-Mycobacterial Small Molecules Based on Adenylate Kinase Release as a Reporter of Cell Lysis. PLoS One 10(6): e0129234.
- Subramanyam CSV, Ahuja JM, Sapra ML (1975) Miliary tuberculosis simulating acute myeloid leukemia- review of literature and report of a case. Ind J Tub 22(4): 136-141.
- Ananthan S, Faaleolea ER, Goldman RC, Hobrath JV, Kwong CD, et al. (2009) High-throughput screening for inhibitors of Mycobacterium tuberculosis H37Rv. Tuberculosis (Edinb) 89(5): 334-353.
- Greenstein RJ, Su L, Shahidi A, Brown WD, Clifford A, et al. (2014) Unanticipated Mycobacterium tuberculosis complex culture inhibition by immune modulators, immune suppressants, a growth enhancer, and Vitamins A and D: clinical implications. Int J Infect Dis 26: 37-43.
- Zhang L, Zheng Y, Callahan B, Belfort M, Liu Y (2011) Cisplatin Inhibits Protein Splicing, Suggesting Inteins as Therapeutic Targets in Mycobacteria. J Biol Chem 286(2): 1277-1282.
- Batista AA, Back DF, Lang ES, Ellena J, Lemos S, et al. (2010) Palladium (II) complexes with thiosemicarbazones. Syntheses, characterization and cytotoxicity against breast cancer cells and Anti-Mycobacterium tuberculosis activity. J Braz Chem Soc 21(7): 1177-1186.
- Datta M, Via LE, Kamoun WS, Liu C, Chen W, et al. (2015) Antivascular endothelial growth factor treatment normalizes tuberculosis granuloma vasculature and improves small molecule delivery. Proc Natl Acad Sci U S A 112(6): 1827-1832.
- Lamb R, Ozsvari B, Lisanti CL, Tanowitz HB, Howell A, et al. (2015) Antibiotics that target mitochondria effectively eradicate cancer stem cells, across multiple tumor types: treating cancer like an infectious disease. Onco target 6(7): 4569-4584.
- Watt B, Rayner A, Harris G (1996) Comparative activity of azithromycin against clinical isolates of mycobacteria. J Antimicrob Chemother 38(3): 539-542.
- Walker NF, Clark SO, Oni T, Andreu N, Tezera L, et al. (2012) Doxycycline and HIV infection suppress tuberculosis-induced matrix metalloproteinases. Am J Respir Crit Care Med 185(9): 989-997.
- Pang H, Li G, Wan L, Jiang Y, Liu H, et al. (2015) In vitro drug susceptibility of 40 international reference rapidly growing mycobacteria to 20 antimicrobial agents. Int J ClinExp Med 8(9): 15423-15431.
- Shu X, Gao YT, Linet MS, Brinton LA, Gao RN, et al. (1987) Chloramphenicol use and childhood leukemia in Shanghai. Lancet 2(8565): 934-937.
- Yuan ZR, Shi Y (2008) Chloramphenicol induces abnormal differentiation and inhibits apoptosis in activated T cells. Cancer Res 68(12): 4875-4881.
- Smith RM, Joslyn DA, Gruhzit 0M, McLean IW, Penner MA, et al. (1948) Chloromycetin: biological studies. J Bacteriol 55: 425.
- Youmans GP, Youmans AS, Osborne RR (1948) Tuberculostatic action of Chloromycetin in vitro and in vivo. Proc. Soc. Exp Biol & Med 67: 426.
- Rattan R, Ali Fehmi R, Munkarah A (2012) Metformin: an emerging new therapeutic option for targeting cancer stem cells and metastasis. J Oncol 2012: 928127.
- Hirsch HA, Iliopoulos D, Tsichlis PN, Struhl K (2009) Metformin selectively targets cancer stem cells, and acts together with chemotherapy to block tumor growth and prolong remission. Cancer Res 69(19): 7507-7511.
- Singhal A, Jie L, Kumar P, Hong GS, Leow MK, et al. (2014) Metformin as adjunct antituberculosis therapy. Sci Transl Med 6(263): 263ra159.
- Esumi H, Lu J, Kurashima Y, Hanaoka T (2004) Antitumor activity of pyrviniumpamoate, 6-(dimethylamino)-2-[2-(2,5-dimethyl-1-phenyl- 1H-pyrrol-3-yl)ethenyl]-1-methyl-quinolinium pamoate salt, showing preferential cytotoxicity during glucose starvation. Cancer Sci 95(8): 685-690.
- Lougheed KEA, Taylor DL, Osborne SA, Bryans JS, Buxton RS (2009) New Anti-tuberculosis agents amongst known drugs Tuberculosis (Edinb) 89(5): 364-370.
- Maitra A, Bates S, Kolvekar T, Devarajan PV, Guzman JD, et al. (2015) Repurposing-a ray of hope in tackling extensively drug resistance in tuberculosis. Int J Infect Dse 32: 50-55.
- Livingston (1972) Virginia Wuerthele-Caspe. Cancer: a new breakthrough, Nash Publishing, Los Angeles, USA.
- Ewing J (1919) Neoplastic diseases. 2nd (edn), WB Saunders, Philadelphia, USA, p. 1027.
- Rusch HP (1985) The beginnings of cancer research centers in the United States. J Natl Cancer Inst 74(2): 391-403.
- Hunter D (1978) The diseases of occupation. 6th (edn), Little Brown and Company, Boston, UK.
- Fraenkel E, Much H (1910) Uber die Hodgkinsche Krankheit (Lymphomatosis granulomatosa), insbesondere derenAtiologie. Z Hyg 67: 159-200.
- L'Esperance E (1931) Studies in Hodgkin's disease. Annal Surg 93(1): 162-168.
- Livingston V, Allen RM (1948) Presence of consistently recurring invasive mycobacterial forms in tumor cells. MicroscopSoc Bull 2: 5-18.
- Sweany HC (1926) Mutation forms of the tubercle bacillus. JAMA 87(15): 1206-1211.
- Beinhauer LG, Mellon RR (1938) Pathogenesis of non-caseating epithelioid tuberculosis of hypoderm and lymph glands. Arch Dermatol Syph 37: 451-460.
- Mellon RR, Fisher LW (1932) New studies on the filterability of pure cultures of the tubercle group of microorganisms. J Infect Dis 51(1): 117-128.
- Livingston V, Alexander-Jackson EA (1950) Cultural properties and pathogenicity of certain microorganisms observed from various proliferative and neoplastic diseases (published under Virginia Wuerthele-Caspe). Am J Med Sci 220: 636-648.
- Boesch M (1960) The long search for the truth about cancer. GP Putnam's Sons, New York, USA.
- Glover T, Scott M (1926) A study of the Rous chicken sarcoma No.1. Can Lancet Practitioner 66(2): 49-62.
- Goodman LS, Gilman A (1975) The pharmacologic basis of therapeutics. 5th (edn), MacMillan, New York, USA.
- Skirvin JA, Relias V, Koeller J (1996) Long term sequelae of cancer chemotherapy. Highlights Oncol Practice 14(2): 26-34.
- Pukkala E, Kyyronen P (2002) Tamoxifen and toremifene treatment of breast cancer and risk of subsequent endometrial cancer: a population- based case-control study. Int J Cancer 100(3): 337-341.
- Mankiewicz E (1965) Bacteriophages that lyse Mycobacteria andCorynebacteria and show cytopathogenic effect on tissue cultures of renal cells of Cercopithecus aethiops. Can Med Assn J 92: 31-33.
- Dubos R (1987) The White Plague: Tuberculosis. New Brunswick, NJ: Man & Society; Rutgers University Press, USA, A Journal of History of Science 78: 615-616.
- Aaronson JD (1926) Spontaneous tuberculosis in salt water fish. J Infect Dis 39(4): 315-320.
- Wuerthele-Caspe VE, Alexander-Jackson E, Smith LW (1971) Some aspects of the microbiology of cancer. J Am Woman's Med Assoc 8: 7.
- Alexander-Jackson EA (1954) A specific type of microorganism isolated from animal and human cancer. Growth 18(1): 37-51.
- Inoue S, Singer M (1970) Experiments on a spontaneously originated visceral tumor in the Newt Trituruspyrrhogaster. Annal NY AcadSci 174(2): 729-764.
- Lwoff A (1962) Biologic order. Karl Taylor Compton Lectures, Cambridge MA: The MIT Press, USA.
- Klieneberger-Nobel E (1949) Origin, development and significance of L-forms in bacterial cultures. J Gen Microbiol 3: 434-442.
- Rogers AD (1953) Erwin Frink Smith: a story of North American plant pathology. Philadelphia: American Philosophical Society 152(9): 879.
- Seibert FB (1968) Pebbles on the Hill of a Scientist. In: Florence B (ed.), Seibert publisher, St. Petersburg, USA.
- Cantwell A (1990) The cancer microbe. Aries Rising Press, USA.
- Mattman LH (1993) Cell wall deficient forms-stealth pathogens. 2nd (edn), Boca Raton (ed.), CRC Press, USA.
- Schneider B (1994) Specific binding of Bacillus Calmette-Guerinin urothelial tumor cells. In- vitro World J Urol 12(6): 337-344.
- Rosenberg SA, Barry JM (1992) The transformed cell/unlocking the mysteries of cancer. GP Putnam's Sons, New York, USA.
- Devados PO, Klegerman ME, Groves MJ (1993) Phagocytosis of Mycobacterium bovis BCG organisms by murine S180 sarcoma cells. Cytobios 74(296): 49-58.
- Moss RW (1997) The independent consumer's guide to non-toxic treatment and prevention. Cancer therapy. Equinox Press, New York, USA.
- Martin W (1997) Medical heroes and heretics. Old Greenwich, The Devin Adair Company, Connecticut, USA.
- Acevedo H, Pardo M, Campbell-Acevedo E, Domingue GJ (1987) Human choriogonadotropin-like material in bacteria of different species: electron microscopy and immunocytochemical studies with monoclonal and polyclonal antibodies. J Gen Microbiol 133(3): 783791.
- (1990) Congress of the United States Office of Technology Assessment. Unconventional Cancer Treatments US Govt Printing Office, Washington, USA.
- Glover TJ (1930) The bacteriology of cancer. Canada Lancet Mar 74(3): 92-111.
- Mazet G (1941) Etude Bacteriologique sur la Maladie d' Hodgkin. Montpellier Med, pp. 1-6.
- Wuerthele-Caspe V (1949) Mycobacterial forms observed in tumors. J Am Med Womens Assoc 4: 135-141.
- Alexander-Jackson E (1976) Progenitor Cryptocides, The Specific Pleomorphic Microorganism Isolated From Cancer. J Int Acad Metab 5: 31-39.
- Alexander-Jackson E (1978) Microscopic and Submicroscopic Phases of P Cryptocides from Fresh Lymphocytic leukemia. J Int Acad Metab 1: 9-18.
- Diller I, Diller W (1965) Intracellular acid-fast organisms isolated from malignant tissues. Trans Am Micr Soc 84: 138-148.
- Diller I, Donnelly A, Fisher M (1967) Isolation of pleomorphic, acid- fast organisms from several strains of mice. Cancer Res 27(8): 1402-1408.
- Seibert F, Feldmann F, Davis R, Richmond I (1970) Morphological, biological, and immunological studies on isolates from tumors and leukemic bloods. Ann N Y Acad Sci 174: 690-728.
- Wang A, Xie J (1998) Infection of mycobacterium tuberculosis in lung cancer. Zhongguo Fei Ai ZaZhi 1(2): 92-94.
- Xie J, Anchao W, Xiazhi Z (1999) Isolation of acid fast bacillus L- forms from carcinoma of Lung. Acta Academiae Medicinae Bengbu 24: 145.146.
- Song LY, Yan WS, Zhao T (2002) Detection of in lung cancer tissue by indirect in situ nested PCR. Di Yi Jun Yi Da XueXueBao 22(11): 992-993.
- Yesong WXQ, Lifa X (2004) A case report on pneumoconio tuberculosis complicated with lung cancer and Mycobacterium tuberculosis- L form infection. Chin J Industrial Med.
- Zhang S, Guang-ling Z, Yan-sheng T (2009) Detection of Mycobacterium tuberculosis L forms infection in tissues of lung carcinoma. Chin J Public Health 25: 1317-1318.
- Sheng TY, Kun CX, Tong H, Guang LH, Wei Z, et al. (2009) Study on the relationship between Mycobacterium tuberculosis L infection and lung cancer. Tumor 29(11): 1085-1089.
- Tian Y, Hao T, Cao B, Zhang W, Ma Y, et al. (2015) Clinical End-Points Associated with Mycobacterium tuberculosis and Lung Cancer: Implications into Host-Pathogen Interaction and Coevolution. Bio Med Research Intern p. 9.
- Alexander-Jackson E (1970) Ultraviolet spectrogramic microscope studies of Rous sarcoma virus cultured in cell-free medium. Ann N Y Acad Sci 174(2): 765-781.
- Shinde SR, Chandawarkar RY, Deshmukh SP (1995) Tuberculosis of the breast masquerading as carcinoma: A study of 100 patients. World J Surg 19(3): 379-381.
- Deng B, Huang W, Tan QY, Fan XQ, Jiang YG, et al. (2011) Breast cancer anti-estrogen resistance protein 1 (BCAR1/p130cas) in pulmonary disease tissue and serum. Mol Diagn Ther 15(1): 31-40.
- Hatwal Deepa, Suri Vijay, Mishra Jai P, Joshi Chitra (2011) Tubercular mastitis is common in garhwal region of uttarakhand: clinico athological features of 14 cases. Journal of Clinical and Diagnostic 5(8):1569-1573.
- Lay G, Poquet Y, Salek-Peyron P, Puissegur MP, Botanch C, et al. (2007) Langhans giant cells from M tuberculosis-induced human granulomas cannot mediate mycobacterial uptake. J Pathol 211(1): 76-85.
- Weiss MG, Sommerfeld J, Uplekar MW (2008) Social and cultural dimensions of gender and tuberculosis. Int J Tuberc Lung Dis 12(7): 829-830.
- Holmes CB, Hausler H, Nunn P (1998) A review of sex differences in the epidemiology of tuberculosis. Int J Tuberc Lung Dis 2(2): 96-104.
- Tsuyuguchi K, Suzuki K, Matsumoto H, Tanaka E, Amitani R, et al. (2001) Effect of estrogen on Mycobacterium avium complex pulmonary infection in mice. Clin Exp Immunol 123(3): 428-434.
- Havlik JA, Horsburgh CR, Metchock B, Williams PP, Fann SA, et al. (1992) Disseminated Mycobacterium avium complex infection: clinical identification and epidemiologic trends. J Infect Dis 165(3): 577-580.
- Tanaka E, Amitani R, Niimi A, Suzuki K, Murayama T, et al. (1997) Yield of computed tomography and bronchoscopy for the diagnosis of Mycobacterium avium complex pulmonary disease. Am J Respir Crit Care Med 155(6): 2041-2046.
- Prince DS, Peterson DD, Steiner RM, Gottlieb JE, Scott R, et al. (1989) Infection with Mycobacterium avium complex in patients without predisposing conditions. N Engl J Med 321(13): 863-868.
- Reich JM, Johnson RE (1991) Mycobacterium avium complex pulmonary disease. Am Rev Respir Dis 143(6): 1381-1385.
- Howlader N, Noone AM, Krapcho M (2012) SEER Cancer Statistics Review, 1975-2009 (Vintage 2009 Populations). National Cancer Institute, Bethesda.
- Philley JV, Kannan A, Griffith DE, Devine MS, Benwill JL, et al. (2017) Exosome secretome and mediated signaling in breast cancer patients with nontuberculous mycobacterial disease. Oncotarget 8(11): 18070-18081.
- Cassidy PM, Hedberg K, Saulson A, Nelley Mc E, Winthrop KL (2009) Nontuberculous mycobacterial disease prevalence and risk factors: a changing epidemiology. Clin Infect Dis 49(12): e124-e129.
- Franson JC, Friend M (1999) Field manual of wildlife diseases, Geological Survey, Washington DC, USA.
- Miller RE, Fowler ME (2012) Fowler's Zoo and Wild Animal Medicine Current Therapy, Volume 7. Elsevier Health Sciences, p. 688.
- Phillips MS, von Reyn CF (2001) Nosocomial Infections Due to Nontuberculous Mycobacteria. Clin Infect Dis 33(8): 1363-1374.
- Nason EN (1898) The Influence of Locality on the Prevalence of Malignant Disease. Br Med J 1(1941): 679-681.
- Jones L (1899) The Influence of Locality on the Prevalence of Cancer. Br Med J 1(1996): 812-813.
- Mason H (1902) A Possible Predisposing Cause of Cancer. Br Med J 1(2142): 139-141.
- Miller BA, Gloeckler Ries LA, Hankey BF (1993) SEER cancer statistics review, 1973-1990. Bethesda, MD: National Institutes of Health.
- Kulldorff M, Feuer EJ, Miller BA, Freedman LS (1997) Breast cancer clusters in Northeastern United States: a geographic analysis. Am J Epidemiol 146(2): 161-170.
- Jenks S (1994) Researchers to comb Long Island for potential cancer factors. J Natl Cancer Inst 86: 88-95.
- Lewis-Michi EL, Melius JM, Kallenbach LR (1996) Breast cancer risk and residence near industry or traffic in Nassau and Suffolk counties, Long Island, New York. Arch Environ Health 51: 255-265.
- Wittenberg C (1994) Long Island breast cancer studies move forward. J Natl Cancer Inst 86: 1501-1503.
- Jacquez GM, Greiling DA (2003) Local clustering in breast, lung and colorectal cancer in Long Island, New York. Int J Health Geogr 2(1): 3.
- Gammon MD, Neugut AI, Santella RM, Teitelbaum SL, Britton JA, et al. (2002) The Long Island Breast Cancer Study Project: description of a multi-institutional collaboration to identify environmental risk factors for breast cancer. Breast Cancer Res Treat 74(3): 235-254.
- Davidson A (1999) Oxford Companion to Food. Oxford: Oxford University Press, India, p. 593.
- Zeng Y, Du J, Pu X, Yang J, Yang T, et al. (2015) Coevolution between Cancer Activities and Food Structure of Human Being from Southwest China. Bio Med Res Int 2015: 497934.
- (2009) Long Island Duck Farm History and Ecosystem Restoration Opportunities Report. US Army Corps of Engineers, New York District. Suffolk County, NY, pp. 1-20.
- Davids HW, Cosulich WF (1968) Water Pollution from Duck Farms and Recent Developments in Treatment. 1968. Paper presented at the June 10, 1968 meeting of the New York Water Pollution Control Association, Montauk, NY, p. 9.
- Partridge MS, Smith WG, Rutz DA (1992) Pest and Pesticide Use Assessment for Poultry Production Systems in New York State for 1998. Pesticide Management Education Program, Cornell University, USA.
- World Health Organization (2008) Guidelines for Drinking- water Quality, Incorporating 1st and 2nd Addenda, Volume 1, Recommendations. 3"1 (edn), WHO, Geneva, Switzerland.
- Fenwick A (2006) Waterborne Diseases-Could they be Consigned to History? Science 313: 1077-1081.
- George I, Crop P, Servais P (2001) Use of ß-D-Galactosidase and ß-D-Glucuronidase Activities for Quantitative Detection of Total and Faecal Coliforms in Wastewater. Can J Microbiol 47(7): 670-675.
- Shivaprasad HL, Palmieri C (2012) Pathology of mycobacteriosis in birds. Vet Clin North Am Exot Anim Pract 15(1): 41-55.
- Song XH, Chen HX, Zhou WS, Wang JB, Liu MF, et al. (2016) Complete Genome Sequence of Mycobacterium avium, Isolated from Commercial Domestic Pekin Ducks (Anas platyrhynchos domestica),Determined Using PacBio Single-Molecule Real-Time Technology. Genome Announc 4(5).
- (2003) Proceedings the U.S. EPA's Research on Microorganisms in Drinking Water Workshop August 5-7, Cincinnati, Ohio.
- Kids Count Data Center-a project of the Annie E. Casey Foundation, Maryland, United States.
- Rosenzweig DY (1979) Pulmonary mycobacteria infections due to Mycobacterium avium complex. Clinical features and course in 100 consecutive patients. Chest 75: 115.
- Wolinsky E (1979) Nontuberculous mycobacteria and associated diseases. Am Rev Respir Dis 119(1): 107-159.
- Rosenzweig DY (1980) A typical mycobacteriosis. Clin Chest Med 1: 273-284.
- Dlugovitzky D, Luchesi S (1995) Circulating immune complexes in patients with advanced tuberculosis and their association with autoantibodies and reduced CD4+ lymphocytes. Braz J Med Biol Res 28(3): 331-335.
- Lamoureux G, Davignon L (1987) Is prior mycobacterial infection a common predisposing factor to AIDS in Haitians and Africans? Ann Inst Pasteur Immunol 138(4): 521-529.
- Hirsch CS, Toossi Z (1999) Apoptosis and T-cell hyporesponsiveness in pulmonary tuberculosis. J Infect Dis 179(4): 945-953.
- Chaouchi N, Arvanitakis L, Auffredou, Blanchard DA, Vazquez, et al. (1995) Characterization of transforming growth factor-B1 induced apoptosis in normal human B cells and lymphoma B cell lines. Oncogene 11(8): 1615-1622.
- McDonald I, Wang H (1996) Transforming growth factor B1 cooperates with anti-immunoglobulin for the induction of apoptosis in group I (biopsy-like) Burkitt lymphoma cell lines. Blood 87: 11471154.
- Fratazzi C, Arbeit RD, Carini C, Balcewicz-Sablinska MK, Keane J, et al. (1999) Macrophage apoptosis in mycobacterial infections. J Leukoc Biol 66(5): 763-764.
- Molloy A, Laochumroonvorapong P, Kaplan G (1994) Apoptosis, but not necrosis, of infected monocytes is coupled with killing of intracellular bacillus Calmette-Guerin. J Exp Med 180(4): 1499-1509.
- Hirsch CS, Toossi Z, Johnson JL, Luzze H, Ntambi L, et al. (2001) Augmentation of apoptosis and interferon-y production at sites of active Mycobacterium tuberculosis infection in human tuberculosis. J of Infectious Dis 183(5): 779-788.
- Bermudez LE, Parker A, Petrofsky M (1999) Apoptosis of Mycobacterium avium-infected macrophages is mediated by both tumour necrosis factor TNF and Fas and involves the activation of caspases. Clin Exp Immunol 116(1): 94-99.
- Edition of the Drinking Water Standards and Health Advisories (2012) Environmental Protection Agency, Office of Water, Washington, US, pp. 1-12.
- Shin GA, Lee J, Freeman R, Cangelosi GA (2008) Inactivation of mycobacterium avium by UV irradiation. Appl Environ Microbiol 74(22): 7067-7069.
- Pedley S, Bartram J, Cotruvo JA, Alfred D, Gareth Rb (2004) Pathogenic mycobacteria in water: a guide to public health consequences, monitoring and management. World Health Organization, London pp. 144-168.
- Nalbandian A, Yan BS, Pichugin A, Bronson RT, Kramnik I (2009) Lung carcinogenesis induced by chronic tuberculosis infection: the experimental model and genetic control. Oncogene 28(17): 19281938.
- Da Silva AL, Bresciani MJ, Karnopp TE, Weber AF, Ellwanger JH, et al. (2015) DNA damage and cellular abnormalities in tuberculosis, lung cancer and chronic obstructive pulmonary disease. Multidiscip Respir Med, pp. 10-38.
- Daley CL, Iseman M (2012) Mycobacterium avium complex and lung cancer: chicken or egg? Both? J Thorac Oncol 7(9): 1329-1330.
- Lande L, Peterson DD, Gogoi R, Daum G, Stampler K, et al. (2012) Association between Pulmonary Mycobacterium Avium Complex Infection and Lung Cancer. J Thorac Oncol 7(9): 1345-1351.
- Hosoda C, Hagiwara E, Shinohara T, Baba T, Nishihira R, et al. (2014) Clinical characteristics of pulmonary Mycobacterium avium complex infection complicated with lung cancer. Kekkaku 89(8): 691-695.
- Griffith DE, Aksamit T, Brown-Elliott BA, Catanzaro A, Daley C, et al. (2007) An official ATS/IDSA statement: diagnosis, treatment, and prevention of nontuberculous mycobacterial diseases. Am J Respir Crit Care Med 175(4): 367-416.
- Bender F (1956) Primary pulmonary carcinoma associated with active pulmonary tuberculosis. Dis Chest 30(2): 207-216.
- Moll A (1949) "Der Bronchialkrebs,” Medizinische Klinik. 44: 916.
- Woodruff CE, Sen-Gupta N, Wallace S, Chapman PT, Martineau PC (1952) Anatomic Relationships between Bronchogenic Carcinoma and Calcified Nodulesin the Lung. Am Rev Tuberc 66(2): 151-160.
- https://training.seer.cancer.gov/
- Sunnetcioglu A, Sunnetcioglu M, Binici I, Baran AI, Karahocagil MK, et al. (2015) Comparative analysis of pulmonary and extrapulmonary tuberculosis of 411 cases. Ann Clin Microbiol Antimicrob, pp. 14-34.
- Ates Guler S, Bozkus F, Inci MF, Kokoglu OF, Ucmak H, et al. (2015) Evaluation of pulmonary and extrapulmonary tuberculosis in immunocompetent adults: a retrospective case series analysis. Med Princ Pract 24(1): 75-79.
- Rasolofo Razanamparany V, Menard D, Auregan G, Gicquel B, Chanteau S (2002) Extrapulmonary and pulmonary tuberculosis in Antananarivo (madagascar): high clustering rate in female patients. J Clin Microbiol 40(11): 3964-3969.
- Sreeramareddy CT, Panduru KV, Verma SC, Joshi HS, Bates MN (2008) Comparison of pulmonary and extrapulmonary tuberculosis in Nepal- a hospital-based retrospective study. BMC Infect Dis p. 8.
- Cooper A (1829) Diseases of the Breast-Part 1. S. McDowall Publisher, London, P. 73.
- Audebert F, Schneidewind A, Hartmann P, Kullmann F, Schölmerich J (2006) Lymph node tuberculosis as primary manifestation of Hodgkin's disease. Med Klin (Munich) 101(6): 500-504.
- Centkowski P, Sawczuk-Chabin J, Prochorec M, Warzocha K (2005) Hodgkin's lymphoma and tuberculosis coexistence in cervical lymph nodes. Leuk Lymphoma 46(3): 471-475.
- Lamden K, Watson JM, Knerer G, Ryan MJ, Jenkins PA (1996) Opportunist mycobacteria in England and Wales: 1982 to 1984. Commun Dis Rep CDR Rev 6(11): 147-51.
- Meissner G, Anz W (1977) Sources of Mycobacterium avium complex infection resulting in human disease. Am Rev Respir Dis 116(6): 1057-1064.
- Schaad UB, Votteler TP, McCracken GH, Nelson JD (1979) Management of atypical mycobacterial lymphadenitis in childhood: a review based on 380 cases. J Pediatr 95(3): 356-360.
- White MP, Bangash H, Goel K, Jenkins PA (1986) Nontuberculous mycobacterial lymphadenitis. Arch Dis Child 61(4): 368-371.?
- Felini M, Johnson E, Preacely N, Sarda V, Ndetan H, et al. (2011) A pilot Case-Cohort Study of Liver and Pancreatic Cancers in Poultry Workers. Ann Epidemiol 21(10): 755-766.
- Johnson ES, Zhou Y, Lillian Yau C, Prabhakar D, Ndetan H, et al. (2010) Mortality from malignant diseases-update of the Baltimore union poultry cohort. Cancer Causes Control 21(2): 215-221.
- Duesberg PH (1987) Retroviruses as carcinogens and pathogens: expectations and reality. Cancer Res 47(5): 1199-1220.
- Demochowski L, Grey CE (1957) Subcellular Structures of Possible Viral Origin in Some Mammalian Tumors. Ann NY Acad Sci, pp. 559615.
- Miller JM, Miller LD, Olson C, Gillette KG (1969) Virus-like particles in phytohemagglutinin-stimulated lymphocyte cultures with reference to bovine lymphosarcoma. J Natl Cancer Inst 43(6): 1297-1305.
- Seibert FB, Yeomans F, Baker JA, Davis RL, Diller IC (1972) Bacteria in Tumors. Trans of the NY Acad of Sci June 34(6): 504-533.
- Buehring GC, Shen HM, Jensen HM, Choi KY, Sun D, et al. (2014) Bovine leukemia virus DNA in human breast tissue. Emerg Infect Dis 20(5): 772-782.
- Buehring GC, Shen HM, Jensen HM, Jin DL, Hudes M, et al. (2015) Exposure to Bovine Leukemia Virus is Associated with Breast Cancer: A Case-Control Study. PLoS One 10(9): e0134304.
- Lysenko AP, Broxmeyer L, Vlasenko VV, Krasochko PA, Lemish AP, et al. (2016) Further Evidence for Cancer as a Cell-Wall-Deficient Mycobacterial Disease. J Mol Path Epidemol 1(1): 1-12.
- Bittner JJ (1936) Some possible effects of nursing on the mammary gland tumor incidence of mice. Science 84(2172): 162.
- Chauveau A. Transmission of virulent diseases by the ingestion of virulent principles in the digestive tract. Gaz de Paris p. 45.
- Klebs E (1870) On the history of tuberculosis. Virchows Arch F path Anat, p. 291.
- Gerlach AC (1870) On the inoculability of tuberculosis and theperilla, and on the transferability of the latter by feeding. Virchows Arch F path Anat 11: 297.
- Pfeiffer DU (1994) The role of a wildlife reservoir in the epidemiology of bovine tuberculosis. New Zealand.
- Dameshek W, Gunz (1965) Leukemia. Am J Med Sci 249: 115.
- Latarjet R, Duplan JF (1962) Experiment and Discussion on Leukemogenesis by Cell-Free Extracts of Radiation-Induced Leukemia in Mice. Int J Rad Biol 5(4): 339-344.
- Dobrindt U, Reidl J (2000) Pathogenicity islands and phage conversion: evolutionary aspects of bacterial pathogenesis. Int J Med Microbiol 290: 519-527.
- Landman OE, Burchard WK, Angelety LH (1962) Lysogeny and bacteriophage adsorption in stable and reverting L-forms of Salmonella paratyphi B and Escherichia coli. Bacteriol Proc, p. 53.
- Nelson EL, Pickett MJ (1951) The Recovery of L Forms of Brucella and their Relation to Brucella Phage. J Infect Dis 89(3): 226-232.
- Kruegar AP, Cohn T, Smith PN, McGuire CD (1948) Observations on the effect of penicillin on the reaction between phage and staphylococc. J Gen Physiol 31: 477-488.
- Takahashi S (1979) L phase growth of Mycobacteria. Cell wall deficient form of Mycobacteria. Kekkaku 54: 63-70.
- Broxmeyer L, Sosnowska D, Miltner E, Chacón O, Wagner D, et al. (2002) Killing of Mycobacterium avium and Mycobacterium tuberculosis by a mycobacteriophage delivered by a nonvirulent mycobacterium: a model for phage therapy of intracellular bacterial pathogens. J Infect Dis 186(8): 1155-1160.
- Halsted WS (1912) W.W. Duke to Halsted. In: William Stewart Halsted papers, Series I. The Alan Mason Chesney Medical Archives of the Johns Hopkins Medical Institutions, Baltimore, USA.






























