Introduction
Precision medicine (PM) has transformed the landscape of cancer care by enabling more accurate diagnosis, targeted treatment, and improved patient outcomes [1-3]. Yet, while scientific innovation in genomics, biomarker testing, and targeted therapies continues to advance at an unprecedented pace, health systems worldwide remain unevenly prepared to adopt these innovations into standard practice. A persistent gap between innovation and access has left millions of patients, particularly in low- and middle-income countries, without equitable access to the benefits of precision medicine in oncology [4,5].
This global implementation gap is largely related to the gaps in policy, infrastructure, financing, and governance. Without systematic approaches to guide national planning, investments, and stakeholder engagement, precision medicine risks reinforcing existing health inequities rather than bridging them. Policymakers, advocacy groups, medical societies, and the private sector increasingly recognize the urgency of embedding PM into health systems, but many lack the tools to assess readiness, prioritize actions, and develop evidence-informed strategies tailored to local contexts [6-8].
To address this need, the Precision Medicine Policy Assessment Toolkit for Oncology (PM Toolkit) was developed. It is a strategic policy guide to help countries integrate PM into their health systems. This resource is designed to help different stakeholders across various World Bank (WB) income levels [9] to evaluate a country’s current level of PM readiness, set realistic priorities, and implement actionable steps in a context-specific and evidence-informed way. By doing so, the PM Toolkit provides a much-needed bridge between the promise of precision medicine and the policies required to make it a reality.
This brief introduces the Toolkit, explains its development process, describes its nine-domain framework and incomelevel stratification, highlights real-world case applications, and discusses its policy implications. The goal is to raise awareness of this practical resource and to help stimulate engagement among stakeholders who can drive forward the equitable implementation of precision medicine.
Methods
The PM Toolkit was created through a structured, iterative process designed to combine scientific evidence with pragmatic policy discussions and stakeholder engagement. The methodology included several sequential stages outlined below:
Evidence Mapping and Literature Review
The process began with an extensive review of peer-reviewed literature, grey literature, policy documents, and case examples across oncology and broader precision medicine implementation. This evidence-based review established a clear picture of existing challenges, policy gaps, and opportunities across diverse health system settings.
Identification of Core Domains
From the literature review, recurring themes were distilled into nine critical dimensions essential for advancing PM at the country level. These domains reflect the multi-dimensional nature of health system readiness, spanning legal frameworks, clinical practice, data infrastructure, financing, and partnerships.
Design of a Tiered Framework
Recognizing that one-size-fits-all solutions do not work in global health, a three-tier approach aligned with the World Bank income-level classifications is proposed. This stratification ensures that the suggested policy paths are realistic, feasible, and tailored to each country’s fiscal space, technological readiness, and governance capacity.
Stakeholder Consultation and Validation
Draft versions of the framework and possible policy avenues were shared with a wide range of stakeholders, advocacy leaders, clinicians, and global health experts. Their feedback was used to refine objectives, framework, and validate relevance across contexts.
Toolkit Assembly and Design
The final Toolkit integrates assessment instruments, such as a self-assessment survey covering the nine domains, as well as income-specific objectives and actionable policy avenues. Practical guidance for different stakeholder groups was included to help translate the framework into action.
Case Study Collection
To demonstrate real-world applicability, illustrative case studies were gathered from countries at different income levels, for each of the nine dimensions. These examples showcase how targeted interventions, across the framework’s domains, have advanced PM implementation.
Framework Description: The Nine Dimensions and Tiered Stratification
The PM Toolkit is based around a comprehensive nine-domain
framework. Each domain represents a pillar of system readiness
and policy action for PM:
1. Policy & Regulatory Frameworks: Establishing PM
enabling laws, standards, and regulations.
2. Patient-Centered Care & Equity: Ensuring that all
patients can access, understand, and benefit from PM equitably.
3. Healthcare Infrastructure & Workforce-Building
laboratories, diagnostic networks, and a trained health workforce.
4. Clinical Guidelines: Translating evidence into practice
through standardized protocols.
5. Access & Reimbursement: Addressing financial
barriers and creating sustainable coverage mechanisms.
6. Research & Innovation: Supporting discovery,
translation, and locally relevant clinical studies.
7. Education & Awareness: Building PM awareness
among providers, patients, and other stakeholder groups.
9. Public-Private Partnerships & Sustainability: Engaging stakeholders to ensure long-term viability.
While all domains are interdependent, the framework is designed to be adaptable. Countries may prioritize domains based on the prevalent and most pressing challenges, resources, and opportunities. The Toolkit segments guidance into three tiers based on WB income classifications:
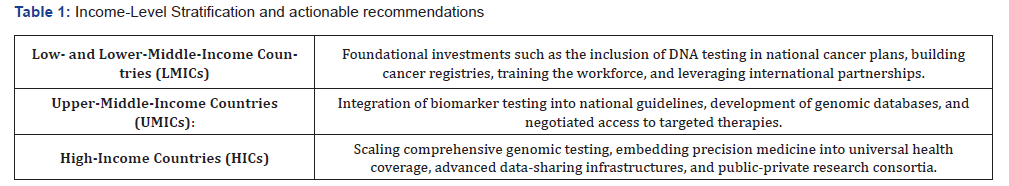
This stratification ensures that the Toolkit is globally relevant while remaining context sensitive. It acknowledges that while the vision of precision medicine is shared, the pathways to achieve it differ significantly depending on health system maturity and implementation capacity.
The PM Toolkit consists of:
• Executive Summary: Provides an overview of the
Toolkit framework and its contents.
• “How-To” Stakeholder Guide: Offers clear, practical
steps to help stakeholders engage meaningfully in advancing
precision medicine. It outlines how different groups, such as
policymakers, patient advocacy organizations, and medical
societies, can use the content and why it is relevant to their
specific roles.
• PM Toolkit Dimensions Summary: Summarizes the
nine essential domains for PM implementation.
• PM Country Readiness Framework: with Objectives
by Income Level Serves as a comprehensive assessment and
planning tool designed to help countries evaluate their current
PM readiness and develop targeted action plans.
• Country Self-assessment survey-by Income Level:
Helps to assess a country’s status on Precision Medicine across
the nine dimensions, identify areas for improvement, and link
these to tailored possible policy avenues to achieve that objective.
• Compilation of Case Studies: Provides country-specific
best practice examples for each dimension, organized by the three
income levels.
Strengths of the PM Toolkit
• Strong Evidence Base: Grounded in an extensive
literature review and expert validation, the Toolkit offers
evidence-informed guidance.
• Practical Policy Framework: The nine-domain
assessment structure provides a comprehensive, adaptable, and
actionable roadmap for stakeholders.
• Income-Level Stratification: By segmenting guidance
into three tiers, the Toolkit ensures relevance across diverse
health systems, from resource-limited to high-income settings.
• Real-World Applications: Case studies from Brazil,
India, and North Africa, among others, demonstrate feasibility
and impact.
• Stakeholder-Focused Approach: With dedicated
guidance for policymakers, advocacy groups, medical societies,
and the pharmaceutical sector, the Toolkit helps each stakeholder
understand its role in advancing PM.
• Implementation Science Focus: It bridges theory and
practice, translating frameworks into action through milestones,
surveys, and monitoring tools.
• Offers a data-driven, adaptable approach: Relevant
to over 190 health systems, the Toolkit enables stakeholders to
assess their country’s status and take targeted, realistic steps to
advance precision medicine readiness.
• Global Health Equity Lens: By embedding equity into
its tiered approach, the Toolkit directly addresses disparities in
access to precision medicine.
These strengths make the Toolkit not only a planning resource but also a catalyst for dialogue, alignment, and action across the global health ecosystem.
Policy Implications
1. Guiding Evidence-Based National Strategies:
Governments can use the PM Toolkit to conduct baseline
assessments, prioritize objectives, and design national precision
medicine roadmaps. This ensures investments are grounded in
local realities while aligned with global best practices.
2. Supporting Equitable Access: By tailoring the policy
paths to income levels, the Toolkit helps countries avoid unrealistic
models and instead focus on context-appropriate actions.
3. Facilitating Multi-Stakeholder Collaboration: The
Toolkit provides a common language and framework for dialogue
among policymakers, clinicians, advocacy groups, and industry.
This fosters alignment, reduces duplication, and enables cocreation
of sustainable solutions.
4. Embedding Precision Medicine in Broader Health
Reform: Precision medicine does not exist in isolation; it intersects
with digital health, universal health coverage, and innovative
ecosystems. The Toolkit helps connect PM implementation to
these wider reforms, amplifying impact.
5. Enabling Monitoring and Accountability: With its
self-assessment survey and milestone-based progress tracking,
the Toolkit empowers stakeholders to measure implementation,
celebrate progress, and course-correct where needed.
6. Informing Global Benchmarking and Donor
Engagement: Because it is aligned with widely recognized WB
classifications, the Toolkit enables benchmarking across countries
and regions. This makes it a valuable tool for international
organizations and donors seeking to target investments effectively.
Conclusion
The Precision Medicine Policy Assessment Toolkit for Oncology represents a timely and practical contribution to global health, PM, and cancer policy. By offering an evidence-based, nine-domain framework stratified by income level, it equips stakeholders to systematically assess readiness, set realistic priorities, and implement tailored actions. Its real-world case studies illustrate that meaningful progress is possible across all resource settings, provided strategies are context-sensitive and inclusive.
The strength of the Toolkit lies in its combination of rigor and pragmatism. It is grounded in evidence, designed for diverse stakeholders, adaptable across income levels, and focused on moving from framework to action. Importantly, it emphasizes global health equity by ensuring that even resource-limited countries have a clear pathway to advance precision medicine.
As cancer incidence continues to rise globally, the need to make precision medicine a standard of care, rather than a privilege, has never been greater. This Toolkit provides policymakers, advocacy groups, clinicians, and industry with the tools they need to support this transformation.
More information and access to the full Toolkit can be found on the Policy Wisdom LLC website. Stakeholders are encouraged to explore, apply, and share this resource to advance equitable precision medicine implementation worldwide.
This Toolkit is intended for use by policymakers, advocacy groups, and medical societies involved in the design, evaluation, or implementation of precision medicine policies and strategies. It is provided for informational purposes only and does not constitute clinical, legal, or regulatory advice.
References
- Litvinova Y, Taskén K, Frøstrup Hansen T, Dahl Steffensen K, Pajusalu S, et al. (2025) Transforming Cancer Care with Precision Oncology 31(1).
- Lip S, Padmanabhan S (2025) Introduction to precision medicine. Medicine 53(7): 476-482.
- Schwartzberg L, Kim ES, Liu D, Schrag D (2017) Precision Oncology: Who, How, What, When, and When not? American Society of Clinical Oncology Educational Book 37: 160-169.
- Radich JP, Briercheck E, Chiu DT, Menon MP, Sala Torra O, et al. (2022) Precision Medicine in Low- and Middle-Income Countries. Annual Review of Pathology 17: 387-402.
- Shih YCT, Pan IW, Teich N (2022) Global Challenges in Access to and Implementation of Precision Oncology: The Health Care Manager and Health Economist Perspective. American Society of Clinical Oncology Educational Book 42: 429-437.
- WEF (2021) Precision Medicine Readiness Principles Resource Guide: Care integration Pp: 1-19.
- WEF (2020) Precision Medicine Readiness Principles Resource Guide: Innovation Loop Pp: 1-35.
- Chanfreau Coffinier C, Peredo J, Russell MM, Yano EM, Hamilton AB, et al. (2019) A logic model for precision medicine implementation informed by stakeholder views and implementation science. Genetics in Medicine 21(5): 1139-1154.
- Eric Metreau, Kathryn Elizabeth Young, Shwetha Grace Eapen (2024) World Bank country classifications by income level for 2024-2025.






























