Background
Radioligand therapy (RLT) represents a major advance in targeted cancer treatment, delivering radiation directly to tumors while sparing healthy tissue [1-3]. Compared with chemotherapy, which is systemic and toxic, [4-6] or external beam radiation therapy (EBRT), which irradiates broader areas from outside the body, RLT offers a more precise and tolerable option that improves patient safety [7, 8].
Advances in targeted radionuclide therapy (TRT) have further refined RLT into an accurate, effective, and well-tolerated therapeutic option, with, for example, Lutetium-based (177Lu) therapies, combining predictable pharmacokinetics with improved benefit-risk profile [9-11]. Evidence from Phase III clinical trials [4,12] and real-world evidence confirm the efficacy, favorable benefit-risk profile with improved cost-effectiveness of RLT [4,13-15]. Standardized fixed-dose treatment regimens simplify and broaden applicability across diverse health systems, including those with limited resources [16-18]. Radiation exposure to the public and healthcare workers after RLT remains minimal and well below established safety thresholds, typically as low as two chest x-rays [19,20].
Yet, despite the growing global cancer burden, RLTs are currently available for only two indications: gastroenteropancreatic neuroendocrine tumors (GEP-NETs) and metastatic castrationresistant prostate cancer (mCRPC) [21]. Outdated and inconsistent policies affect RLT expansion across different domains, from discharge criteria, [19, 22-24] to infrastructure requirements, [9] and radioactive waste management protocols, [24] among others. Most strikingly, hospitalization requirements vary from discharge within hours, in some countries, to others imposing up to 96 hours of inpatient isolation (See Table 1). These divergences reflect policy choices rather than medical necessity and create unnecessary barriers, resulting in inequities in patient access and disincentives for institutions to expand RLT services [24].
This report aims to bridge the gap between clinical evidence and policy by providing actionable recommendations to expand equitable access to RLT by strengthening health system readiness, optimizing care models, and supporting safe and sustainable implementation. (Table 1)
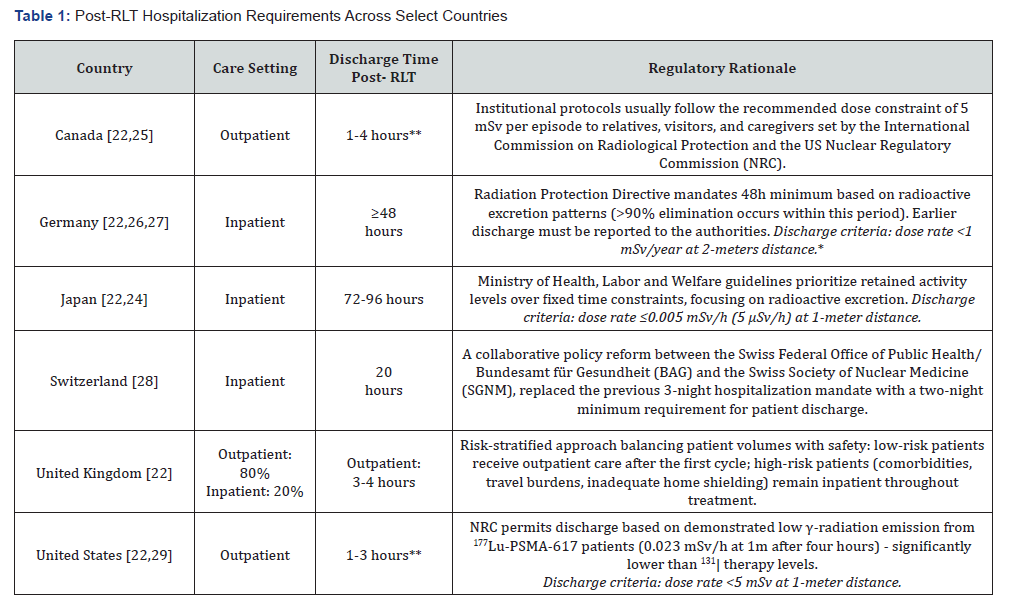
*Dose limit rate of <1 mSv/year originates from European Council Directive 2013/59/Euratom & International Commission on Radiological
Protection (IRCP) directives.
**Depending on institutional protocol.
Methodology
This report employed a broad approach to synthesize evidence on RLT radiation safety and hospitalization optimization. The methodology was designed to provide a structured assessment of current practices, identify policy gaps, and develop evidencebased recommendations for improving RLT accessibility while maintaining safety standards.
Research Design
The analysis used a narrative synthesis approach, integrating findings from diverse data sources to build a comprehensive picture of today’s RLT landscape. This method accommodated the varied nature of available evidence, from clinical trial data to regulatory documents, independent reports, and real-world practice patterns. Multiple sources were cross-referenced to ensure findings were reliable and valid, with results organized using the RLT Readiness Assessment Framework across seven focus areas [30].
Data Collection and Analysis Methods Literature Review
The analysis drew on a structured review of current evidence on RLT, focusing specifically on radiation safety, healthcare system readiness, and hospitalization practices. Searches of PubMed, Google Scholar, and institutional repositories used targeted keywords including ‘radioligand therapy’, ‘hospitalization’, ‘radiation safety’, and ‘health system readiness’. Given the rapidly evolving nature of RLT evidence, no date restrictions were applied to capture the most current developments.
The review also examined recent analyses commissioned by third parties, summarizing their findings in alignment with the broader evidence base. These sources were selected for the breadth and solidity of their research designs, which integrated different research methodologies to generate complementary insights across diverse contexts that enriched the overall evidence base. Source prioritization focused on materials that examined new evidence in the context of next-generation RLT, and health system implementation needs. Relevant data, frameworks, and recommendations underwent thematic synthesis to support discussions on optimizing RLT access while maintaining radiation safety.
Evidence Analysis
Policy gaps and challenges underwent identification through thematic analysis of the evidence collected. This analytical approach enabled a comprehensive assessment of policy barriers across multiple dimensions and identified practical reform opportunities that policymakers can implement in their national contexts. The specific focus areas that emerged from this analysis are presented in the Key Findings section.
Key Findings
This section includes the main findings organized according
to the seven focus areas and their corresponding policy gaps:
• Governance and policy frameworks
• Health system readiness and capacity
• Radiation safety
• Workforce development and training
• Financing, reimbursement, and economic assessment
• Evidence generation and data infrastructure
• Stakeholder education and awareness
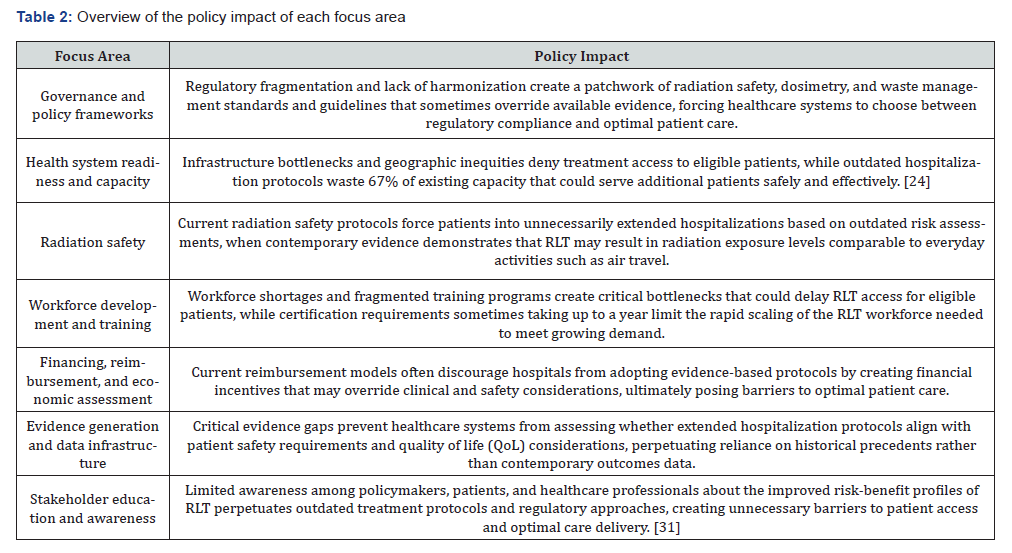
(Table 2) provides a summary of the policy impact of each of the seven focus areas.
Governance and Policy Framework for RLT
Fragmented governance frameworks result in patients with similar cancer diagnoses receiving dramatically different treatment experiences-from same-day discharge to mandatory 96-hour hospital stay-based solely on geography rather than medical need. While international frameworks such as the International Atomic Energy Agency (IAEA) Basic Safety Standards and the European Council Directive 2013/59/Euratom provide foundational radiation protection and operational requirements, [32,33] the absence of harmonized implementation guidelines allows each country to interpret these standards differently, creating a regulatory patchwork that prioritizes historical risk perceptions and conservative interpretations of risk-benefit profiles over patient welfare. This fragmentation directly contradicts the goals established by the IAEA, which in its 2023 resolution urged Member States to strengthen nuclear and radiation safety infrastructure, scientific capabilities, and international cooperation [34].
Critical Gaps in Policy and Policy Implementation
• Lack of updated, evidence-based radioprotection
guidelines reflecting actual 177Lu-based RLT risk-benefit profiles.
• Limited integration of RLT in national and regional
cancer-care frameworks despite growing clinical importance and
lack of dedicated committees or working groups at the national
level to guide RLT’s safe use in health systems.
• Across countries, there is no uniform, patient-centered
approach to post-RLT treatment assessment that informs
discharge decisions, with most relying solely on radiation safety
as the primary criterion.
• Lack of dedicated, harmonized regulations for the
local small-scale radiopharmaceutical preparation, leading to
inconsistent implementation of Good Manufacturing Practices
and potentially regional quality variability.
• Conflicting guidance on dosimetry requirements despite
clinical evidence supporting fixed-dose protocols.
• Lack of appropriate regulations for standardized
radiopharmaceutical waste disposal.
• Insufficient patient and patient advocacy group
involvement in RLT policy development processes.
Health System Readiness & Capacity for RLT
Health system readiness and infrastructure capacity represent critical determinants of equitable RLT access, with current limitations creating significant barriers to treatment availability across healthcare settings. The delivery of RLT requires specialized theranostics infrastructure encompassing radiationshielded treatment rooms, secure radiopharmaceutical storage areas, dose calibrators, radioligand imaging capabilities (e.g., 68Ga/18F-PSMA PET, 99mTc-PSMA SPECT/scintigraphy) and comprehensive waste management systems. These capabilities are often concentrated in urban areas, forcing patients to travel long distances or cross borders for treatment [31].
Economic modeling of hospital operations demonstrates that reducing hospitalization from 48-to72-hour protocols to 24 hours could increase patient throughput by 67%. This would enable typical 3-bed facilities to treat 612 patients annually instead of 367, while maintaining equivalent safety standards [24](b). However, healthcare systems continue operating under divergent approaches that limit access. In Germany and Japan, RLT is typically administered in inpatient settings using shielded rooms with attached washrooms, following conservative radiation safety protocols. In contrast, Australia and the United States routinely administer RLT on an outpatient basis, with patients discharged once radiation levels fall below 0.023 mSv/h at 1 meter, typically within four hours p.i. [9,35,36] Outpatient care is often delivered in standard nuclear medicine or infusion rooms, offering greater flexibility and efficiency, especially in high-demand settings.
Critical Gaps in Policy and Policy Implementation
• Absence of systematic health system readiness
assessments and data-driven capacity planning, particularly in
resource-constrained settings.
• Bottlenecks in PSMA-PET/CT imaging capacity and
other modalities of tumor expression assessment creating
cascading delays in the RLT treatment pathway.
• Lack of harmonized infrastructure requirements and
standards across healthcare systems.
• Underdeveloped referral networks resulting in
geographic inequities and delayed treatment access.
• Insufficient strategic investment in RLT infrastructure
relative to projected demand and clinical benefits.
Radiation Safety
Radiation safety is one of the central concerns in RLT scale-up and implementation. Research confirms that modern RLT technologies result in exposure levels well below safety thresholds requiring extended inpatient stays mandated in several countries (see Table 1). The clinical safety and efficacy of Lu-based treatments have been well established through landmark trials such as NETTER-1 [37] and VISION, [38] while evidence on radiation safety continues to accumulate. Studies report that discharging patients 48 hours after receiving an average dose of 6.3 gigabecquerel (GBq) resulted in a maximum public exposure dose of just 0.294 mSv, well below the 1 mSv/ year limit recommended by international guidelines [19]. When patients are released 24 hours p.i., the expected public dose remains [19]. Additional research demonstrates that radiation levels measured at 1 meter from patients had already fallen below 0.030 mSv/hour within 4-6 hours p.i., a threshold deemed safe for release [23]. These empirical measurements significantly diverge from the conservative discharge requirements widely practiced across countries, which fail to reflect the improvements in the risk-benefit profiles of RLTs.
Critical Gaps in Policy and Policy Implementation
• Lack of harmonized radiation exposure thresholds for
patients, caregivers, healthcare professionals, and the public
across regions during and after treatment.
• Absence of radiation safety exposure thresholds specific
to RLTs that reflect their actual risk-benefit profile and support
evidence-based discharge criteria.
RLT Workforce Development & Training
Workforce development and training are critical enablers of effective RLT implementation across healthcare systems [25]. The delivery of RLT depends on a multidisciplinary team of healthcare professionals, each with specialized competencies, whose coordination directly affects patient access, care quality, and healthcare system readiness. RLT practitioners encompass nuclear medicine physicians, urologists, oncologists, radiologists, nurses, pathologists, radiation safety officers, medical physicists and hospital administrators each contributing uniquely to the patient journey from diagnosis and referral to treatment and follow-up.
Current workforce limitations create cascading access barriers across the treatment pathway. With 21% of the nuclear medicine workforce in Europe expected to retire within five years, [39] and certification for handling radioactive materials typically requiring one year for untrained staff, healthcare systems face a growing gap between clinical demand and available expertise [24]. Simultaneously, safety guidelines mandate strict limits on staff radiation exposure-for example, nurses can attend approximately 110 sessions of ¹⁷⁷Lu-PSMA-617 per year [23, 24] to remain within the 1 mSv/year exposure limit defined by EURATOM [19]. Post-treatment patient exposure also contributes to the 1 mSv limit, [25] posing remarkable limitations and human resources management challenges, especially in settings with severe limitation in workforce and resources.
Critical Gaps in Policy and Policy Implementation
• Lack of harmonized, specialized training programs for
RLT practitioners across all healthcare disciplines involved in
patient care.
• Limited availability of multidisciplinary teams and
coordination frameworks for RLT delivery. Inadequate systematic
workforce planning mechanisms for RLT healthcare professionals.
RLT Financing, Reimbursement, & Economic Assessment
Economic analysis reveals that hospital financial outcomes vary dramatically based on hospitalization duration due to the underlying payment models. These financial pressures frequently conflict with clinical evidence and patient welfare, creating systematic barriers to optimal care delivery. Payment systems can either support or undermine evidence-based practice adoption, with profound implications for both healthcare system efficiency and patient access to life-extending treatments [24]. Estimates from financial modeling of RLT administration in a standard 3-bed facilities showed that reducing hospitalization from 48- to 72-hour protocols to 24-hour protocols generate annual hospital profits of approximately €510,700 (~US $600, 800) in Germany and JPY 224 million (~US$1.53 million) in Japan, while simultaneously enabling treatment of significantly more patients [24].
However, the financial impact varies significantly due to prevailing reimbursement mechanisms. Under Germany’s DRG system, hospitals anticipate a 20% reduction in reimbursement rates with shortened stays yet maintain profitability through increased patient volume. Conversely, Japan’s DPC model, which combines per-diem payments with procedure-specific fees, creates more favorable financial situation for reduced hospitalization [24]. The cost structure analysis reveals that the predominant expense component of RLT is the radiopharmaceutical itself, which amounts at JPY 6.2 million for six treatment cycles in Japan. Variable costs associated with extended stays contribute additional, unnecessary treatment expenses, further limiting system capacity and creating inefficiencies that could be eliminated with the implementation of evidence-based protocols [24].
Critical Gaps in Policy and Policy Implementation
• Current reimbursement structures penalize evidencebased
hospitalization protocols which allow for earlier discharge.
• Absence of RLT-specific HTA frameworks capturing full
value propositions and facilitating value-driven reimbursement.
• Limited real-world economic evidence validating the
theoretical efficiency gains of reduced hospitalization for RLT.
• Lack of consideration for patients’ financial burden
associated with extended hospitalization for RLT treatment.
RLT Evidence Generation & Data Infrastructure
Healthcare systems lack the real-world data (RWD) generation mechanisms needed to prove that shorter hospitalizations are safe, perpetuating outdated policies despite growing clinical and radiation safety evidence.
Despite specialized assessment tools such as the Functional Assessment of Cancer Therapy-Radionuclide Therapy (FACT-RNT) being developed to monitor relevant symptoms and toxicities in radiopharmaceutical trials, [40] implementation in routine practice remains sporadic. Critically, no prospective studies have systematically evaluated whether 24-hour, 48-hour, or 72- hour hospitalization protocols result in differential outcomes for patient safety, QoL, or healthcare system efficiency. Without integrated data systems linking diagnostic imaging results, treatment records, post-treatment monitoring, and long-term follow-up outcomes, policymakers cannot assess the full clinical and economic impact of different RLT delivery models.
Critical Gaps in Policy and Policy Implementation
• Limited availability of RWE for RLT.
• Insufficient policy and funding support for rigorous
research into differential outcomes for patient safety, QoL, or
healthcare system efficiency of different hospitalization protocols.
• Limited consideration for patient perspective and
preferences.
• Absence of policy requirement to systematically collect,
analyze, and share post-marketing RLT usage data, including
radiation exposure metrics, safety incidents, and compliance with
radiation protection standards.
Stakeholder Education & Awareness
Robust stakeholder education and public awareness are critical enablers for equitable and safe RLT implementation across healthcare systems. Despite growing therapeutic relevance and demonstrated clinical benefits, global understanding of RLT-particularly among policymakers, patients, and caregiversremains limited [25]. This knowledge gap affects not only informed decision-making but also regulatory support, societal perception, and the successful transition toward evidence-based care delivery models. Policymakers often have limited awareness of the full value and benefits of RLT, particularly regarding improved risk-benefit profiles of the most recent RLT treatments, like the Lu-based RLTs, and minimal post-treatment risk to the public. Similarly, patients and caregivers may not fully understand RLT’s safety and efficacy compared to conventional treatments, or the importance of adhering to radiation safety precautions postdischarge. This creates hesitancy, hindering treatment acceptance and limiting the shift toward shorter hospitalization models that could benefit both patients and healthcare systems [31].
Public and patient education is essential for any transition toward reduced post-RLT hospitalization. Comprehensive guidance must be provided to patients and caregivers regarding radiation safety measures, including avoiding prolonged close contact with vulnerable individuals (e.g., pregnant women, infants), safely managing waste, [41] and understanding the excretion kinetics of isotopes like 177Lu-PSMA-617 [24, 25, 42].
Critical Gaps in Policy and Policy Implementation
Insufficient awareness and understanding among the general public and key stakeholders, including policymakers, healthcare professionals and patients, regarding the value and benefits of RLT and radiation safety post-RLT administration.
High-Level Recommendations
Based on the policy gaps identified across seven focus areas and a comprehensive review of evidence, the following strategic recommendations provide actionable guidance for policymakers and other stakeholders to optimize RLT implementation while maintaining safety standards:
Governance and Policy Framework for RLT
• Establish global coordination mechanisms. Facilitate
the creation of a multi-stakeholder technical task force under
IAEA leadership, including EANM, national radiation authorities,
leading radiation academic and research institutions, and oncology
societies, to advance risk-based radioprotection frameworks that
reflect the actual risk-benefit profiles of RLT.
• Develop evidence-based discharge criteria. Promote
global consensus on updated discharge methodologies that
incorporate individual risk assessment and balance radiation
safety with patient-centered outcomes.
Example: The UK successfully transitioned to outpatient
treatment for most patients based on evidence and clinical
leadership (see Best Practices in Appendix 1) [24, 28].
• Integrate RLT into national cancer frameworks. Support
health ministries in recognizing RLT as integral to cancer care and
establishing national RLT advisory committees for its integration
into national cancer control plans (NCCPs).
Example: Belgium developed a national RLT plan through
multistakeholder collaboration (see Best Practices in Appendix 1)
[43].
• Harmonize regulatory approaches. Facilitate
collaboration at the global level between multisectoral
stakeholders and regulatory agencies to standardize small-scale
radiopharmaceutical preparation regulations and establish
suitable, evidence-based radiopharmaceutical waste management
protocols.
• Include patient voices in policy development. Create
structured mechanisms for patient advocacy group involvement
in the RLT policymaking to ensure transparency, trust, and
alignment with patient needs.
Health System Readiness & Capacity for RLT
• Develop readiness assessment frameworks. Develop
systematic health system evaluation tools with data-driven
capacity planning to ensure equitable access to RLT, particularly
in resource-constrained settings.
• Implement systematic capacity monitoring. Develop
and adopt systematic methodologies to assess and track imaging
capacity bottlenecks over time, with data collected globally to
assess progress and inform capacity planning decisions.
• Establish minimum infrastructure standards. Promote
global consensus on basic minimum infrastructure requirements,
focusing on essential elements for safe and effective RLT delivery
adaptable to local resource contexts in LMICs.
• Support the establishment of an international
multistakeholder coalition that supports existing efforts and
provide countries with practical guidance to scale up RLT capacity,
including facility requirements, technical assistance, and referral
network development.
• Build the economic case for investment. International
organizations and academic institutions involved in RLT should
partner to develop the economic case for global investments
in RLT and advocate for inclusion in the scope of multilateral
development banks, bilateral aid institutions, and other
international financing mechanisms.
RLT Workforce Development & Training
• Support integration of workforce assessment into
readiness frameworks. Ensure that critical resources developed
for countries to assess their RLT readiness include comprehensive
healthcare workforce capacity and training evaluations to
facilitate systematic assessment of workforce capacity gaps.
• Develop comprehensive training guidance. Ensure that
international multistakeholder coalition guidance for RLT capacity
scaling includes detailed healthcare workforce requirements, skill
specifications, and frameworks for developing education curricula
and recurrent training opportunities.
Example: Europe’s INSPIRE Program provides a proactive,
system-wide approach to workforce sustainability (see Best
Practices in Appendix 1) [39].
• Support training material development. Facilitate
collaboration between international organizations, academic
institutions, and funding agencies to develop education and
training materials that support countries in scaling up RLT
capacity.
Radiation Safety
• Develop comprehensive safety protocols review.
Collaborate with leading international organizations such as
the Organization for Economic Co-operation and Development
(OECD), Nuclear Energy Agency (NEA) and key RLT stakeholders
to develop a comprehensive review report on radiation safety
protocols implemented by frontrunner countries (UK, US,
Australia), including detailed analyses of lessons learned and
actionable guidance for adaptation to different national contexts.
• Create permanent coordination mechanisms. Support
the establishment of a Standing Radiation Safety Coordination
Group under IAEA auspices, modeled on European Heads of
Medicines Agencies or African CDC to enable structured dialogue
and cooperation among national regulators and implementing
agencies.
• Create awareness-raising materials. Promote the
development of evidence dissemination materials through
multistakeholder coalitions highlighting progress in the RLT
field - particularly the evolving benefit-risk profile of RLTs-to
strengthen stakeholder understanding and prepare for evidencebased
policies.
RLT Financing, Reimbursement, & Economic Assessment
• Support appropriate payment models. Advocate for
the development and implementation of payment models that
accelerate RLT integration and favor cost-containment, through
evidence-based discharge criteria.
Example: Switzerland conducted pilot projects assessing 20-
hour admission feasibility and revised incentive schemes to remove
systemic barriers (see Best Practices in Appendix 1) [28].
• Establish specialized HTA frameworks. Support
development of theranostics-specific evaluation criteria
capturing diagnostic and therapeutic value, healthcare system
capacity gains, and patient QoL improvements within integrated
evaluation frameworks.
Example: Belgium created the Technical Council for
Radioisotopes as an independent evaluation body (see Best Practices
in Appendix 1) [43].
• Advocate for standardized economic data collection.
Support requirements for healthcare institutions to capture
comprehensive economic outcomes, including direct costs,
system capacity improvements, and patient QoL measures under
different hospitalization scenarios.
• Support patient financial impact research. Advocate for
prospective programs comparing economic outcomes between
traditional extended hospitalization and evidence-based early
discharge protocols across multiple healthcare systems.
RLT Evidence Generation & Data Infrastructure
• Create global RLT research coordination mechanisms.
Advocate for the establishment of research coordination
mechanisms under the leadership of the IAEA, World Health
Organization, or Organization for Economic Co-operation and
Development/Nuclear Energy Agency to define global research
priorities, harmonize data collection protocols, and synthesize
real-world evidence and patient-reported outcomes.
• Support the development of innovative financing for
research. Explore options to secure financing for evidencegeneration
through blended models leveraging: (i) international
development bank allocations under health systems innovation
frameworks; (ii) voluntary member state contributions for nuclear
medicine capacity-building; and (iii) strategic philanthropic
partnerships with global health donors (e.g., Bill & Melinda
Gates Foundation, Wellcome Trust, Gavi-style consortia). Explore
innovative options such as a Radiopharmaceutical Innovation
Fund for multi-country implementation research.
• Expand safety research in diverse settings. Prioritize
evidence generation across LMICs and proactively disseminate
findings to support scalable protocol adoption.
Stakeholder Education & Awareness
• Support targeted awareness campaigns. Partner with
leading patient advocacy organizations to design RLT-focused
sessions at major patient engagement events highlighting the
improvements in the risk-benefit profiles of RLT.
• Engage scientific and policy publications. Work through
multisectoral stakeholder coalitions to ensure systematic
inclusion of RLT content in high-reach journals and professional
networks, raising awareness among decision-makers, payers, and
healthcare professionals.
• Promote country-specific material development. Codevelop
tailored awareness resources with advocacy groups,
clinical leaders, and communication specialists for use at national
and subnational levels.
Conclusion
Radioligand therapy (RLT) faces a policy paradox: strong clinical evidence supports its safety and efficacy, yet outdated regulations and misaligned incentives continue to restrict access. Instead of evidence gaps, barriers stem from institutional inertia, excessive hospitalization requirements, and fragmented frameworks that strain health system capacity, inflate costs, and slow adoption. These inefficiencies also delay the generation of real-world data that could further strengthen the case for reform, perpetuating a cycle of limited access.
Experiences from focus countries show that policy change is possible when regulators prioritize contemporary evidence over precedent. Their approaches highlight key enablers of progress: evidence-based regulatory alignment, active clinical leadership, multi-stakeholder engagement, financial reform, and structured implementation guidance. To achieve sustainable integration, immediate priorities include forming an IAEA-led task force to modernize global radioprotection frameworks, conducting readiness assessments to address workforce and infrastructure gaps, and adopting evidence-based discharge criteria to enable shorter, safer hospital stays. Medium-term reforms should integrate RLT into national cancer control plans, harmonize regulatory standards, and align payment systems to support efficient and equitable delivery.
Financial Declaration
This policy report was developed by Policy Wisdom, initiated and financially supported by Novartis. The views expressed are based on an independent analysis of publicly available evidence, expert opinions, and a synthesis of research findings. Input from Novartis experts was incorporated to ensure technical accuracy and completeness during the review and finalization of the report.
Acknowledgement
The authors thank Amit Mehto, Elvira Forconesi, and Gina De Villiers of Policy Wisdom for their assistance with data analysis and manuscript drafting, conducted under the direction of the authors and funded by Novartis.
Appendices
Appendix 1: Best Practices
Several countries have successfully navigated the complex challenges associated with RLT implementation, offering valuable lessons in policy innovation and system readiness. The following examples demonstrate how different healthcare systems have successfully implemented evidence-based RLT policies, overcoming regulatory barriers and optimizing patient care delivery. These cases provide concrete evidence that policy reform is both feasible and beneficial, offering practical models for other countries to adapt to their specific contexts.
Switzerland
Switzerland transformed its hospitalization requirements through collaborative policy reform between the Federal Office of Public
Health (BAG) and the Swiss Society of Nuclear Medicine (SGNM). The previous 3-night hospitalization mandate was replaced with
flexible protocols following a systematic pilot project that demonstrated safety and feasibility. New regulations implemented in 2024
eliminated the financial incentives for the minimum hospital stay of three nights and legally mandated a two-night (48 hours) hospital
stay for 177Lu-therapies. A pilot study started by BAG and SGNM to evaluate discharge after 20 hours. Implementation was supported
through patient radiation protection leaflets developed by BAG and SGNM [28].
• Key success factors: Strong professional advocacy by SGNM and key opinion leaders, regulatory receptiveness, evidencebased
pilot testing, and coordinated financial incentive reform.
• Main Stakeholders: Federal Office of Public Health (BAG), Swiss Society of Nuclear Medicine (SGNM), Swiss Association of
Physicians (FMH), key opinion leaders, and hospitals
United Kingdom
The UK transformed RLT delivery from inpatient to outpatient models through evidence-based protocol implementation. King’s College
Hospital pioneered the transition, supported by 2019 audit data demonstrating equivalent safety outcomes. Most centers now use
day-case models with discharge guided by radiation thresholds (<25 μSv/h at 1m) rather than time-based requirements [28]. The
transition addressed healthcare capacity pressures while improving patient experience. Today, 80% of RLT is outpatient-based, [22]
demonstrating successful system-wide transformation.
• Key Success Factors: Clinical leadership, audit-supported evidence generation, risk-stratified protocols, and healthcare
capacity optimization priorities.
• Main Stakeholders: NHS centers, King’s College Hospital, Medicines and Healthcare products Regulatory Agency (MHRA),
clinical researchers, and patient groups.
United States
Federal guidance enables evidence-based outpatient protocols through clear regulatory frameworks. Nuclear Regulatory Commission
(NRC) rules permit discharge when exposure limits remain below 5 mSv, typically achieved within 1-3 hours post-treatment [22, 29,
44]. The approach prioritizes actual radiation exposure data over historical precedents, supported by robust evidence demonstrating
low dose rates following administration [22, 29].
• Key Success Factors: Clear federal guidance, evidence-based safety thresholds, institutional implementation flexibility, and
systematic safety monitoring.
• Main Stakeholders: Nuclear Regulatory Commission (NRC), hospital systems, medical physicists, and clinical researchers.
Italy
Legislative reform shifted from time-based to risk-based discharge criteria through comprehensive policy change [44]. The 2020
Legislative Decree 101 eliminated mandatory hospitalization except for high-dose iodine-131 therapies, implementing individual risk
assessment protocols with 6-hour minimum monitoring. Discharge involves radiometric evaluation and personalized safety protocols.
Nationwide guidelines ensure standardized application across facilities [45].
• Key Success Factors: Legislative support, evidence-based risk stratification, national professional collaboration, and
systematic implementation guidance.
• Main Stakeholders: Italian Ministry of Health, Italian Association of Nuclear Medicine (AIMN), Italian Association of Medical
Physics (AIFM), Italian Scientific Institutes for Research, Hospitalization and Healthcare (IRCCS), and radiation protection
specialists.
Japan
Payment system adaptation supported evidence-based protocol transition from inpatient to hybrid care models. Initial Xofigo®
administration under traditional inpatient settings evolved to outpatient therapy as clinical confidence grew. The Diagnosis Procedure
Combination (DPC) system financially rewards shorter stays, aligning economic incentives with evidence-based protocols. This
experience also highlighted the critical role of workforce development and infrastructure planning [24].
• Key Success Factors: Payment system alignment, institutional adaptation flexibility, clinical confidence building through
experience.
• Main Stakeholders: Hospitals, payer agencies, Ministry of Health, and nuclear medicine departments.
Belgium
Dedicated reimbursement framework overcame financing barriers through targeted policy innovation. The Technical Council for
Radioisotopes (TCRI) within national healthcare institute RIZIV/INAMI specifically addresses RLT reimbursement challenges. Belgium
became the first EU country reimbursing 177Lu-PSMA-617 (Pluvicto®) in April 2024, following national RLT plan development with
multi-stakeholder input [43].
• Key Success Factors: Dedicated institutional infrastructure, early stakeholder alignment, multi-stakeholder planning
processes, early reimbursement pathway establishment, and integrated capacity development support.
• Main Stakeholders: TCRI, RIZIV/INAMI, hospitals, oncologists, nuclear medicine physicians, radiopharmaceutical suppliers,
patient organizations, and regulatory authorities.
Inspire Program (Europe)
The European Association of Nuclear Medicine (EANM) launched INSPIRE in 2024 as a comprehensive workforce development
initiative addressing anticipated nuclear medicine professional shortages. The program employs a three-pillar strategy: awareness
outreach to attract new professionals, hands-on educational experiences for skill development, and innovation to modernize training
pathways. Implementation leverages digital engagement platforms and targeted grant schemes supporting national initiatives and
student engagement programs [39].
• Key Success Factors: Proactive workforce planning, multi-modal educational approaches, digital platform utilization, and
coordinated national implementation support.
• Main Stakeholders: EANM, national nuclear medicine societies, universities, student associations, patient groups, and
industry partners.
Appendix 2: Key Terms and Concepts
Alara Principle (As Low as Reasonably Achievable)
A fundamental radiation protection principle that requires that radiation exposures be kept as low as reasonably achievable, considering relevant economic and societal factors. These principal guides decision-making in radiation protection by requiring optimization of protection measures to minimize doses while considering practical constraints, costs, and benefits. In the context of RLT, ALARA influences protocols for patient hospitalization duration, shielding requirements, and discharge criteria.
Diagnosis-Related Group (DRG)
A patient classification system that groups hospital cases into categories based on clinical conditions and procedures that are expected to require similar hospital resources. Under DRG-based payment systems, hospitals receive a fixed payment for each patient case within a specific DRG category.
Diagnosis Procedure Combination (DPC)
A Japanese hospital payment system that combines elements of per-diem and fee-for-service reimbursement. Under DPC, hospitals receive a daily fixed payment that covers basic hospital services, with decreasing rates for longer stays, plus additional fees for specific procedures and expensive medications. This hybrid approach aims to balance cost control with appropriate compensation for resource intensive treatments.
Discharge Criteria
Specific measurable parameters, for RLT primarily radiation dose rates, that must be met before a patient can be safely released from medical supervision. These criteria typically specify maximum admissible radiation exposure levels at defined distances from the patient (e.g., ≤0.005 mSv/hour at 1 meter in Japan, or <1 mSv/year at 2 meters in Germany). Discharge criteria vary significantly between countries, directly impacting hospitalization duration and healthcare system capacity needs.
Dosimetry
The scientific discipline concerned with the measurement, calculation, and assessment of ionizing radiation doses received by the human body. In RLT context, dosimetry encompasses both radiation protection dosimetry (measuring external radiation exposure to healthcare workers and the public) and therapeutic dosimetry (calculating radiation doses delivered to tumors and healthy organs).
Fixed-dose Protocol
A standardized treatment regimen where all eligible patients receive the same predetermined amount of radiopharmaceutical activity, regardless of individual characteristics such as body weight, tumor burden, or organ function.
Health Technology Assessment (HTA)
A multidisciplinary process that systematically evaluates clinical effectiveness, cost-effectiveness, and broader impact of health technologies, including medicines, medical devices, and procedures. HTA bodies analyze scientific evidence to inform coverage and reimbursement decisions, considering factors such as comparative effectiveness, budget impact, and societal values.
Multidisciplinary Team (MDT)
A collaborative group of healthcare professionals from different specialties who work together to plan and deliver comprehensive patient care. In RLT, the MDT typically includes urologists or uro-oncologists, medical or clinical oncologists, nuclear medicine physicians, radio pharmacists, medical physicists, specialized nurses, and radiation safety officers. The MDT reviews patient cases, determines treatment eligibility, coordinates care delivery, monitors treatment response, and manages adverse events, ensuring that complex treatment decisions incorporate diverse clinical perspectives.
Millisievert (mSv)
The mSv (millisievert) is a unit of ionizing radiation dose. It is a subunit of the sievert (Sv), which is the International System of Units (SI) measurement used to express the biological effect of ionizing radiation on human tissue. One millisievert is one-thousandth of a sievert (1 mSv=0.001Sv). The sievert measures the “effective dose” of radiation, which means it quantifies the risk of biological harm from an absorbed dose, considering both the type of radiation and the varying sensitivities of different tissues and organs in the body. The mSv is commonly used because a full sievert is a very large dose and rare outside of accidents or medical treatment; typical medical and environmental exposures are often in the range of microsieverts (μSv) or millisieverts (mSv) [46].
Post-infusion (p.i.)
The time immediately following the intravenous administration of a radioligand therapy agent. This period is critical for radiation safety considerations as the patient’s body contains the highest levels of radioactivity, with gradual elimination occurring primarily through renal excretion. Radiation exposure measurements during the post-infusion period (e.g., 4 hours p.i., 24 hours p.i.) inform discharge decisions and safety protocols.
Radioligand Therapy (RLT)
A precision cancer treatment modality that combines targeted molecular therapy with therapeutic radiation. RLT consists of three components: i) a ligand that binds target receptors and/or other biomarkers with high affinity; ii) a chelator and linker that enable the attachment of a radionuclide to the ligand, and iii) a radionuclide/radioisotope that enables the visualization or treatment of tumor lesions. This approach enables selective irradiation of cancer cells while minimizing exposure to healthy tissues, offering therapeutic benefits for patients with advanced cancers.
Real-World Evidence (RWE)
Clinical evidence regarding the usage and potential benefits or risks of a medical product derived from analysis of real-world data collected outside the controlled environment of clinical trials. RWE sources include electronic health records, claims databases, patient registries, and patient-reported outcome measures. For RLT, RWE provides insights into treatment patterns, effectiveness in diverse patient populations, long-term risk-benefit profiles, and healthcare resource utilization, complementing clinical trial data and informing policy decisions.
Theranostics
An integrated approach to personalized medicine that combines diagnostic imaging and targeted therapy using the same or similar molecular targeting vectors. In this paradigm, a diagnostic radiopharmaceutical (e.g., 68Ga-PSMA for PET imaging) first identifies whether a patient’s tumor expresses the target of interest, followed by a therapeutic radiopharmaceutical (e.g., 177Lu-PSMA) that delivers treatment to the same target.
Theranostics Infrastructure
The specialized facilities, equipment, and systems required for the safe and effective delivery of theranostic procedures. Essential components include radiation-shielded treatment rooms with dedicated ventilation systems, secure radiopharmaceutical storage areas, dose calibrators and radiation monitoring equipment, waste management systems for radioactive materials, imaging equipment (PET/ CT, SPECT/CT), and information systems for scheduling and tracking. Infrastructure requirements vary based on local regulations and service delivery models (inpatient vs. outpatient).
Appendix 3: Glossary of acronyms
ADCs: Antibody Drug Conjugates; ALARA: As Low as Reasonably Achievable; AR: Androgen Receptor; CT: Computed Tomography; DPC: Diagnosis Procedure Combination; DRG: Diagnosis-Related Group; EANM: European Association of Nuclear Medicine; EBRT: External Beam Radiation Therapy; EMA: European Medicines Agency; EURATOM: European Atomic Energy Community; FACT-RNT: Functional Assessment of Cancer Therapy-Radionuclide Therapy; FDA: Food and Drug Administration (United States); GBq: Gigabecquerel; GEPNETs: Gastroenteropancreatic Neuroendocrine Tumors; HTA: Health Technology Assessment; IAEA: International Atomic Energy Agency; ICRP: International Commission on Radiological Protection; LMICs: Low- and middle-income Countries; 177Lu: Lutetium-177; MCRPC: Metastatic Castration-Resistant Prostate Cancer; MoA: Mechanism of Action; MDT: Multidisciplinary Team; MDTs: Multidisciplinary Teams; MHRA: Medicines and Healthcare products Regulatory Agency; MTBs: Multidisciplinary Tumor Boards; MHLW: Ministry of Health, Labour and Welfare (Japan); mSv: Millisievert; mSv/h: Millisieverts Per Hour; μSv: Microsievert; NETs: Neuroendocrine Tumors; NICE: National Institute for Health and Care Excellence (United Kingdom); NRC: Nuclear Regulatory Commission; OECD: Organization for Economic Co-operation and Development; PET: Positron Emission Tomography; PET/CT: Positron Emission Tomography/Computed Tomography; p.i.: post-infusion; PSA: Prostate-Specific Antigen; PSMA: Prostate-Specific Membrane Antigen; QoL: Quality of Life; RLI: Radioligand Imaging; RLT: Radioligand Therapy; RWD: Real World Data; RWE: Real-World Evidence; SGNM: Swiss Society of Nuclear Medicine; SNMMI: Society of Nuclear Medicine and Molecular Imaging; SPECT-CT: Single Photon Emission Computed Tomography; Sv: Sievert ; TRT: Targeted Radionuclide Therapy; WHO: World Health Organization
References
- Holik HA, Ibrahim FM, Elaine AA, Putra BD, Achmad A, et al. (2022) The Chemical Scaffold of Theranostic Radiopharmaceuticals: Radionuclide, Bifunctional Chelator, and Pharmacokinetics Modifying Linker. Molecules 27(10): 1-34.
- Creating a ready health system for radioligand therapy in the US Pp: 1-4.
- Dadgar H, Pashazadeh A, Norouzbeigi N, Assadi M, Al Balooshi B, et al. (2025) Targeted radioligand therapy: physics and biology, internal dosimetry and other practical aspects during 177Lu/225Ac treatment in neuroendocrine tumors and metastatic prostate cancer. Theranostics 15(10): 4368-4397.
- Sartor O, Bono J de, Chi KN, Fizazi K, Herrmann K, et al. (2021) Lutetium-177–PSMA-617 for Metastatic Castration-Resistant Prostate Cancer. New England Journal of Medicine 385(12): 1091-1103.
- Morris MJ, Castellano D, Herrmann K, de Bono JS, Shore ND, et al. (2024) 177Lu-PSMA-617 versus a change of androgen receptor pathway inhibitor therapy for taxane-naive patients with progressive metastatic castration-resistant prostate cancer (PSMAfore): a phase 3, randomized, controlled trial. The Lancet 404(10459): 1227-1239.
- Hofman MS, Emmett L, Sandhu S, Iravani A, Buteau JP, et al. (2024) Overall survival with [177Lu] Lu-PSMA-617 versus cabazitaxel in metastatic castration-resistant prostate cancer (TheraP): secondary outcomes of a randomized, open-label, phase 2 trial. The Lancet Oncology 25(1): 99-107.
- Yeong CH, Cheng M hua, Ng KH (2014) Therapeutic radionuclides in nuclear medicine: Current and future prospects. Journal of Zhejiang University: Science B 15(10): 845-863.
- Zhang Z, Liu X, Chen D, Yu J (2022) Radiotherapy combined with immunotherapy: the dawn of cancer treatment. Signal Transduction and Targeted Therapy 7(1): 1-34.
- Ladrière T, Faudemer J, Levigoureux E, Peyronnet D, Desmonts C, et al. (2023) Safety and Therapeutic Optimization of Lutetium-177 Based Radiopharmaceuticals. Pharmaceutics 15(4): 1-22.
- Saracyn M, Durma AD, Bober B, Kołodziej M, Lubas A, et al. (2022) Long-Term Complications of Radioligand Therapy with Lutetium-177 and Yttrium-90 in Patients with Neuroendocrine Neoplasms. Nutrients 15(1): 1-11.
- Shah HJ, Ruppell E, Bokhari R, Aland P, Lele VR, et al. (2023) Current and upcoming radionuclide therapies in the direction of precision oncology: A narrative review. European Journal of Radiology Open 10: 1-19.
- Strosberg J, El Haddad G, Wolin E, Hendifar A, Yao J, et al. (2017) Phase 3 Trial of 177Lu-Dotatate for Midgut Neuroendocrine Tumors. New England Journal of Medicine 376(2): 125-135.
- Strosberg J, Wolin E, Chasen B, Kulke M, Bushnell D, et al. (2018) Health-Related Quality of Life in Patients with Progressive Midgut Neuroendocrine Tumors Treated With 177Lu-Dotatate in the Phase III NETTER-1 Trial. Journal of Clinical Oncology 36(25): 2578-2584.
- Recommendations (2018) Lutetium (177Lu) oxodotreotide for treating unresectable or metastatic neuroendocrine tumors. Guidance NICE Pp: 1-21.
- Mehrens D, Kramer KKM, Unterrainer LM, Beyer L, Bartenstein P, et al. (2023) Cost-Effectiveness Analysis of 177Lu-PSMA-617 Radioligand Therapy in Metastatic Castration-Resistant Prostate Cancer. Journal of the National Comprehensive Cancer Network 21(1): 43-50.
- Konijnenberg M, Herrmann K, Kobe C, Verburg F, Hindorf C, et al. (2021) EANM position paper on article 56 of the Council Directive 2013/59/Euratom (basic safety standards) for nuclear medicine therapy. European Journal of Nuclear Medicine and Molecular Imaging 48(1): 67-72.
- Aerts A, Eberlein U, Holm S, Hustinx R, Konijnenberg M, et al. (2021) EANM position paper on the role of radiobiology in nuclear medicine. European Journal of Nuclear Medicine and Molecular Imaging 48(11): 3365-3377.
- Dieudonné A, Bailly C, Cachin F, Edet Sanson A, Kraeber Bodéré F, et al. (2024) Dosimetry for targeted radionuclide therapy in routine clinical practice: experts’ advice vs. clinical evidence. European Journal of Nuclear Medicine and Molecular Imaging 51(4): 947-950.
- Kurth J, Krause BJ, Schwarzenböck SM, Stegger L, Schäfers M, et al. (2018) External radiation exposure, excretion, and effective half-life in 177Lu-PSMA-targeted therapies. EJNMMI Research 8(1): 1-11.
- Garje R, Hope TA, Rumble RB, Parikh RA, et al. (2023) Systemic Therapy Update on 177Lutetium-PSMA-617 for Metastatic Castration-Resistant Prostate Cancer: ASCO Guideline Rapid Recommendation Q and A. JCO Oncology Practice 19(3): 132-135.
- George SC, Samuel EJJ (2023) Developments in 177Lu-based radiopharmaceutical therapy and dosimetry. Frontiers in Chemistry 11: 1-13.
- Novartis (2024) Internal analysis on Time Spent in Hospital for RLT.
- Demir M, Abuqbeitah M, Uslu Beşli L, Yildirim O, Yeyin N, et al. (2016) Evaluation of radiation safety in 177Lu-PSMA therapy and development of outpatient treatment protocol. Journal of Radiological Protection 36(2).
- Novartis (2024) Internal Analysis on Radio safety and Hospitalization Optimization.
- Mench A, Winters C, Mittra E, Szidonya L, Hess C, et al. (2025) Navigating Radiation Safety After Radiopharmaceutical Therapies: Proposed Workflow and Essential Guidelines for Nonspecialists. Journal of Nuclear Medicine 66(7): 990-994.
- RS Handbuch Stand 07/14 (2015) Durchführung der Strahlenschutzverordnung (StrlSchV) Strahlenschutz in der Medizin-Richtlinie zur Strahlenschutzverordnung (StrlSchV) Pp: 1-82.
- (2011) GMBl Nr 44-47 2011. FragDenStaat Pp: 1-88.
- Novartis (2024) Internal Analysis on Country Best Practices.
- Calais J, Eulau SM, Gardner L, Hauke RJ, Kendi AT, et al. (2023) Incorporating radioligand therapy in clinical practice in the United States for patients with prostate cancer. Cancer Treatment Reviews 115: 1-12.
- RLT Readiness assessment framework-Health System Readiness.
- Merkel C, Whicher CH, Bomanji J, Herrmann K, Ćwikła J, et al. (2020) Realizing the potential of radioligand therapy: policy solutions for the barriers to implementation across Europe. European Journal of Nuclear Medicine and Molecular Imaging 47(6): 1335-1339.
- European Commission (2014). Radiation Protection and Safety of Radiation Sources: International Basic Safety Standards Pp: 1-436.
- (2013) Directive 2013/59.European union. EUR-Lex.
- International Atomic Energy Agency (2023) GC (67)/RES/DEC (2023) Resolutions and Other Decisions of the General Conference Sixty-Seventh Regular Session Pp: 1-150.
- Benke RR, Hamby DM, Boozer DL, Rose CT, Mangini CD, et al. (2021) Activity Thresholds, Patient-Specific Modifying Factors, Breastfeeding Interruption Times, and Other Supporting Data. RCD Radiation Protection Associates Pp: 1-133.
- Calais PJ, Turner JH (2014) Radiation safety of outpatient 177Lu-octreotate radiopeptide therapy of neuroendocrine tumors. Annals of Nuclear Medicine 28(6): 531-539.
- (2022) A Study Comparing Treatment With 177Lu-DOTA0-Tyr3-Octreotate to Octreotide LAR in Patients with Inoperable, Progressive, Somatostatin Receptor Positive Midgut Carcinoid Tumors (NETTER-1). ClinicalTrials.gov.
- (2025) Study of 177Lu-PSMA-617 In Metastatic Castrate-Resistant Prostate Cancer. ClinicalTrials.gov.
- Inspire by EANM. The EANM Community.
- Calais J, Morris MJ, Kendi AT, Kalebasty AR, Tutrone R, et al. (2024) Best Patient Care Practices for Administering PSMA-Targeted Radiopharmaceutical Therapy. Journal of Nuclear Medicine 65(11): 1666-1671.
- Kratochwil C, Fendler WP, Eiber M, Hofman MS, Emmett L, et al. (2023) Joint EANM/SNMMI procedure guideline for the use of 177Lu-labeled PSMA-targeted radioligand-therapy (177Lu-PSMA-RLT). European Journal of Nuclear Medicine and Molecular Imaging 50(9): 2830-2845.
- de Bakker M, Dominicus N, Meeuwis A, Janssen M, Konijnenberg MW, et al. (2023) Urinary excretion kinetics of [177Lu] Lu-PSMA-617. European Journal of Nuclear Medicine and Molecular Imaging 50(12): 3572-3575.
- Deroose PC, Leuven KU. RLT Academy Project Result 4 Policy Recommendations-To Ensure Wider Uptake of Radioligand Therapies in Europe Pp: 1-34.
- Zagni F, Vetrone L, Farolfi A, Vadalà M, Rizzini EL, et al. (2024) Feasibility of 177Lu-PSMA Administration as Outpatient Procedure for Prostate Cancer. Journal of Nuclear Medicine 65(12): 1848-1849.
- Anna B, Carlo C, Laura A, Sara F, Mahila Esmeralda F, et al. Aspetti di radioprotezione nelle terapie con 177Lu-DOTATATE e 177Lu-PSMA-617 Pp: 1-26.
- National Environment Agency. Health Effects of Ionising Radiation on People.






























