Abstract
Introduction: Severe Acute Respiratory Syndrome (SARS), also known as acute respiratory distress syndrome (ARDS), represents a critical clinical condition characterized by refractory hypoxemia, bilateral pulmonary infiltrates and decreased lung compliance. Mechanical ventilation is an essential intervention in the management of SARS, aiming to ensure adequate oxygenation and minimize the risk of ventilator-induced lung injury (VILI).
Objective: To perform a systematic review of the scientific evidence on the effects of Positive End-Expiratory Pressure in Severe Acute Respiratory Syndrome, seeking to provide support for evidence-based clinical practice and contribute to the optimization of ventilatory management of these critically ill patients.
Methods: This study is a systematic review, classified as exploratory and descriptive. The research was carried out through bibliographic research in electronic databases on methods associated with SLR (Systematic Literature Review) and applications of SMARTER (Simple Multi-Attribute Rating Technique using Exploiting Rankings).
Results: A comprehensive systematic search of the literature yielded a total of 4490 articles related to the topic addressed, of which 19 articles were eligible to compose this systematic review.
Conclusion: Although PEEP is a valuable tool in the ventilatory support of patients with SARS, its application should be personalized, considering the individual characteristics of each patient.
Keywords: Positive End-Expiratory Pressure (Peep); severe acute respiratory syndrome (Sars); Mechanical Ventilation; Oxygenation
Abbreviations: SARS: Severe Acute Respiratory Syndrome; ARDS: Acute respiratory distress syndrome; SMARTER: Simple Multi-Attribute Rating Technique using Exploiting Rankings; VILI: Ventilator-induced lung injury; PEEP: Positive End-Expiratory Pressure
Introduction
Severe Acute Respiratory Syndrome (SARS), also known as acute respiratory distress syndrome (ARDS), represents a critical clinical condition characterized by refractory hypoxemia, bilateral pulmonary infiltrates and decreased lung compliance[1,2]. This syndrome can be triggered by several etiologies, such as sepsis, severe pneumonia, pulmonary aspiration and trauma, and is frequently observed in intensive care units (ICU) due to its high morbidity and mortality [3]. Mechanical ventilation is an essential intervention in the management of SARS, aiming to ensure adequate oxygenation and minimize the risk of ventilator-induced lung injury (VILI) [4,5]. Among the ventilatory strategies, the use of Positive End-Expiratory Pressure (PEEP) stands out, which aims to keep the alveoli open during the respiratory cycle, preventing alveolar collapse and improving gas exchange [6,7].
Appropriate application of PEEP is critical to optimize oxygenation and reduce the risk of VILI [8]. However, determining the optimal PEEP level remains a clinical challenge, as inadequate levels may result in alveolar overdistension or lung collapse, worsening existing lung injury [8-10]. Several studies have investigated the effects of different PEEP levels in patients with severe acute respiratory syndrome (SARS), including those with COVID-19 [11-13]. Some evidence suggests that higher PEEP strategies may improve oxygenation and reduce mortality in specific patient subgroups [14-16]. However, other studies have not demonstrated significant benefits, indicating the need for individualization of therapy [10,17,18].
Furthermore, the response to PEEP may vary depending on the severity of SARS and individual patient characteristics, such as the capacity of the lungs to expand and the presence of comorbidities [19]. Therefore, careful assessment of the clinical and hemodynamic response to PEEP is essential to guide the personalized adjustment of this intervention, aiming to optimize oxygenation and minimize the risks associated with mechanical ventilation. Given the existing controversies and the clinical importance of PEEP in the management of SARS, it is pertinent to carry out a systematic review of the literature to summarize the available evidence on the effects of this intervention on relevant clinical outcomes, such as mortality, duration of mechanical ventilation, incidence of barotrauma and improvement in oxygenation. This study aims to carry out a systematic review of the scientific evidence on the effects of Positive End-Expiratory Pressure in Severe Acute Respiratory Syndrome, seeking to provide support for evidence-based clinical practice and contribute to the optimization of ventilatory management of these critically ill patients.
Methods
This study is a systematic review, classified as exploratory and descriptive. The research was carried out through bibliographic research in electronic databases on methods associated with SLR (Systematic Literature Review) and applications of SMARTER (Simple Multi-Attribute Rating Technique using Exploiting Rankings). The work carried out is of a qualitative and quantitative nature. The qualitative analysis of the data was carried out intuitively and inductively during the survey of the theoretical framework. It is also quantitative by employing the multicriteria method. In addition, there is also a numerical experimental study in order to simulate a situation of article selection based on the observed criteria. The bibliographic research was carried out in the following databases: Web of Science; Science Direct; Wiley; Springer Link; Taylor and Francis and PubMed. In addition, searches were carried out using bibliographic references of studies that addressed the topic in a relevant manner on the Google Scholar search platform.
The search in the databases was carried out using the terminologies registered in the Health Sciences Descriptors created by the Virtual Health Library developed from the Medical Subject Headings of the US National Library of Medicine, which allows the use of common terminology in Portuguese, English and Spanish. The present study sought to investigate the literature on the effects of positive end-expiratory pressure in severe acute respiratory syndrome. For this purpose, the descriptors “positive end-expiratory pressure (PEEP)”, “severe acute respiratory syndrome (SARS)”, “Mechanical Ventilation” and “oxygenation” were used, initially in English, and complementary in Spanish and Portuguese. As a tool to support decision-making in the selection and prioritization of articles, a set of criteria were considered essential to represent the state of the art of the research topic. This method has the following characteristics:
(i) rigorous logic allows the method to be accepted as a decision-making support tool;
(ii) simple to understand and apply with results that are easy to interpret.
References of selected papers were also searched for other documents of potential interest. Once eligible for full text in the evaluation, articles were included in the qualitative review if they met the following inclusion criteria:
a) contained data on positive end-expiratory pressure (PEEP);
b) contained data on severe acute respiratory syndrome (SARS);
c) contained data on mechanical ventilation;
d) contained data on oxygenation.
Articles were excluded if they were reports, banners, or conference abstracts. There was no review of confidential health information and the study was not interventional. Therefore, ethics committee approval was not required. In the end, the result obtained totaled 19 articles that contemplated the desired characteristics for the study. Four independent researchers extracted data from articles that met the inclusion criteria and entered them into a Microsoft Excel-generated “data extraction form” on the effects of positive end-expiratory pressure in severe acute respiratory syndrome. From this form, the authors and year of publication, study abstract, study type, outcome measure, limitations, and conclusions of the main studies selected were included, as shown in (Table 1-3).
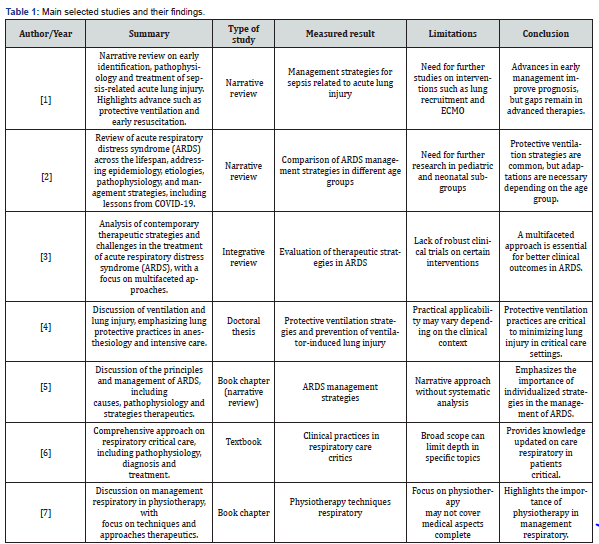
Source: Authors (2025)
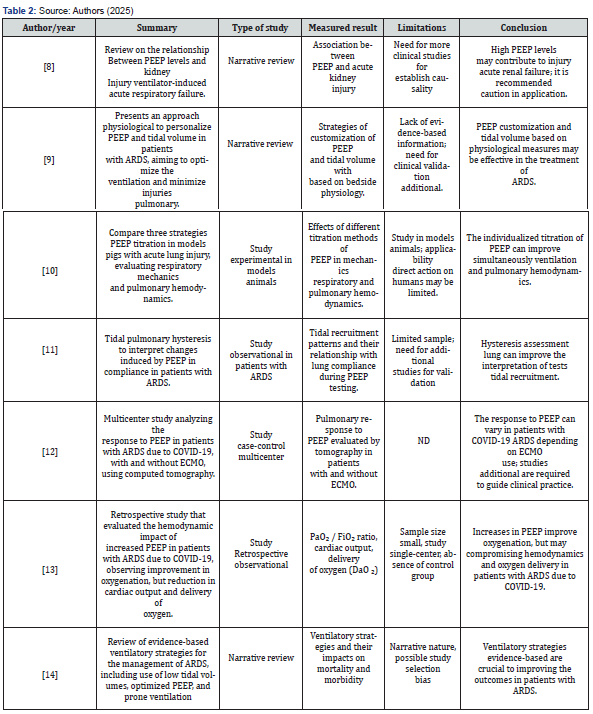
ND: Not Described
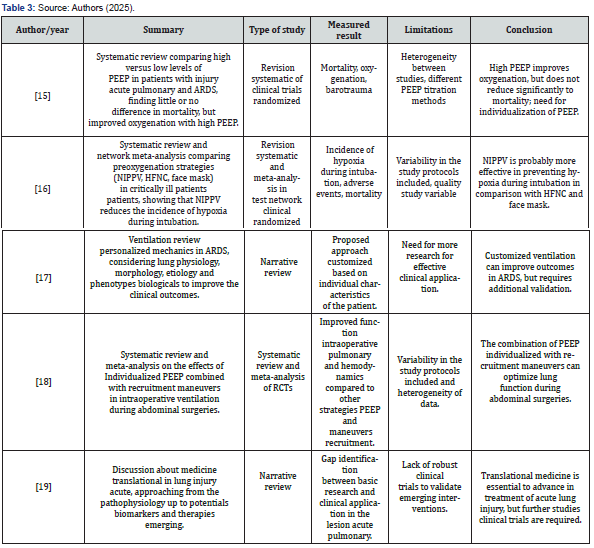
Results
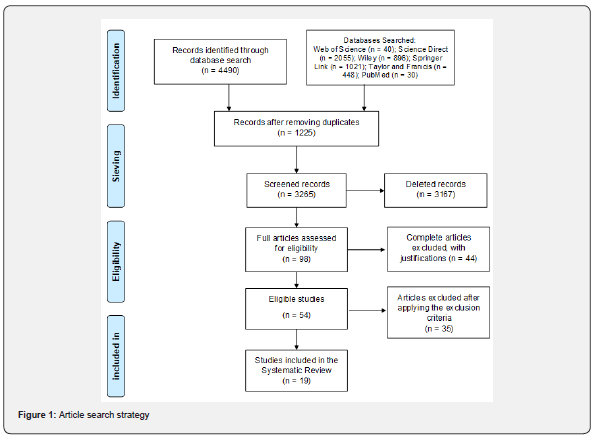
A comprehensive systematic literature search yielded a total of 4490 articles on the effects of positive end-expiratory pressure in severe acute respiratory syndrome. Of these, 1225 studies were excluded due to overlapping data. From this, the SMARTER (Simple Multi-Attribute Rating Technique using Exploiting Rankings) and 98 articles were selected that were suitable for full-text screening, of which 54 articles were included for data extraction, of which 35 were excluded by the exclusion criteria, making 19 articles eligible and included for systematic review. In (Figure 1), we describe the strategy for selecting articles on the topic in question.
Discussion
The effects of Positive End-Expiratory Pressure (PEEP) in severe acute respiratory syndrome (SARS) highlight the complexity of ventilatory management in critically ill patients. Recent studies highlight the importance of individualizing PEEP to optimize oxygenation and minimize lung injury induced by mechanical ventilation.
Zhang et al. [1], emphasize that in cases of sepsis-related SARS, lung-protective ventilation, including adequate application of PEEP, is essential to improve oxygenation and reduce mortality. However, the authors highlight the need for additional research on strategies such as lung recruitment and prone ventilation to improve clinical outcomes.
Cave et al. [2], analyze SARS over the life course and highlight that, although protective ventilation strategies are common in different age groups, the response to PEEP can vary significantly. During the COVID-19 pandemic, it was observed that the application of high PEEP did not always result in benefits, especially in patients with low lung recruit ability.
Oliveira et al. [3], identified that factors such as advanced age, comorbidities, and high PEEP levels are associated with higher mortality in patients with SARS on mechanical ventilation. These findings suggest that the indiscriminate application of high PEEP may be harmful in certain subgroups of patients.
Kecskés [4], highlights that mechanical ventilation can cause additional lung injuries if not carefully adjusted. The author emphasizes the importance of monitoring parameters such as lung compliance and plateau pressure to avoid alveolar overdistension and barotrauma.
Scaramuzzo et al. [5], suggest that PEEP application should be guided by individual physiological data, such as lung compliance response and the presence of alveolar collapse. This personalized approach may improve ventilation efficacy and reduce associated complications.
The study carried out by de Mattos Oliveira et al. [3], highlights that the management of SARS requires a multifaceted approach, which goes beyond the simple application of standardized ventilatory parameters. PEEP, in this context, emerges as a crucial tool to maintain oxygenation and prevent alveolar collapse, but its indiscriminate application can bring risks, such as pulmonary hyperinflation and hemodynamic compromise.
The work of Schreuder et al. [7], in line with this view, reinforces that effective respiratory management must take into account factors such as lung compliance, ventilation distribution and the systemic effects of positive pressure. For these authors, PEEP adjustment must be carefully monitored, using clinical and laboratory indicators that allow ventilation to be adapted to the patient’s individual physiological needs. In this sense, Mauri’s [9], findings support the need for personalizing PEEP based on physiological parameters at the bedside, such as the dynamic compliance response and the behavior of the pressure-volume curve. The author argues that PEEP titration should be done in order to maximize alveolar opening without inducing ventilator-induced lung injury (VILI). This approach based on the patient’s physiology contributes to improving oxygenation and reducing the risk of barotrauma and volutrauma.
In addition, Sousa et al. [10], demonstrated, through a clinical study, that the application of individualized PEEP was able to significantly improve pulmonary hemodynamics and respiratory function in patients with acute lung injury. The authors suggest that the use of strategies guided by images or respiratory mechanics parameters can optimize the effects of PEEP and reduce complications associated with invasive ventilation, such as pulmonary overdistension and dysfunction of adjacent organs. A relevant point addressed by Benites et al. [8], concerns the systemic repercussions of mechanical ventilation, particularly the impact of high PEEP on renal function. The study indicates that the elevation of intrathoracic pressure resulting from high PEEP levels can compromise venous return and, consequently, reduce renal perfusion, contributing to the development of ventilator-induced acute kidney injury. This reinforces the need for caution in choosing PEEP levels, especially in patients at increased risk of renal dysfunction.
Thus, the literature shows that, although PEEP is a fundamental intervention in the ventilatory support of SARS, its efficacy and safety depend on careful and individualized application. The use of tools to assess the pulmonary response to PEEP, combined with continuous monitoring of systemic parameters, can contribute to safer and more effective management. The integration of these strategies into clinical practice requires professional training and evidence-based protocols to ensure personalized care and optimization of clinical outcomes.
Final Consideration
The systematic review shows that the application of higher levels of PEEP can improve oxygenation and reduce the incidence of hypoxemia in patients with SARS. However, the benefits in terms of mortality reduction remain inconclusive, especially in patients with lower alveolar recruitment potential. The heterogeneity of the results suggests that the response to PEEP varies significantlyamong patients, being influenced by factors such as the severity of SARS, the presence of comorbidities and the capacity of alveolar recruitment. Therefore, careful titration of PEEP, based on individual parameters and continuous monitoring, is essential to optimize clinical results.
Furthermore, complementary strategies, such as the use of the prone position and electrical impedance tomography monitoring, can help identify the ideal PEEP level, minimizing the risks of alveolar overdistension and hemodynamic compromise. Although PEEP is a valuable tool in ventilatory support for patients with SARS, its application should be personalized, considering the individual characteristics of each patient. Additional studies focusing on specific subgroups and using advanced monitoring technologies are essential to improve clinical guidelines and maximize the benefits of this intervention.
References
- Zhang J, Yan W, Dong Y, Luo X, Miao H, et al. (2024) Early identification and diagnosis, pathophysiology, and treatment of sepsis-related acute lung injury: a narrative review. J Thorac Dis 16(8): 5457-5476.
- Cave C, Samano D, Sharma AM, Dickinson J, Salomon J, et al. (2024) acute respiratory distress syndrome: A review of ARDS across the life course. J Investig Med 72(8): 798-818.
- de Mattos Oliveira CW, Guimarães EM, Guimarães MQ, Pereira MVRA, et al. (2024) Advances and challenges in the management of acute respiratory distress syndrome: a multifaceted approach for better clinical outcomes. Obs LA Econ Latinoam 22(5): 4528-4528.
- Kecskés G (2024) Ventilation and Lung Injury. Lung Protection in Anesthesiology and Intensive Care Practice. University of Pécs Pp: 1-23.
- Scaramuzzo G, Pellegrini M, Borgstedt L (2023) Principles and Management of ARDS. In: Best 2022 Clinical Cases in Intensive Care Medicine: From the ESICM NEXT Committee Clinical Case Contest Pp: 181-194.
- Singh S, Pelosi P, Morris AC (2023) Oxford Textbook of Respiratory Critical Care. Suveer Singh, Paolo Pelosi ACM, editor. Oxford University Press Pp: 1-584.
- Schreuder SEIFM, Schreuder IM, Jiandani MP (2024) Management of Respiratory. Tidy's Physiother South Asia Ed
- Benites MH, Suarez Sipmann F, Kattan E, Cruces P, Retamal J (2025) Ventilation-induced acute kidney injury in acute respiratory failure: Do PEEP levels matter? Crit Care 29(1): 1-15.
- Mauri T (2021) Personalized Positive End-Expiratory Pressure and Tidal Volume in Acute Respiratory Distress Syndrome: Bedside Physiology-Based Approach. Crit Care Explorer 3(7): 1-11.
- Sousa MLA, Menga LS, Schreiber A, Docci M, Vieira F, et al. (2025) Individualized PEEP can improve both pulmonary hemodynamics and lung function in acute lung injury. Crit Care 29(1): 1-11.
- Mojoli F, Pozzi M, Arisi E, Mongodi S, Orlando A, et al. (2023) Tidal lung hysteresis to interpret PEEP-induced changes in compliance in ARDS patients. Crit Care 27(1): 1-10.
- Richard JC, Sigaud F, Gaillet M, Orkisz M, Bayat S, et al. (2022) Response to PEEP in COVID-19 ARDS patients with and without extracorporeal membrane oxygenation. A multicenter case-control computed tomography study. Crit Care 26(1): 1-13.
- Barthélémy R, Beaucoté V, Bordier R, Collet M, Le Gall A, et al. (2021) Haemodynamic impact of positive end-expiratory pressure in SARS-CoV-2 acute respiratory distress syndrome: oxygenation versus oxygen delivery. Br J Anesthesia 126(2): 70-72.
- Liaqat A, Mason M, Foster BJ, Kulkarni S, Barlas A, et al. (2022) Evidence-Based Mechanical Ventilatory Strategies in ARDS. J Clin Med 11(2): 1-12.
- Santa Cruz R, Villarejo F, Irrazabal C, Ciapponi A (2021) High versus low positive end-expiratory pressure (PEEP) levels for mechanically ventilated adult patients with acute lung injury and acute respiratory syndrome. Cochrane Database Syst Rev 3: 1-62.
- Pitre T, Liu W, Zeraatkar D, Casey JD, Dionne JC, et al. (2025) Preoxygenation strategies for intubation of patients who are critically ill: a systematic review and network meta-analysis of randomized trials. Lancet Respir Med
- Pelosi P, Ball L, Barbas CS V, Bellomo R, Burns KEA, et al. (2021) Personalized mechanical ventilation in acute respiratory distress syndrome. Crit Care 25(1): 1-10.
- Li X, Ni ZL, Wang J, Liu XC, Guan HL, et al. (2022) Effects of individualized positive end-expiratory pressure combined with recruitment maneuver on intraoperative ventilation during abdominal surgery: a systematic review and network meta-analysis of randomized controlled trials. J Anesth 36(2): 1-13.
- Zhang J, Guo Y, Mak M, Tao Z (2024) Translational medicine for acute lung injury. J Transl Med 22(1): 1-19.






























