From Roadblocks to Milestones: Assessing the Path Forward for Precision Medicine Policy for Cancer Care in Latin America
Maria Isabella Grueso1, Ana Rita Gonzalez2*, Patricia Salazar2, Margarita Quijano Serrano2, Luisa Morales Cabral2 and Luis Alberto Suarez1
1Pfizer, New York, USA
2Policy Wisdom LLC, Miami, FL, USA
Submission: January 31,; Published: February 13, 2025
*Corresponding author: Ana Rita Gonzalez, Policy Wisdom LLC, Miami, FL, USA
How to cite this article: Grueso, M. I., Gonzalez, A. R., Salazar, P., Quijano-Serrano, M., Morales Cabral, L., & Suarez, L. A. (2025). From roadblocks to milestones: Assessing the path forward for precision medicine policy for cancer care in Latin America. Juniper Online Journal of Public Health, 9(4). 555766. DOI: 10.19080/JOJPH.2025.09.555766
Abstract
Background/Objectives: Precision medicine, an innovative approach in healthcare, utilizes individual genetic information, environmental factors, and lifestyle choices to guide personalized decisions for disease prevention, diagnosis, and treatment. This article explores the role of a comprehensive policy framework to support the successful implementation of precision medicine in Latin America, addressing the region’s unique challenges and opportunities.
Methods: This paper analyses the current landscape of precision medicine in Latin America, identifies barriers to its implementation, and reviews relevant literature and case studies from other countries. Through this analysis, actionable recommendations are formulated to advance precision medicine in the region.
Results: The study identifies key challenges, including high costs, limited access, and the need for robust data management systems. It highlights opportunities such as the integration of genomic data, infrastructure development, and capacity building. Case studies from countries with advanced precision medicine programs are examined to draw lessons on successful policy frameworks. The recommendations emphasize the importance of legal frameworks, public-private partnerships, and the training of healthcare professionals.
Conclusion: Advancing precision medicine in Latin America requires a multisectoral approach that addresses systemic challenges and ensures equitable access. The implementation of data privacy measures, investment in healthcare training, and the fostering of international partnerships are critical for overcoming barriers. By adapting global best practices to the region’s context, Latin America can build an inclusive and effective precision medicine infrastructure, ultimately improving patient outcomes and advancing healthcare systems across the region.
Keywords: Precision Medicine; Policy; Oncology
Abbreviations: WHO: World Health Organization; CDC: Centres for Disease Control and Prevention; IARC: International Agency for Research on Cancer; PAHO: Pan American Health Organization; LAC: Latin America and the Caribbean; WEF: World Economic Forum; HTA: Health Technology Assessment; NICE: National Institute for Health and Care Excellence; GMS: Genomic Medicine Sweden; GAPP: Genomic Ap-plications Partnership Program; GHGA: German Human Genome Phenome Archive; FISMA: Federal Information Security Modernization Act; IRB: Institutional review board;
Introduction
Precision medicine, also known as personalized medicine, is an innovative approach to public health. According to the World Health Organization (WHO), precision medicine is an evolving strategy that uses an individual's genetic information, environmental factors, and lifestyle choices to guide tailored decisions regarding disease prevention, diagnosis, and treatment [1]. As with any innovative approach in healthcare, precision medicine offers the potential to transform medical practices in various ways. Considering genetic profiles, along with environmental and lifestyle factors, allows for the customization of treatments to achieve the most effective outcomes for each patient [2-4]. Precision medicine contributes to scientific advances by improving the understanding of disease mechanisms and represents a transition from empirical to patient-targeted medicine and patient entered care. It has the potential to optimize the accuracy of diagnosis and treatments while minimizing adverse effects [5-7]. This article examines the critical role of establishing a comprehensive policy frame-work to support the successful implementation of precision medicine in Latin America. It explores the current landscape, identifies key challenges, and highlights opportunities for advancing precision medicine in the region. Additionally, the brief analyses case studies from other countries to draw insights from their experiences. Based on this analysis, a set of actionable recommendations is presented to promote effective policy solutions that can facilitate the progress of precision medicine in Latin America.
Background
The Value of Precision Medicine in Oncology
One of the sectors with the highest potential for precision medicine is oncology. Given the complexity of cancer, which involves intricate interactions at multiple lev-elsgenomic, transcriptional, proteomic, and metabolic precision medicine can provide critical insights into cancer care [8,9]. Pharmacogenomics is vital in identifying patients at risk of adverse drug reactions or those most likely to benefit from specific therapies [10]. Additionally, biomarkers used to assess disease risk, prognosis, and treatment response represent a positive impact by informing prevention strategies, updating treatment guide-lines, strengthening healthcare systems, and guiding reimbursement models [11] Ongoing research already demonstrates that innovations in precision medicine have the potential to improve health outcomes and cost-effectiveness in cancer care, highlighting their transformative impact on oncology [12].
Nevertheless, it is crucial to recognize that cancer is influenced not only by genetic factors but also by lifestyle, social behaviour, and environmental factors. The Centres for Disease Control and Prevention (CDC) emphasizes the importance of integrating social-behavioural and genomic sciences to enhance the effectiveness of precision medicine in oncology. Precision medicine has become a standard approach for managing certain cancers in countries such as the United States, Australia, Israel, and some regions of Europe, where treatment is guided by molecular profiling based on tumour genomics [13,14]. In countries with less advanced systems for precision medicine, which is the case of Latin America, the application of biomarkers is constrained by challenges such as high costs, inadequate laboratory infrastructure, limited access to advanced therapies (including targeted therapies), and gaps in patient and biomedical education [15,16].
The Role of Policy to Advance Precision Medicine
Policy plays a crucial role in advancing precision medicine to optimize public health, with governments being key actors in funding foundational research, setting standards, and establishing regulations on data privacy, interoperability, and integration with health surveillance systems [17]. Effective policy and prioritization ensure that the benefits of precision medicine, such as targeted treatments and early disease detection, are equitably distributed and implemented effectively. Agile governance is essential to address the technological and ethical challenges associated with precision medicine, including data management and regulatory efficiency, while enabling innovation and protecting public welfare [18]. Additionally, collaboration between governments, non-profit organizations, and the private sector is vital for maximizing the potential of precision medicine. The convergence of advanced technologies, such as genome editing and artificial intelligence, presents both opportunities and challenges [18]. Governments should adopt flexible and responsive governance frameworks to tackle these challenges and support the equitable integration of precision medicine into healthcare systems. By drawing on global case studies and best practices, policymakers can develop strategies that enhance the delivery of precision medicine, ultimately improving health outcomes at both individual and population levels [17-19].
Oncology Policy Landscape in Latin America
Latin America's cancer burden is significant, and cancer is one of the leading causes of mortality in the region. In 2022, there were 1.5 million new cancer cases and nearly 749,000 cancer-related deaths [20]. Policymakers in the region recognize that timely diagnosis and fast access to treatment with the best available interventions are crucial for enhancing patient quality of life and optimizing health outcomes [21,22]. In 2023, the International Agency for Research on Cancer (IARC) and the Pan American Health Organization (PAHO) gathered experts from Latin America and the Caribbean (LAC) to develop a set of evidence-based cancer prevention measures, resulting in a code with 17 preventive actions and public policy recommendations for effective implementation [22]. Oncology is prioritized in most countries; however, challenges remain in defining pathways and ensuring that resources are effectively translated into effective access to cancer care [21]. At the regional level, PAHO has a Strategic Plan for 2020-2025 that prioritizes non-communicable diseases, including cancer. Despite this, the plan has limitations in specifying routes for effective cancer care access [23].
At the national level, most Latin American countries have established oncology-related plans and programs and include cancer as a priority in their health strategies. Nevertheless, there is variability in policy comprehensiveness and the countries' capacity to make operable and effective cancer strategies [24-29]. The main challenges are delays that hinder timely diagnosis, treatment, and care, which are caused by financial constraints, the geographic distribution of healthcare services, and fragmented healthcare systems, among other factors [30–34].
Policy Landscape for Precision Medicine and Cancer Care in Latin America
The policy landscape for integrating precision medicine into cancer care across Latin America is evolving, showing progress and opportunities for further development. Countries in the region increasingly recognize the importance of precision medicine and genetic diagnostic testing, with several innovative initiatives and legislative efforts reflecting this growing commitment. Latin American countries have introduced several innovative programs and legislative measures to support precision medicine. For example, Brazil's National Program for Genomics and Precision Health- Genomas Brazil leverages genomic sequencing for diagnosing and treating diseases, including cancer [35]. Complementing this national initiative, various state governments in Brazil have implemented programs to enhance access to genetic testing for breast and ovarian cancers [35]. Similarly, in Argentina, the recent consensus on precision medicine, presented in the Protocol on the Role of Genetic Oncology Counselling [36], and the establishment of the Reference Program and Genomic Biobank of the Argentine Population (Polar), highlight the country's commitment to creating a genetic data repository and developing specific oncology guidelines [34,37]. However, given the recent publication of the guidelines, there is no clear evidence of the operationalization of the protocol and how it will be translated into effective access for the population.
In Mexico and Colombia, progress in precision medicine remains limited because current oncology-related policies and programs do not adequately address access to innovative therapies, genetic diagnostic testing, or precision medicine on a large scale. In Mexico, initiatives such as the TRASLADA program at the Mexican Institute of Social Security (IMSS) and collaborations between the National Institute of Genomic Medicine (INMEGEN) and the National Institute of Cancerology (INCAN) offer genetic diagnostic testing and precision medicine on a smaller scale [38-40]. Likewise, in Colombia, the 10-Year Public Health Plan (2022-2031) [41] and the National Development Plan (2022-2026) highlight the importance of health technology assessments and prioritize early cancer detection and prevention [42]. However, there is no specific policy addressing genetic diagnostic testing or precision medicine within these frameworks [29].
While Latin American countries are making progress in integrating precision medicine into cancer care, with notable initiatives in Brazil, Argentina, Colombia, and Mexico, there is a long road ahead. Each nation is at a different stage in operationalizing these advancements, from comprehensive national programs to emerging regulatory frameworks and pilot projects. The evolving policy landscape presents both opportunities and obstacles, highlighting the need for continued commitment, strategic implementation, and collaborative efforts to ensure equitable access to precision medicine across the region.
Challenges for Policy in Precision Medicine in Latin America
As an innovation in the healthcare sector, precision medicine presents several policy related challenges that must be addressed to ensure its effective implementation. Key issues include managing complex data, developing the necessary infrastructure, enhancing the capacity of healthcare professionals, and allocating resources for innovative treatments. To overcome these challenges, it is essential to develop and implement initiatives that address the regulatory and policy frameworks, enabling the most efficient and effective operationalization of precision medicine strategies. The World Economic Forum (WEF) discusses a range of challenges facing precision medicine, including technological, ethical, and regulatory issues, which constrain its effectiveness [43]. The WEF, mentions that precision medicine has been affected by a “pacing problem” which in technology refers to the issue where technological advancements are advancing faster than the ability of laws and regulations to keep up [43,44]. Addressing this pacing problem from a policy perspective is crucial to ensure sustainable and lasting changes, even in unstable political scenarios. Even though the regulatory and policy frameworks demonstrate that precision medicine for cancer care is starting to appear in the public health agenda of countries in Latin America, challenges and barriers remain. Some identified include:
Limited Legislative Frameworks
Effective legislative frameworks are crucial for the advancement and equitable distribution of precision medicine. These frameworks establish the legal and regulatory groundwork necessary for integrating genomic information into healthcare systems, ensuring both ethical practices and broad access to cutting-edge treatments. While there is growing interest in genomic regulation across Latin America, significant challenges remain in developing comprehensive legislative frameworks for precision medicine. Mexico has demonstrated interest in genomic regulation through the General Health Law, which addresses genomic testing and emphasizes genome ownership and related rights. However, this law is focused on protecting ownership but is not specifically intended to ensure equitable access to precision medicine. Two proposed amendments are currently under consideration to broaden access [45,46]. Similarly, in Brazil, a federal bill is under review to further enhance access to genetic diagnostics, reflecting a growing commitment to integrating precision medicine into healthcare [47,48]. Finally, in Colombia, the recently enacted Law 2360 of 2024 updates cancer care regulations, presenting an opportunity to incorporate precision medicine and innovative therapies into the regulatory framework [29]. However, translating this legislative update into tangible improvements in precision medicine access will require careful implementation and sustained political will. Overall, the challenge across Latin America lies not only in creating and updating legislative frameworks but also in ensuring that these frameworks effectively address the practical and equitable integration of precision medicine.
Barriers in Healthcare Infrastructure and Integration
Effective healthcare infrastructure and integration are essential for the successful implementation of precision medicine. A well-coordinated infrastructure ensures that advanced diagnostic and treatment options, such as those offered by precision medicine, are accessible across diverse geographic and socioeconomic settings. Without a robust and equitable infrastructure, the benefits of precision medicine may be unevenly distributed, limiting its impact and efficacy. Across the region, there are significant barriers in healthcare infrastructure that impede the equitable integration of precision medicine. In Brazil, despite a well-established cancer care network, services are concentrated in specific geographic areas, particularly in affluent regions, leaving vast parts of the country underserved, restricting access to advanced care and precision medicine for many patients [31,49]. Colombia similarly experiences disparities in the distribution of oncological services, with a significant concentration in the capital city despite nationwide coverage [50]. This centralization can limit ac-cess to precision medicine and other advanced treatments for individuals living outside the capital. Also, Argentina faces challenges with fragmented provincial management, further complicating the equitable distribution of services. This fragmentation can create barriers to accessing the latest diagnostic and therapeutic innovations, further exacerbating disparities in healthcare delivery [34].
Delays in Technology and Treatment Access affecting the patient journey
Timely access to new technologies and treatments is crucial for effectively delivering precision medicine. Delays in integrating advanced therapies can significantly impact patient outcomes, as precision medicine often relies on rapidly adopting cutting-edge technologies and treatments. Efficient processes for evaluating and approving new interventions are essential to ensure that patients benefit from the latest advancements without unnecessary delays. In Latin America, the slow integration of new technologies is a widespread issue. One major issue is the lack of standardized and transparent Health Technology Assessment (HTA) processes. A significant challenge in this region is that the unclear process and criteria of the HTA agencies complicate the application and submission process. This confusion leads to more burdensome and expensive application processes for sponsors, resulting in slower approval and adoption of new technologies.
This is the case in countries like Brazil and Mexico, where dedicated HTA agencies exist, their criteria and decision-making processes are often unclear, undermining the fairness and effectiveness of reimbursement decisions. The absence of specific HTA pro-cesses for oncology drugs and precision medicine exacerbates these issues, creating gaps in the structured evaluation of advanced therapies [51-55]. For example, in Brazil, oncology treatments can take up to 1,100 days to become fully available in the public sector, even after regulatory approvals [52,53]. Mexico experiences similar delays, with uneven access to innovative treatments concentrated in major urban centres. Colombia also struggles with delays in receiving confirmed diagnosis and treatment, exacerbated by a perception of long waiting periods and instances of denied care [50].
Funding and Financial Barriers
Adequate funding is essential for the successful implementation of precision medicine, as it enables the development, approval, and distribution of advanced therapies. Without sufficient financial resources, the adoption of innovative treatments and technologies is severely constrained, impacting patient access and outcomes. Financial barriers, including budget constraints and reimbursement limits, can create significant obstacles to integrating precision medicine into healthcare systems. In Latin America, insufficient funding for cancer care and advanced treatments is a critical issue. The lack of clarity on the approval process, combined with budget constraints and reimbursement limits, poses significant barriers. In Mexico, public health funding for cancer treatment is diminishing, leading to disparities in access to advanced treatments like targeted therapies [33,56]. Colombia also faces financial barriers, with many patients incurring out-of-pocket costs due to delays and limited coverage options [32]. Additionally, in Brazil, and Mexico, financial shortfalls in covering innovative cancer treatments, like precision medicine, lead to substantial judicialization costs, with Brazil incurring approximately $307 million in such costs in 2017 [57] and Mexico utilizing only 27.4% of its budget for high-cost medicines in 2022 [58]. In line with this, evidence from Colombia in 2022, shows that accessing cancer treatments frequently involves judicial actions, with diagnoses of tumours and neoplasms being the most common cases invoking the individual´s right to health, accounting for 13.18% of such cases [59].
Fragmentation within healthcare systems exacerbates these financial challenges. For instance, in Mexico, the lack of a unified list of covered medicines and transparency issues result in inconsistent access to treatments [60]. Similarly, Argentina’s fragmented health system complicates stakeholder alignment and policy implementation [34]. Furthermore, regulatory and procedural delays, along with complex approval processes in Argentina and the slow integration of new technologies into public healthcare in Brazil, impede timely access to new therapies [34,52,53,57,61]. Addressing these issues requires a focus on improving transparency, efficiency, and overall effectiveness in healthcare policies across the region.
Data Management and Data Privacy
Effective data management and robust data privacy protections are essential for the successful implementation of precision medicine. Secure handling of genomic and health data is critical to ensuring accurate diagnoses, personalized treatments, and maintaining patient trust. Without strong data management infrastructure and clear privacy regulations, integrating genomic information into health records can be compromised, leading to potential risks in data security and patient confidentiality.
In Latin America, disparities in technology access and varying data protection laws hinder the equitable implementation of precision medicine. Key challenges include inadequate infrastructure for secure data handling, as many countries lack the necessary technology to manage and store sensitive genomic data safely. Additionally, there is limited standardization in the management and sharing of genomic data across regions, with inconsistent data formats and interoperability issues impeding the seamless integration of genomic data with health records [62-64]. Insufficient regulatory frameworks also con-tribute to the problem, with many countries having underdeveloped regulations for the protection and use of genomic data. Furthermore, variations in the availability of advanced data management technologies across the region exacerbate these challenges. Regions with limited technological resources may struggle to adopt and maintain secure and effective data management systems. These issues complicate the integration of genomic data with health records, raising concerns about data security and patient confidentiality [62-64].
Training Deficiencies and Capacity Building
Effective implementation of precision medicine depends on well-trained healthcare professionals and access to specialized physicians. Without adequate training and specialization, the potential benefits of precision medicine cannot be fully realized, impacting the quality of care and access to advanced treatments. Across the region, a common problem facing precision medicine implementation is the inadequate training of healthcare professionals in precision medicine and limited specialized physicians [65]. In Brazil, the implementation is hindered by a shortage of trained medical geneticists and an uneven distribution of genetic services, resulting in only 30% of the population having access to specialists [66]. Argentina also struggles with limited training in genomics and precision medicine within healthcare education, affecting the ability of professionals to effectively use these tools [34]. Similarly, Colombia's progress is hindered by outdated clinical practice guidelines and a general lack of knowledge among healthcare providers about precision medicine [65,67]. These issues are compounded by insufficient professional societies dedicated to the field.
These challenges highlight the need for systemic reforms and strategic investments to improve cancer care and integrate precision medicine across Latin America. Addressing these barriers will be crucial for enhancing healthcare delivery and ensuring equitable ac-cess to advanced treatments throughout the region.
Case studies
This article presents a case study to identify effective policy solutions for advancing precision medicine in Latin America. The analysis focuses on the best practices from around the world. Specifically, it examines policies and programs that countries have de-signed and implemented to advance precision medicine, as well as initiatives that integrate precision medicine specifically into cancer care. The case studies were examined to identify strategies addressing the main challenges associated with precision medicine in cancer care in Latin America. These challenges include a range of critical issues, including the need for an effective legislative framework, improvements in data management and privacy, and the enhancement of health infrastructure. Additionally, addressing delays in treatment and access, promoting capacity building, and overcoming financial barriers are essential for advancing precision medicine.
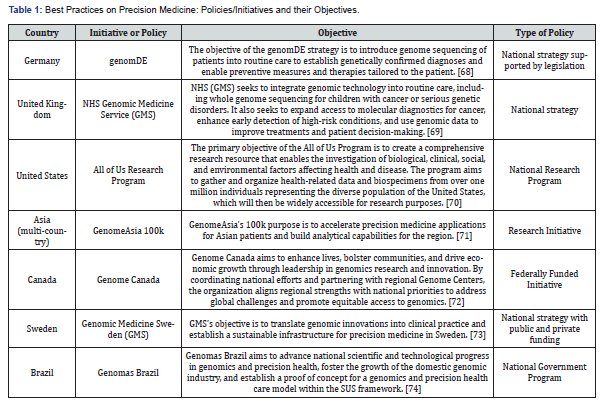
Table 1 summarizes the best practices identified, outlining the relevant policies or initiatives and their objectives.
Key Components of Successful Initiatives/Policies in Precision Medicine
To address the challenges of keeping pace with advancements, countries have developed initiatives focused on both research and clinical applications to integrate precision medicine into their healthcare practices. The best practices analysed use a holistic approach to adapting healthcare systems to the innovations in precision medicine. Many of these practices began as targeted initiatives funded by governments and, with supporting evidence, have evolved into national strategies and even legislation. This process rep-resents the "bottom-up" approach, where specific initiatives are used to build competence networks and infrastructure, ultimately enabling equitable access to precision medicine [19].
When analyzing common elements of the best practices the following can be high-lighted:
Legal Framework and Standardization
One of the latest goals of precision medicine policies is that they are supported by legislation making them effective and translatable to facilitate access. Some of the best practices demonstrate this approach. Genom DE exemplifies this approach by establishing a legislative framework that mandates insurance coverage for precision medicine-associated diagnostics and therapies, particularly for rare and oncological diseases. This framework, tailored to the German health system, serves as a valuable model for other countries, including those in Latin America, by demonstrating how to design and implement similar initiatives through strategic advocacy and collaboration with existing providers and networks [75]. Similarly, the NHS GMS policy focuses on extending reimbursement to genomically informed care and treatment, though specific technologies are still under evaluation by the National Institute for Health and Care Excellence (NICE) [69]. Both examples highlight the importance of developing comprehensive legal and policy frameworks to ensure the effective integration and reimbursement of precision medicine technologies.
Healthcare Infrastructure and Integration
The advancement of precision medicine infrastructure is one of the common elements identified in the best practices. Genome DE plays a pivotal role in strengthening infrastructure by funding the design of data systems essential for precision medicine. Although it does not directly provide funding to service providers or networks, these entities receive support from other sources, ensuring the development of a robust data infrastructure [68]. Meanwhile, the NHS GMS initiative strengthens the clinical landscape by establishing and expanding laboratory and clinical networks, including Genomic Laboratory Hubs and national directories, which are crucial for integrating genomic insights into routine healthcare and facilitating the widespread implementation of precision medicine [69]. Similarly, Genomic Medicine Sweden (GMS) has established regional Genomic Medicine Centres at university hospitals throughout Sweden, promoting the adoption of genomic based diagnostics developed in collaboration with the Swedish research infrastructure Science for Life Laboratory [19].
Complementing these efforts, GenomDE Asia 100k contributes by advancing scientific understanding by researching genetic variants and populations with high homozygosity rates. This research enhances global resources and underpins the scientific foundation of precision medicine [76]. Additionally, Genomas Brazil focuses on building scientific capacity and bolstering the national genomic medicine industry, promoting sustainable development within the field [74]. The GenomDE Canada initiative further supports infra-structure development by providing funding for essential equipment, aiding the expansion and modernization of physical facilities [77]. Collectively, these initiatives address various critical components of precision medicine infrastructure, from data systems and clinical networks to scientific research and equipment, thus enhancing the overall effectiveness and integration of precision medicine into healthcare systems.
Optimizing Patient Journey
The best practice examples that are already concentrating on clinical applications, such as GenomDE and NHS GMS, have been successful in optimizing the patient journey through the integration of genomic medicine into healthcare pathways [69,75]. In the GenomDE initiative, patients can be referred to the Model Project by their treating physician or an eligible provider, whether at the primary or specialized care levels [75]. Similarly, the NHS GMS initiative allows oncologists, hematologists, and neurologists, along with clinical genomics services, to refer patients for genomic testing. Furthermore, the NHS Genomic Medicine Service (GMS) improves this process by integrating genomic medicine throughout every stage of patient care, from initial diagnosis to treatment and follow-up, in collaboration with seven specialized alliances. These approaches ensure that genomic insights are seamlessly incorporated into patient care, thereby improving the precision and effectiveness of treatment throughout the patient journey [69].
Innovative Funding and Collaboration
Effective funding and collaboration are crucial for advancing precision medicine, and several successful initiatives highlight key best practices in both patient-focused and pro-gram-focused strategies. For example, GenomDE exemplifies the implementation of com-prehensive reimbursement models, which ensure that eligible patients receive full cover-age for precision medicine diagnostics and advanced therapies. This patient-focused approach removes financial barriers and promotes a broader adoption of precision medicine at the individual level [78]. Alternatively, program-focused funding initiatives aim to support the broader development and application of precision medicine. Genomas Brazil illustrates this with its multifaceted funding strategy, which includes direct allocations from the Ministry of Health, parliamentary amendments, and contributions from tax ex-emption programs [74]. These diverse funding sources help advance the field of precision medicine by supporting research and development on a larger scale. Similarly, GenomDE Canada supports its initiatives, such as the All for One project, through the Genomic Ap-plications Partnership Program (GAPP), an owned initiative that funds projects aiming to apply genomic research to real-world problems and practical solutions. The purpose of GAPP is to bridge the gap between research and practical application, reflecting a commitment to sustaining progress through ongoing financial support [79,80]. Additionally, Genomic Medicine Sweden exemplifies program-focused funding by securing resources from both public and private sources, further supporting the advancement of precision medicine through diverse financial streams [73].
Collaborative efforts also play a significant role. Genome Asia 100k demonstrates the power of public-private partnerships, involving a wide range of stakeholders including Med Genome, Nanyang Technological University, and numerous international partners like Genentech and Illumina [81]. This is also the case of GenomDE with the participation of 15 stakeholders that represent different sectors [82]. This collaborative model pools resources and expertise to drive innovation and expands the reach of precision medicine. In summary, the integration of comprehensive reimbursement models to enhance patient access, diverse funding sources to support diverse programmatic goals, and collaborative partnerships evidenced by initiatives such as GenomDE, Genomas Brazil, Genome Canada, and Genome Asia 100k-provides a robust framework for advancing precision medicine and achieving sustained success.
Advanced-Data Management and Data Privacy
Data privacy is a critical challenge for precision medicine. The best practices that tackle data privacy concerns were examined. These strategies include integrating secure data systems, adhering to legal and ethical standards, and employing techniques that promote anonymity to safeguard patient information. Such measures are evident in both research and clinical practice. Strategies such as genomDE ensure data protection by collaborating with bioinformatics institutions such as the German Human Genome Phenome Archive (GHGA) and the Fraunhofer Institute for Applied Information Technology (FIT) [82]. This collaboration is underpinned by SGB V § 64e, which mandates the confidentiality and anonymization of data. Therefore, adhering to international legal standards for data management. Similarly, the NHS GMS strategy addresses privacy by using the National Genomic Research Library, which stores patient data in a secure environment while developing interoperable data infrastructure and cutting-edge analytical tools [69].
In the All of Us Research Program, privacy is safeguarded using data systems that meet the Federal Information Security Modernization Act (FISMA) moderate standards and are authorized by the NIH Office of the Chief Information Officer (OCIO), there by addressing potential data confidentiality risks [83]. Although Genome Asia 100k does not provide explicit details, its adherence to Institutional review board (IRB) standards suggests it employs confidentiality and anonymity measures to protect participant data [76]. Finally, Genomas Brazil upholds data privacy through its commitment to confidentiality and ethical responsibility, guided by principles that ensure the privacy of personal information and adherence to legal and social norms [74]. Although the specific measures to ensure this commitment are not outlined, the law regulating the initiative states it as one of its objectives.
Another crucial aspect of effective data management in best practices is the interoperability of informatics infrastructure, which focuses on integrating genomic data with other health information like clinical records including detailed information about the patient’s family medical history, or when possible, lifestyle information (e.g., diet, exercise habits), and data from wearable devices (e.g., heart rate, activity levels). Initiatives such as the NHS GMS and GenomDE illustrate this approach by collaborating with multiple bio-informatics institutions to improve data management and enhance interoperability [69,82].
Capacity Building and Research
Another common element in best practices is the emphasis on developing evidence-based strategies through research and capacity building for personnel involved in precision medicine initiatives. Capacity building is a key focus, with various programs integrating training for future medical professionals and providing ongoing education. For example, GenomDE includes training and continuous education as core components of its national strategy [68]. Similarly, the NHS GMS initiative focuses on educating laboratory professionals through collaborations with Health Education England and the Ge-nomics Education Program to upskill a diverse workforce [69]. Genomas Brazil is dedicated to training its workforce in genomic medicine and precision health [74], while Genome Canada has supported 6,800 trainees since 2000, reflecting a long-term commitment to capacity building [84]. Research-focused strategies across the United States, Canada, Asia, and Brazil highlight the importance of prioritizing research to build evidence-based knowledge and advance precision medicine, particularly by addressing geographic and demographic gaps in genetic studies to drive innovation and ensure diverse representation.
Policy Recommendations for Latin America
Considering the current precision medicine landscape in Latin America, and the key components identified in the best practices, this section presents recommendations designed to support the development of comprehensive national strategies, with the goal of them being supported by legislative and policy frameworks. To effectively advance precision medicine, countries should adopt a holistic and evidence-based policy approach.
Legislative support
To facilitate the successful integration of precision medicine, it is essential to develop and enact supportive legislation. National strategies for precision medicine should be underpinned by laws that ensure coverage for precision medicine diagnostics and therapies. Additionally, effective legislation must also extend to data privacy to safeguard patient information. Clear guidelines and standards for both coverage and data protection will reinforce the adoption of precision medicine into the broader healthcare system, ultimately enhancing uptake and confidence among both patients and providers.
Healthcare Infrastructure
Countries should prioritize the development of healthcare infrastructure to support the implementation of precision medicine. This involves investing in essential components such as data management systems, advanced data analysis tools, biobanks, and patient engagement platforms [19]. Additionally, it is important to consider the role of smaller local laboratories in enhancing access to timely diagnostics. For example, local labs that can process patient samples, such as biopsies, may significantly speed up the diagnostic process and reduce the need for patients to travel to specialized city clinics. Evaluating and upgrading existing healthcare infrastructure should include strategies for integrating these local facilities, which can improve access to precision medicine, contribute to overcoming the geographic distribution barriers for cancer care, and ensure that patients receive timely and efficient care. Focused research-oriented initiatives, which can later be integrated into national policies, are crucial. The experiences from best practices demonstrate that building robust and locally integrated infrastructure is essential for the effective implementation and utilization of precision medicine.
Translating Research into Clinical Applications to optimize patient journey
Given that many initiatives in Latin America are still research-focused rather than clinically applied, it is crucial to support efforts that bridge this gap. Programs should facilitate the translation of research findings into practical applications, ensuring that evidence-based interventions improve patient care effectively. The goal of precision medicine should be improving patient outcomes, for this reason, evidence generation should be focused on understanding the tangible impact of treatments and interventions on actual patient outcomes.
Public-Private Partnerships and Collaborative Funding
Countries should foster public-private partnerships to leverage expertise and resources in precision medicine policy. These collaborations can drive innovation and enhance funding efforts. Additionally, countries should seek opportunities to align with successful initiatives and secure funding from various sources, including government grants, private investments, and international organizations. Given the current landscape in Latin America, governments may also consider engaging private funders for specific precision medicine initiatives.
Data Management and Privacy
Precision medicine policies must establish clear guidelines and standards for data management and privacy to safeguard patient in-formation. Adhering to and complying with international data protection regulations is essential for this purpose. Additionally, initiatives should implement robust security measures to build trust and facilitate the necessary data sharing for research and clinical use.
Education and Capacity Building
To ensure the effective implementation of precision medicine, government policies, and strategies must invest in education and training programs for healthcare professionals to develop expertise in this field. Additionally, they should support ongoing professional development and specialization in genomics and related areas. Countries should also implement education and awareness campaigns to enhance public and professional understanding of precision medicine. It is essential to engage communities and stakeholders early in this process to ensure that policies align with their needs and expectations. Additionally, incorporating feedback into the development and implementation of precision medicine programs is crucial for improving patient engagement and satisfaction [19].
International Cooperation
Given the current landscape in Latin America and the long road ahead, international collaboration should be encouraged to share knowledge, resources, and best practices. Latin American countries should engage in global research initiatives and networks to stay informed about advancements and incorporate successful practices from other regions.
Conclusion
In conclusion, advancing precision medicine in Latin America needs a multisector approach that addresses systemic and operational challenges. As outlined, the integration of precision medicine into healthcare systems worldwide has been significantly enhanced by adopting holistic policies that include data management, infrastructure development, and capacity building. For Latin America to realize the full potential of precision medicine, it must overcome barriers such as high costs and limited access by implementing robust data privacy measures, investing in healthcare professional training, and fostering public-private partnerships. Additionally, establishing comprehensive legal frameworks will ensure equitable access and effective implementation. By following these recommendations and adapting global best practices to their unique context, Latin America can build a more inclusive and effective precision medicine infrastructure, ultimately im-proving patient outcomes and advancing healthcare in the region. To achieve these goals, governments need to collaborate with the private sector, leverage best practices, and engage in international partnerships to drive ethical and innovative advancements in precision medicine.
Author Contributions
Conceptualization, A.G, P.S, M.G, and M.Q; methodology, A.G, M.G, P.S, and MQ; writing original draft preparation, L.M, and M.Q; writing review and editing, M.Q, P.S and L.M; supervision, A.G, M.G, L.S, and P.S. All authors have read and agreed to the published version of the manuscript.
Funding
The authors disclose receipt of financial support from Pfizer for the research and the discussion process that was part of developing this document. The funding organization was not involved in the design, data analysis, interpretation of the results, or manuscript writing. Some of the authors, Maria Isabella Grueso and Luis Alberto Suarez, are employed by Pfizer. This affiliation did not influence the content of this article, and no conflict of interest exists. The opinions and conclusions presented in this article are based solely on an evidence-based literature review and do not reflect the views of Pfizer.
Acknowledgment
The authors acknowledge the contributions of Allira Attwill (AA), Araceli Fernandez (AF), and João Rocha (JR) and the Policy Wisdom team for their role in researching and helping finalize the manuscript. AA, AF, and JR assistance were covered by regular functions at Policy Wisdom LLC.
Conflicts of Interest
Pfizer provided funding for the time spent by researchers in developing this final document; however, they were not involved in its drafting. The content of the document is entirely based on an evidence review and a thorough analysis of the literature. The recommendations are derived from a review of existing literature, publicly available evidence, and the author's own analysis. The views expressed herein are solely those of the authors and are not influenced by any external parties or sponsors.
References
- World Health Organization (WHO) (2023) Health Technologies Accessed.
- National Human Genome Research Institute (2024) Precision Medicine. NIH. Accessed.US Food and Drug Administration (FDA) (2018). Precision Medicine.
- US Food and Drug Administration (FDA) (2018) Precision Medicine.
- Muharremi G, Meçani R, Muka T (2023) The Buzz Surrounding Precision Medicine: The Imperative of Incorporating It into Evidence-Based Medical Practice. J Pers Med 14(1): 1-11.
- Lu CY, Terry V, Thomas DM (2023) Precision medicine: affording the successes of science. npj Precision Oncology 7(3): 1-8.
- Seyhan AA, Carini C (2019) Are innovation and new technologies in precision medicine paving a new era in patients centric care? J Transl Med 17(1): 1-28.
- Kasztura M, Richard A, Bempong NE, Loncar D, Flahault A (2019) Cost-effectiveness of precision medicine: a scoping review. Int J Public Health 64(9): 1261-1271
- Schwartzberg L, Kim ES, Liu D, Schrag D (2017) Precision Oncology: Who, How, What, When, and When Not? American Society of Clinical Oncology Educational Book (37): 160-169.
- Tebani A, Afonso C, Marret S, Bekri S (2016) Omics-Based Strategies in Precision Medicine: Toward a Paradigm Shift in Inborn Errors of Metabolism Investigations. Int J Mol Sci 17(9): 1-27.
- Bhat AH, Khaja UM, Ahmed M, Khan WY, Ganie SA (2023) Pharmacogenomics in cancer. In: Pharmacogenomics Pp: 195-221.
- CDC (U.S. CENTERS FOR DISEASE CONTROL AND PREVENTION). Precision Health: Treat and Manage Disease.
- Chen W, Anothaisintawee T, Butani D, et al. (2022) Assessing the cost-effectiveness of precision medicine: protocol for a systematic review and meta-analysis. BMJ Open 12(4): 1-7.
- Stein MK, Oluoha O, Patel K, VanderWalde A (2021) Precision Medicine in Oncology: A Review of Multi-Tumour Actionable Molecular Targets with an Emphasis on Non-Small Cell Lung Cancer. J Pers Med11(6): 1-33.
- The Lancet (2021). 20 years of precision medicine in oncology. The Lancet 397(10287): 1-1781.
- Dienst Mann R (2021) WS04.02 Access to Biomarker Testing in Latin America. Journal of Thoracic Oncology 16(10): 1.
- Calderon Aparicio A, Orue A (2019) Precision oncology in Latin America: current situation, challenges and perspectives. Ecancermedicalscience 13: 1-14.
- Whitsel LP, Wilbanks J, Huffman MD, Hall JL (2019) The Role of Government in Precision Medicine, Precision Public Health and the Intersection with Healthy Living. Prog Cardiovasc Dis 62(1):50-54.
- Doxzen K, Signe L, Bowman DM (2022) Advancing precision medicine through agile governance Bridging innovation and regulation for the greater good. Brookings.
- Stenzinger A, Moltzen EK, Winkler E, et al. (2023) Implementation of precision medicine in healthcare A European perspective. J Intern Med 294(4): 437-454.
- Pan American Health Organization (PAHO) (2024). Topics: Cancer. PAHO.
- Pan American Health Organization (PAHO) (2023) IARC and PAHO launch the 1st edition of the Latin American and Caribbean Code Against PAHO.
- Pan American Health Organization (PAHO) (2023) Latin America and the Caribbean Code against Cancer.
- Pan American Health Organization (PAHO) (2020) Strategic Plan of the Pan American Health Organization.
- Colombian Congress (2010) Law 1384 de 2010 Función Pública Pp: 1-6.
- Presidency of the Republic Brazil (2023) Law No. 14,758.
- Presidency of the Republic Brazil (2021) Law No. 14,238.
- Health Secretariat Mexico (2021) Specific Action Program of Prevention and Control of Cancer 2021-2024 Pp: 1-44.
- Ministry of Health and Social Development Argentina (2018) National Plan for Cancer Control 2018-2022 Pp: 1-137.
- Colombian Congress (2024) Law 2360 of 2024. Sistema Único de Información Normativa.
- Brand NR, Qu LG, Chao A, Ilbawi AM (2019) Delays and Barriers to Cancer Care in Low- and Middle-Income Countries: A Systematic Review. Oncologist 24(12): 1371-1380.
- da Silva MJS, O Dwyer G, Osorio deCastro CGS (2019) Cancer care in Brazil: structure and geographical distribution. BMC Cancer 19(1): 1-11.
- All Can (2022) Survey: Barriers for patients with Cancer in Colombia. Centro Nacional de Consultorí
- Campos Mariana (2023) The Decline of Fonsabi.
- Economist Impact (2022) The Journey towards Health Improvement in Argentina: A Roadmap for Precision Medicine.
- Ministry of Health Brazil (2024) Genomes Brazil. Government Brazil.
- National Cancer Institute Argentina (2024) Protocol on the Role of Genetic Oncology Counselling within the Framework of Precision Oncology Pp: 1-32.
- Chief of Staff of the Ministers’ Cabinet Argentina (2024) Reference Program and Genomic Biobank of the Argentine Population (PoblAr). Argentina Government.
- Mexican Institute of Social Security (IMSS) (2023) Precision and cutting-edge treatments in breast cancer research. Gobierno de Mexico.
- Mexican Institute of Social Security (IMSS) (2023) IMSS presents the Translational Research Program to fight paediatric cancer. Gobierno de Mexico.
- Health Secretariat Mexico (2023) National Institutes of Cancerology and Genomic Medicine, Leaders in Hereditary Cancer Care.
- Ministry of Health Colombia (2012)10-Year Cancer Control Plan 2012-2021.
- Ministry of Health Colombia (2021) MinSalud and Cancerology Institute Will Integrate Public Health and Cancer Control Plans.
- World Economic Forum (WEF) (2022) 4 Agile Ways Policy-Makers Can Advance Precision Medicine.
- Downes L (2009) The Laws of Disruption: Harnessing the New Forces That Govern Life and Business in the Digital Age. 1st
- Senate of the Republic Mexico (2023) Bill with a draft decree by which various provisions are added to the general health law, regarding precision medicine. Gobierno de Mexico.
- Deputy Chamber of the Union Congress Mexico (2023) Bill adding various provisions of the General Health Law, regarding precision medicine, signed by members of the parliamentary group of the PAN. Sistema de Información Legislativa de la Secretaría de Gobernación.
- Mastology Brazilian Society (SBM) (2024) Genetic testing for breast cancer in the SUS has the potential to save lives SBM.
- Deputy Chamber Brazil (2021) Commission approves bill that obliges SUS to perform genetic testing for cancer predisposition. Agencia Câmara de Notí
- The Economist Intelligence Unit (2020) Personalised Healthcare in Latin America Universalising the Promise of Innovation.
- Ministry of Health Colombia (2016) Oncological Services in Colombia Bulletin.
- Yuba TY, Novaes HMD, de Soárez PC (2018) Challenges to decision-making processes in the national HTA agency in Brazil: operational procedures, evidence use and recommendations. Health Res Policy System 16(1): 1-9.
- Oncoguia (2023) Oncology Drugs Await Measures from the Ministry.
- Stoddart K, Newton M, Ballalai A, Troein P (2022) FIFARMA Patients W.A.I.T Indicator 2022 Survey Pp: 1-40.
- Ribeiro AA, Acosta A, Pontes MA, Machado Beltran MA, Peixoto RT, et al. (2023) Transparency of data on the value chain of medicines in Argentina, Brazil, and Colombia. Front Pharmacology Pp: 1-18.
- CENETEC (2024) Health Technology Assessment Documents. Gobierno de Mé
- Economic Research and Budget Center (CIEP) (2023) Health Fund for Wellbeing: Costs, coverage, and projections 2035.
- Pereira LC, Sturzenegger DVR, Ortiz J, et al. (2019) Challenges in the Regulation of High-Cost Treatments: An Overview from Brazil. Value Health Reg Issues 20:191-195.
- Finance Secretariat Mexico (2023) Health care and Medicines at No Cost for the Population Without Employment-based Social Security Transparencia Presupuestaria Mexico.
- Ombudsman Office Colombia (2023) Ombudsman Office, concerned about the Number of Legal Claims Filed by Cancer Patients.
- Government of Mexico (2023) Organic Statute of Health Services of the Mexican Institute of Social Security for Well-being (IMSS-Wellbeing) Diario Official de la Federation.
- Hauegen R, Colacino L, Feitosa M (2022) Comparative Analysis on Access to Advanced Therapy Medicined Products in Latin America.
- Sussman L, Garcia-Robledo JE, Ordonez-Reyes C, et al. (2022) Integration of artificial intelligence and precision oncology in Latin America. Front Med Technol 4: 1-12.
- McGrath S, Ghersi D (2016) Building towards precision medicine: empowering medical professionals for the next revolution. BMC Med Genomics 9(23): 1-6
- Ruiz de Castilla EM, Mayrides M, Gonzalez H, et al. (2024) Implementing precision oncology in Latin America to improve patient outcomes: the status quo and a call to action for key stakeholders and decision-makers. Ecancermedicalscience 18: 1-11.
- World Bank (2021) Physicians (per 1,000 people)-Colombia. World Bank Indicators.
- Bonilla C, Albuquerque Sortica V, Schuler-Faccini L, Matijasevich A, Scheffer MC (2022) Medical geneticists, genetic diseases and services in Brazil in the age of personalized medicine. Per Med 19(6): 549-563.
- National Health Institute Colombia (2022) Colombia is designated by the WHO as regional reference for genomic surveillance National Health Institute.
- German Federal Ministry of Health (2022) genomDE- National Strategy for Genomic Medicine.
- NHS England (2022) Accelerating genomic medicine in the NHS. NHS England.
- National Institutes of Health (NIH) (2022) All of Us Research Program Protocol Summary.
- PR Newswire (2016) Genome Asia 100K Initiative Announced to Sequence 100,000 Genomes in South, North and East Asia. PR Newswire Association LLC.
- Genome Canada (2022) 20 years collaborating on the future: Explore Genome Canada’s 20-year success story and our vision for the future. Genome Canada.
- Genomic Medicine Sweden (2024) Genomic Medicine Sweden About us. Genomic Medicine Sweden.
- Ministry of Health Brazil (2020) 20 years of the International Health Affairs Office of the Ministry of Health of Brazil.
- International Labour Organization (ILO) (1988) Social Code - Book V - Statutory Health Insurance. Federal Ministry of Justice.
- Wall JD, Stawiski EW, Ratan A, et al. (2019) The Genome Asia 100K Project enables genetic discoveries across Asia. Nature 576(7785): 106-111.
- Genome Canada (2018) Genome Canada Guidelines for Funding.
- Koleva Kolarova R, Buchanan J, Vellekoop H, et al. (2022) Financing and Reimbursement Models for Personalised Medicine: A Systematic Review to Identify Current Models and Future Options. Appl Health Econ Health Policy 20(4): 501-524.
- Genome Canada (2022). All for One: Canada’s precision health partnership. Genome Canada.
- Lemay M (2019) The Role of Expectations of Science as Promissory Discourses in Shaping Research Policy: A Case Study of the Creation of Genome Canada. A thesis submitted in conformity with the requirements for the degree of Doctor of Philosophy. University of Toronto Pp: 1-287.
- Genome Asia 100k. Genome Asia 100k Collaborate. Genome Asia 100k. 2023.
- genomDE genomDE-Consortium (2024) [Translated from German: genomDE-Konsortium].
- National Institutes of Health (NIH) (2021) All of Us Research Program-Protocol V1: 1-129.
- Genome Canada (2022) Genome Canada Our Model. Genome Canada.






























