Glucoscore: An Innovative Metric for Evaluating Glycemic Impact in Precision Nutrition
Alberto Conde Mellado* and Nere Arróniz
Glucovibes, Juan Fermín Gilisagasti, Donostia & Founder and Scientic Director, Spain
Submission: August 22, 2024; Published: August 29, 2024
*Corresponding author: Alberto Conde Mellado, Founder and Scientic Director, Spain. Email: alberto@glucovibes.com
How to cite this article: Alberto Conde Mellado* and Nere Arróniz. Glucoscore: An Innovative Metric for Evaluating Glycemic Impact in Precision Nutrition. JOJ Pub Health. 2023; 9(2): 555759. DOI: 10.19080/JOJPH.2024.09.555759
Abstract
The GlucoScore is an innovative metric developed to quantify the glycemic impact of various foods and meals, essential for precision nutrition strategies. Calibrated using over 100,000 hours of metabolic data from more than 1,500 users, the GlucoScore ranges from 1 to 10, with higher scores indicating minimal glycemic impact. This metric incorporates key parameters such as maximum glucose peak, amplitude of the glucose curve, variation over time, and area under the curve (AUC) as main variables to parametrize Artificial Intelligence for an automation of the knowledge. Validation against 10,000 meals demonstrated a high repeatability with an error margin of less than 10%. GlucoScore’s adaptive learning capabilities continuously enhance its accuracy, processing glycemic data within a few seconds after the analysis window. This paper explores the development, calibration, and implications of the GlucoScore in metabolic health with some examples.
Keywords: Glucoscore; Glycemic Impact; Continuous Glucose Monitoring; Precision Nutrition; Metabolic Health
Abbreviations: CGM: Continuous glucose monitoring; AUC: Area Under the Curve
Introduction
Continuous glucose monitoring (CGM) has revolutionized our understanding of individual metabolic responses, revealing significant variability in how different foods impact glucose levels. Previous studies have shown that postprandial glucose responses vary greatly among individuals consuming the same foods, driven by factors such as microbiota composition, genetics, and lifestyle [1,2]. This variability underscores the need for advanced tools that can integrate and visualize the complex interplay of these factors. To address this need, Glucovibes developed the GlucoScore-a comprehensive metric designed to quantify the glycemic impact of meals. This metric provides actionable insights into how dietary choices affect metabolic health, supporting personalized nutrition strategies that optimize glycemic control. The development of such tools is part of a broader effort to understand and manage metabolic heterogeneity, a concept explored further in the recent introduction of the Glycaemic Matrix and Metabolic Segmentation by Arroniz, et al [3]. This new approach combines glucose and lifestyle data to create functional profiles that can help cluster metabolic types and adapt nutrition or clinical interventions accordingly. The GlucoScore offers a more nuanced measure of glycemic impact compared to traditional methods. While previous studies have demonstrated the variability in postprandial glucose responses [1,2], the GlucoScore integrates multiple dimensions of the glucose response into a single, interpretable score. This chapter details the scientific principles, calibration process, and validation of the GlucoScore, highlighting its significance in the context of personalized nutrition.
Materials and Methods
GlucoScore Calibration Process
The calibration of the GlucoScoress involved an extensive analysis of over 100,000 hours of metabolic data from more than 1,500 users. This data set provided comprehensive information on individual glycemic responses, enabling the development of a robust clustering model. The GlucoScore was calibrated to reflect glycemic impact on a scale from 1 to 10, with 10 representing minimal impact (flat glucose curve) and 1 indicating the biggest impact as glycemic response.
Key parameters used in the GlucoScore calibration includes:
Maximum Glucose Peak: The highest glucose level observed within 2 hours post-ingestion.
Amplitude of the Glucose Curve: The difference between the glucose level at t=0 time and the biggest peak trough glucose values during the 2-hour window.
Delta between t=0 and t=+2 hours glucose values: The change in glucose levels from baseline to the value at the end of the 2-hour period.
Area Under the Curve (AUC): The total glycemic exposure during the 2-hour period.
These parameters were clustered to create the GlucoScore using a Random Forest approach, which was then tested against an additional 10,000 meals to validate its accuracy.
Validation and adaptive learning
To ensure repeatability and accuracy, the GlucoScore was validated against +4,500 meals across different +500 users, demonstrating a high repeatability with an error margin of less than 10% for the 98,6% of the meals. Additionally, the GlucoScore is designed to continuously learn from new data, refining its accuracy over time. This adaptive learning capability allows the metric to adjust to individual variations in metabolism, thereby improving its predictive power.
Results
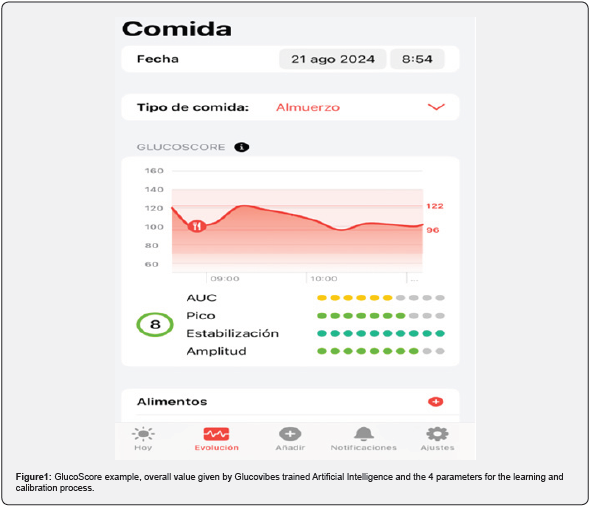
The validation process confirmed that the GlucoScore is a reliable measure of glycemic impact, with an error margin of less than 10%. The metric was able to process and analyze glycemic responses within a few seconds after the 2-hour postmeal period, making it highly efficient for real-time application. Some examples obtained from the sample show that foods like Natural yogurt exhibited a high GlucoScore (8.89) with minimal variation (standard deviation of 0.59), indicating a consistently low glycemic impact. In contrast, items like Banana showed more variability in GlucoScore, 6.28 with a standard deviation of 1.93, suggesting the need for personalized dietary recommendations. (Figure 1) shows an example of the GlucoScore after one meal, showing both the overall score given by the Artificial Intelligence algorithm developed and described in this paper and the [4] parameters used for the calibration of the algorithm.
Discussion
The GlucoScore ability to capture the complexity of glycemic responses through a single metric offers significant advantages in precision nutrition. The variability observed across different foods and among users highlights the importance of personalized approaches in dietary management. The consistent performance of some foods across different individuals suggests that certain foods may universally produce minimal glycemic impact, while others require more individualized consideration [5,6]. The GlucoScore’s adaptive learning feature is particularly noteworthy, as it allows the metric to improve its accuracy over time by learning from new data. This ensures that the GlucoScore remains a relevant and powerful tool in personalized nutrition and metabolic health management helping in the selection of the best food options for precision nutrition strategies.
Conclusion
The GlucoScore represents a significant advancement in the assessment of glycemic impact, offering a robust and reliable metric for use in precision nutrition. Its development and validation through extensive data analysis ensure that it provides accurate and actionable insights into the glycemic impact of foods. The adaptive learning capability of GlucoScore enhances its utility, making it a dynamic tool for managing metabolic health.
Acknowledgements
The author acknowledges the contributions of the Glucovibes research and development team together with all the early adopters and testers of the different studies that were being addressed to get these results and new technologies and concepts. Their invaluable support in the development and validation of the GlucoScore metric has been key to generating these new tools for precision nutrition.
Conflict of Interest
The authors are employees of Glucovibes, the entity responsible for the development of the GlucoScore metric and the Glycemic Matrix and Segmentation concept. Dr. Alberto Conde Mellado is the founder and Scientific Director of Glucovibes.
References
- Zeevi D, Korem T, Zmora N, David I, Michal R, et al. (2015) Personalized Nutrition by Prediction of Glycemic Responses. Cell 163(5): 1079-1094.
- Zhao X, Chen L, Li S (2020) Precision Nutrition in Diabetes Management: A Review of Current Evidence and Future Directions. Journal of Diabetes Research P: 1-10.
- Arroniz N, Conde Mellado A, Francés L (2024) Glycaemic Matrix and Segmentation: A New Metabolic Visualisation and Analysis Tool. Proceedings of the 14th European Nutrition Conference FENS 2023 91(1): 140.
- Jenkins DJ, Wolever TM, Taylor RH, H Barker, DV Goff, et al. (1981) Glycemic index of foods: a physiological basis for carbohydrate exchange. Am J Clin Nutr 34(3): 362-366.
- Mendes Soares H, Raveh Sadka T, Azulay S, Kim Edens, Lihi Segal, et al. (2019) Assessment of a Personalized Approach to Predicting Postprandial Glycemic Responses to Food Among Individuals Without Diabetes. JAMA Netw Open 2(2): e188102.
- Chen L, Magliano DJ, Zimmet PZ (2020) The worldwide epidemiology of type 2 diabetes mellitus-present and future perspectives. Nat Rev Endocrinol16(2): 63-74.






























