Evaluating Quality of Care for Hereditary Angioedema in a Health Care System
Andrew Yeich1, Kristen Park2, Peter Kruse3, Dinh Van Nguyen4, Timothy Craig5*
1Penn State College of Medicine, Hershey
2Penn State College of Medicine, Hershey
3Oak Ridge National Laboratory, University of Tennessee, Oak Ridge
4Vinmec Healthcare System and College of Health Sciences, Vin University, Hanoi, Vietnam, Dept of Medicine, Penn State, Hershey
5Pediatrics and Biomedical Sciences, Penn State University, Hershey, USA
Submission: August 30, 2023; Published: September 13, 2023
*Corresponding author: Timothy Craig, Pediatrics and Biomedical Sciences, Penn State University, Hershey, USA
How to cite this article:Andrew Y, Kristen P, Peter K, Dinh Van N, Timothy C. Evaluating Quality of Care for Hereditary Angioedema in a Health Care System. JOJ Pub Health. 2023; 8(1): 555729. DOI: 10.19080/JOJPH.2023.08.555729
Abstract
Background: Hereditary angioedema (HAE) is an orphan disease that inflicts significant morbidity, post-traumatic stress, anxiety, productivity loss, and potential mortality on its affected patients. For this reason, treatment must be uniquely designed to ensure comprehensive and holistic quality of care.
Objective: To evaluate HAE care in a health care system (HCS) for adequacy and disparities.
Methods: We reviewed the HAE Guidelines and literature and from this developed a survey to assess quality of care for HAE in a HCS. This study met Institutional Review Board exception since it was for quality improvement. RedCap (Vanderbilt, Nashville, TN, USA) was used for collection and evaluation of the data. Chi-square testing was used to compare demographic groups.
Results: The entire population had high rates of their quality of life, attack frequency, rescue medication use, and short- and long-term prophylaxis being recorded. Surgical history, dental hygiene, and avoidance of triggering medications were infrequently addressed. Discrepancies across age and sex categories exist for medication choices, decisions to leave attacks untreated, and patient education. The number of African American patients with HAE in the HCS were too few to determine if disparity of care exists.
Conclusion: Assessment of the care provided suggests that the use of a standardized instrument to gauge HAE control and educate patients during follow-up should be encouraged.
Keywords: Hereditary Angioedema; Orphan Diseases; Quality Improvement; Healthcare Systems; Healthcare Disparities
Introduction
Hereditary angioedema (HAE) is a category of rare genetic disorders originating from excess bradykinin-mediated stimulation of bradykinin B2 receptors leading to severe, symptomatic vasodilation. [1-3] There are three main subtypes of HAE. Type I, the most common, is caused by a deficiency of C1-inhibitor protein (C1-INH), while in type II the protein is present but dysfunctional [4,5] Recently, HAE patients with normal C1-INH levels and function have been identified as type III HAE, better referred to as HAE with normal C1-inhibitor (HAE nl-C1-INH), which has a varied etiology [6] Clinical manifestations are relatively consistent regardless of genotype and include recurrent episodes of nonpruritic swelling of skin and mucosal tissues. Although the swelling is self-limited, laryngeal involvement can compromise the airway and become rapidly fatal if not treated. These episodes begin during childhood, or adolescence for most patients, with symptoms increasing in severity and frequency after puberty [7] Attacks are often spontaneous, but triggers such as stress, trauma, dental, medical and surgical procedures, infections, angiotensin converting enzyme inhibitors (ACE-I), and estrogen containing medications have been shown to cause episodes in patients with HAE [8-10]
Once diagnosed, the decision tree for subsequent management of those diagnosed with HAE is quite wide as many new therapies have recently entered the market [11] The United States Hereditary Angioedema Association (US-HAEA) has identified four guiding principles for treatment of HAE (1) availability of acute therapy for all patients, (2) early treatment of attacks to prevent progression, (3) treatment of all attacks regardless of site, and (4) incorporation of long-term prophylaxis (LTP) based on shared patient-physician decision making [12] C1-INH concentrates, ecallantide, and icatibant are approved for rescue therapy; C1-INH concentrate drugs, lanadelumab, berotralstat, and androgens are approved for LTP. Beyond medical treatment, patient education, ensuring regular follow-up with an expert HAE physician, and coordination of care with other providers are all crucial to reducing illness burden [13]
The purpose of this quality assurance study was to characterize the population of type I and II HAE patients managed by an academic health care system to assess the quality-of-care HAE patients receive. Herein, we evaluated the demographic breakdown of the patients, the medication plans prescribed to them, the quality of medical history taken, and whether adequate HAE education was provided. These data were then stratified by age, sex, and race to see if such factors affected the identified metrics.
Methods
This quality assurance project met criteria for Institutional Review Board exclusion. A master list was generated that included all patients with confirmed or suspected HAE treated from 2019 to 2022 by the academic health care system. A total of 121 patients were identified by the 10th revision of the International Statistical Classification of Diseases and Related Health Problems (ICD10) code D84.1. Using the most recent note in their electronic medical record (EMR) corresponding to an allergy immunology exam, patients were manually classified into those with HAE-C1- INH confirmed by laboratory C1-INH testing and those with either HAE nl-C1-INH, acquired angioedema, or those without a clear diagnosis at the time. 83 patients were identified with confirmed HAE-C1-INH; all others were excluded from data collection. If a patient’s diagnosis was not clearly outlined in the most recent note, previous allergy immunology notes (AIN) were examined in reverse chronological order to determine the final diagnosis.
The selected patients were de-identified and basic demographic information was collected from the EMR including HAE diagnosis, age, sex, and race. Using only the most recent AIN, we recorded the HAE medications currently prescribed and evaluated the quality of the AIN based on 10 standardized binary metrics. The survey used is outlined in Table 1 RedCap (Vanderbilt, Nashville, TN, USA) was used for collection and evaluation of the data [14,15] Chi-square test was used to assess significant differences [16]
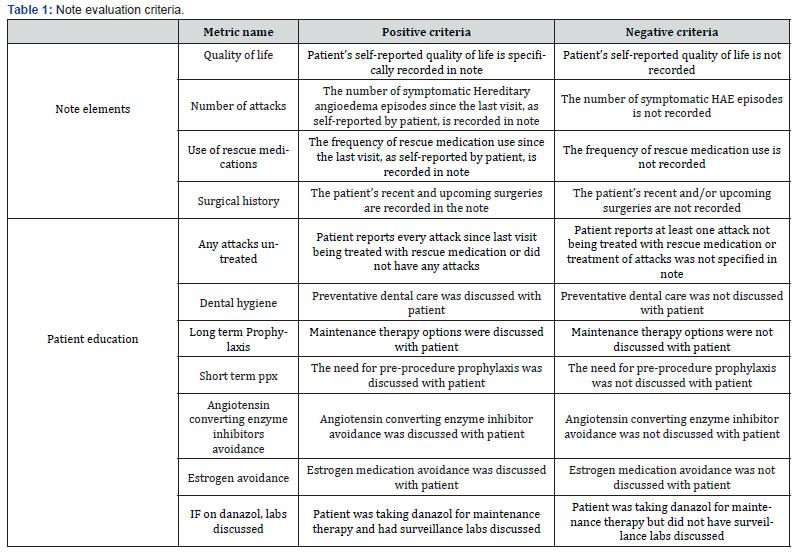
Results
There were 83 patients of the 121 with the ICD10 84.1 code with confirmed HAE-C1-INH included in the analysis. Among these, 71 had type I HAE, 5 had type II, and 7 did not have a clearly labeled diagnosis in any AIN but had confirmatory C1-INH testing. The majority of patients were White females over the age of 18; there were only 34 male patients, 13 patients under the age of 18, and 6 African American patients. There were no patients of other race categories, and 10 patients did not have any race recorded in the EMR. Table 2 shows patient characteristics.
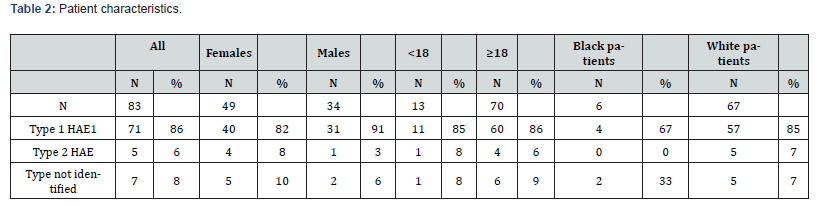
Table 3 shows the quality metrics for all included patients and stratifies by age, sex, and race. The overwhelming majority of AINs contained information on the patient’s quality of life, number of HAE attacks, and the use of rescue medications. Most patients also received education regarding maintenance therapy options and were reminded on the importance of pre-procedure prophylaxis. All 8 patients receiving danazol for long term maintenance therapy were informed of the need for surveillance lab work. 69% of patients reported treating every attack regardless of severity and site of symptoms. Records of surgical history were only mentioned in about half of all patients. The importance of dental hygiene (45%), ACE-I avoidance (23%), and estrogen medication avoidance (28%) were inconsistently addressed.
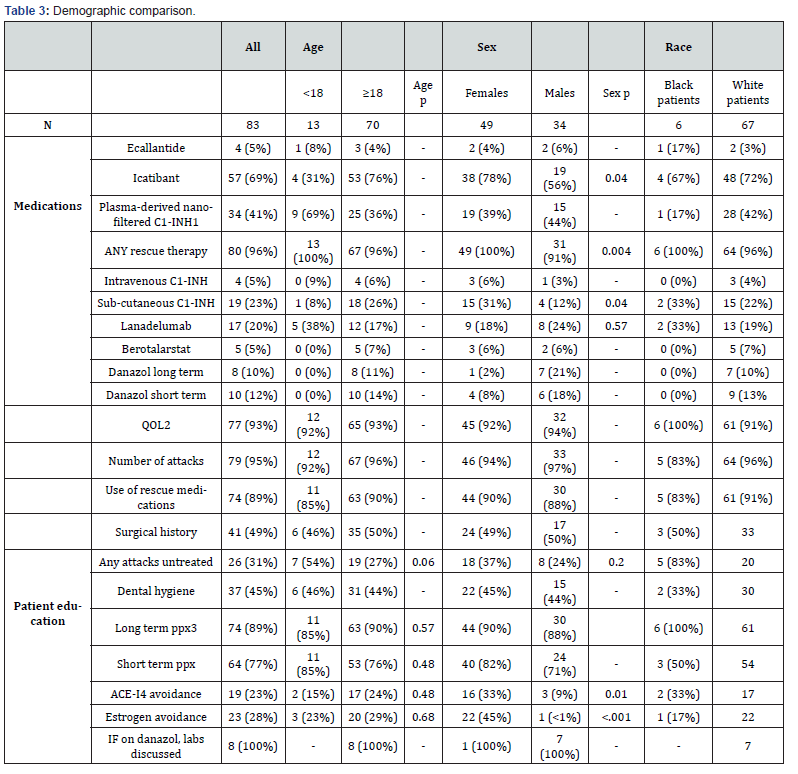
When separated by age, rates of use of almost every medication varied markedly. The frequency by which AINs met note completeness metrics was very similar (within ≤ 5%). Patients with HAE-C1-INH under the age of 18 more frequently let at least one attack go untreated in the interval history. A smaller percentage of children received education on avoiding ACE-I and estrogen containing medication. Maintenance therapy was discussed at a similar rate, but a higher proportion of children were counseled on pre-procedure prophylaxis.
Analysis by sex reveals that men were less likely to be prescribed icatibant or have any rescue therapy on hand. Overall prevalence of maintenance therapy was relatively similar, but women were prescribed sub-cutaneous C1-INH (SC-C1-INH) at a much higher rate, while more men were using lanadelumab and danazol. The contents of the note history were very consistent between sex categories. Many more women had HAE episodes without treatment, but they were more likely to receive education concerning short term prophylaxis, ACE-I avoidance, and estrogen avoidance. All other patient education categories were consistent between the subgroups. When assessing for racial disparity, ten patients were excluded from the subgroups as they did not have racial demographic data recorded in the EMR. Only six patients treated for HAE were listed as African American in the EMR.
Discussion
Congruent with existing literature, most patients were female. Even though “genetic HAE” is equal in sexes, symptomatic disease is greater in females, and this may account for the asymmetry between the number of men and women receiving care. Our prior data suggest care for African American patients is not disparate, however, secondary to the demographics of our area care for African Americans and other races is underrepresented [17]
Our analysis suggests that several aspects of HAE care were consistently addressed in patient visits. Note elements such as recording the number of attacks and the use of rescue medications, as well as inquiring into the patient’s quality of life, are basic components of any complete history that can be analogized to the treatment of other chronic diseases. It is reassuring that these are recorded in almost all our patient notes. The US-HAEA 2020 guidelines recommend that each patient have at least 2 doses of rescue medication regardless of disease severity and use of maintenance therapy. While it is likely that some patients may have expired or missing medications, over 90% of every subpopulation studied had some form of rescue medication prescribed. High rates of discussions concerning both long- and short-term prophylaxis are similarly encouraging; both aspects of HAE care are listed as “strong” recommendations in the updated guidelines. All patients receiving regular androgen treatment were counseled on the results of their surveillance lab work or directed to get it done if out of date. This is a biannual requirement for administration given the class’s side effect profile [18].
Unfortunately, some metrics were not as common across all patient groups. Most patients did not have their surgical history recorded. While surgical history taking is not an explicit recommendation by the US-HAEA, this is important to ensure preprocedure prophylaxis discussions. Additionally, AINs frequently failed to include discussions about dental hygiene. Maintaining regular dental care has been shown to mitigate the need for dental procedures which themselves can cause HAE exacerbations [19] Discussion of HAE triggers is an included guideline component for follow-up visits, and yet only 23% of patients received ACE-I education with 28% receiving warnings about estrogen containing medication.
Statistical testing for both age and race proved impossible due to the limited size of the HAE patient population. When studying by sex, however, several trends are noted. Despite the overall high prevalence of rescue therapy, male patients were less likely to have access to such medication (P=.004). Men were also less likely to be prescribed icatibant (P=.04). Overall prevalence of maintenance therapy was relatively similar, but women were prescribed SC-C1-INH at a much higher rate (P=.04). This preference may be explained by greater adverse event risks from danazol in females and the concern of using lanadelumab in females attempting to become pregnant due to lack of safety data. HAE is a complicated chronic disease with divergent treatment options, and the guidelines stress treatment plans need to be individualized according to each patient’s needs. Patient education metrics were similarly disparate, with men being less likely to receive education on trigger avoidance for both ACE-I and estrogen (P=.01), (P<.001). Considering the possibility of patients concurrently undergoing gender affirming therapy, physicians may consider counseling all HAE patients on the risk of estrogen containing medication.
Taken together, these analyses demonstrate the need for implementation of a standardized follow-up procedure for HAE patient care. While most elements of a general “history of present illness” were included in the notes (i.e., recording attack frequency and its impact on patient quality of life), important aspects of patient education were inconsistently addressed. A physician with years of experience may perceive certain aspects of this education as redundant, but many patients without such training stand to benefit. The current US-HAEA treatment guidelines thus emphasize the need for patient education as a “strong” recommendation. Furthermore, several facets of HAE care were found to be statistically different between the sexes. Discrepancies of the specific treatment selected is likely due to patient preference, but an emphasis should be placed on ensuring equitable care. Further work is needed to stratify by age and race.
Conclusion
Assessment of the care provided suggests that the use of a standardized instrument to gauge HAE control and educate patients during follow-up should be encouraged.
Conflicts of interest
None.
References
- Reshef A, Kidon M, Leibovich I (2020) The Story of Angioedema: from Quincke to Bradykinin. Clin Rev Allergy Immunol 51(2): 121-139.
- Busse PJ, Christiansen SC (2020) Hereditary Angioedema. N Engl J Med 382(12): 1136-1148.
- Frank MM, Zuraw B, Banerji A, Bernstein JA, Craig T, et al. (2016) Management of Children with Hereditary Angioedema Due to C1 Inhibitor Deficiency. Pediatrics 138(5): e20160575.
- Zuraw BL, Christiansen SC (2016) HAE Pathophysiology and Underlying Mechanisms. Clin Rev Allergy Immunol 51(2):216-229.
- Prada AE, Zahedi K, Davis AE (1998) Regulation of C1 inhibitor synthesis. Immunobiology 199(2): 377-388.
- Bork K (2013) Hereditary angioedema with normal C1 inhibitor. Immunol Allergy Clin North Am 33(4): 457-470.
- Bork K, Meng G, Staubach P, Hardt J (2006) Hereditary angioedema: new findings concerning symptoms, affected organs, and course. Am J Med 119(3): 267-274.
- Bork K, Fischer B, Dewald G (2003) Recurrent episodes of skin angioedema and severe attacks of abdominal pain induced by oral contraceptives or hormone replacement therapy. Am J Med 114(4): 294-298.
- Ricketti AJ, Cleri DJ, Ramos Bonner LS, Vernaleo JR (2007) Hereditary angioedema presenting in late middle age after angiotensin-converting enzyme inhibitor treatment. Ann Allergy Asthma Immunol 98(4): 397-401.
- Zotter Z, Veszeli N, Kőhalmi KV, Varga L, Imreh É, et al. (2016) Bacteriuria increases the risk of edematous attacks in hereditary angioedema with C1-inhibitor deficiency. Allergy 71(12): 1791-1793.
- Cicardi M, Aberer W, Banerji A, Bas M, Bernstein JA, et al. (2014) Classification, diagnosis, and approach to treatment for angioedema: consensus report from the Hereditary Angioedema International Working Group. Allergy 69(5): 602-616.
- Busse PJ, Christiansen SC, Riedl MA, Banerji A, Bernstein JA, et al. (2021) US HAEA Medical Advisory Board 2020 Guidelines for the Management of Hereditary Angioedema. J Allergy Clin Immunol Pract 9(1): 132-150.
- Betschel S, Badiou J, Binkley K, Borici Mazi R, Hébert J, et al. (2019) The International/Canadian Hereditary Angioedema Guideline. Allergy Asthma Clin Immunol 15: 72.
- Harris PA, Taylor R, Thielke R, Payne J, Gonzalez N, et al. (2009) Research electronic data capture (REDCap)--a metadata-driven methodology and workflow process for providing translational research informatics support. J Biomed Inform 42(2): 377-381.
- Harris PA, Taylor R, Minor BL, Elliott V, Fernandez M, O'Neal L, et al. (2019) The REDCap consortium: Building an international community of software platform partners. J Biomed Inform 95: 103208.
- McHugh ML (2013) The chi-square test of independence. Biochem Med (Zagreb) 23(2): 143-149.
- Sylvestre S, Craig T, Ajewole O, Craig S, Kaur S, et al. (2021) Racial and Ethnic Disparities in the Research and Care of Hereditary Angioedema Patients in the United States. J Allergy Clin Immunol Pract 9(12): 4441-4449.
- Zuraw BL, Davis DK, Castaldo AJ, Christiansen SC (2016) Tolerability and Effectiveness of 17-α-Alkylated Androgen Therapy for Hereditary Angioedema: A Re-examination. J Allergy Clin Immunol Pract 4(5): 948-955
- Kranz AM, Rozier RG, Preisser JS, Stearns SC, Weinberger M, et al. (2014) Preventive Services by Medical and Dental Providers and Treatment Outcomes. J Dent Res 93(7):633-638.






























