The Potential of a Plant-Based Diet to Prevent New Influenza Viruses
Stewart Rose and Amanda Strombom*
Plant-Based Diets in Medicine, USA
Submission: 2022; Published: September 23, 2022
*Corresponding author: Amanda Strombom, Plant-Based Diets in Medicine, Bellevue, WA 98006, Washington, USA
How to cite this article:Rose S, Strombom A. The Potential of a Plant-Based Diet to Prevent New Influenza Viruses . JOJ Pub Health. 2022; 7(1): 555702. DOI: 10.19080/JOJPH.2022.07.555702
Abstract
Influenza is a highly contagious disease that is responsible for significant morbidity and mortality worldwide. Influenza A viruses (IAVs) have the ability to cross interspecies barriers from avian carriers and then rapidly circulate among and infect crowded livestock creating a breeding ground for the emergence of zoonotic viruses with epidemic and pandemic potential. They are susceptible to antigenic shift and drift and hence are the cause of recurring major epidemics and pandemics. Intensive animal farming creates conditions for the emergence and amplification of epidemics because of the physical and genetic proximity of the billions of animals, often in frail health, raised indoors every year. In particular, because swine and poultry are susceptible to infection with both avian and human influenza viruses, novel influenza viruses can be generated by reassortment of influenza viral segments. These are then transmitted via farm workers into the human population. Increased globalization is a significant factor in the worldwide spread of human influenza viruses that spillover from poultry and swine. The efficacy of influenza vaccination as a public health measure is limited by both the efficacy of the vaccine, which needs to be reformulated biannually, and the degree of public access to the vaccine. The most effective public health prophylaxis would therefore be to encourage less consumption of animal products, thus reducing the need for intensive animal agriculture. This will cut the link in the chain of emergence of influenza viruses into the human population, while at the same time, improving public health more directly.
Keywords: Animal agriculture; Novel influenza virus; Reassortment; Transmission; Vaccine
Abbreviations: IAVs: Influenza A viruses
Introduction
Influenza is a highly contagious respiratory illness that is responsible for significant morbidity and mortality. Approximately 9% of the world’s population is affected annually, with up to 1 billion infections, 3 to 5 million severe cases, and 300,000 to 500,000 deaths each year [1-3].
In the U.S. alone, nearly 20% of the population is affected annually. On average, 25 to 50 million documented influenza cases, 225,000 hospitalizations, and ultimately more than 20,000 deaths occur every year [1,2,4-7]. The estimated average annual total economic burden of influenza to the American healthcare system and society is $11.2 billion [8].
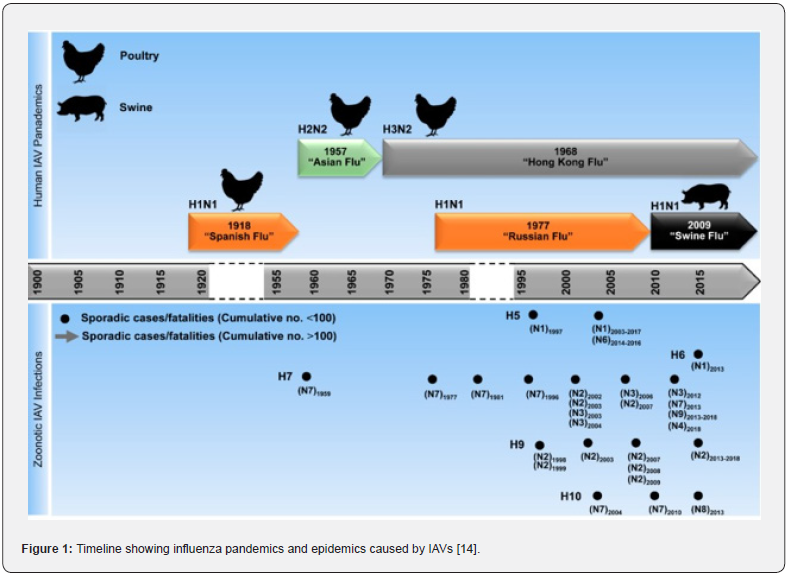
These figures only show the impact of influenza on society in a typical year. Since the beginning of the 20th century, zoonotic spillover events have given rise to the generation of multiple, well-documented pandemic influenza viruses [9-11]. Four influenza pandemics, or global epidemics, in particular have affected humanity in the past century. The most devastating of these occurred in 1918, causing influenza in about half the world population, with 30–50 million deaths worldwide, affecting principally the young and otherwise healthy [12]. The subsequent influenza pandemics of 1957 (“Asian flu”), 1968 (“Hong Kong flu”) and 2009 (“Mexican flu”) were milder, each claiming the lives of 0.3–2 million individuals. [13,14] In addition to these major pandemics, the chart below shows sporadic cases/fatalities (Figure 1) [14].
According to one study, since 1940, agricultural drivers were associated with more than 25% of all, and more than 50% of specifically zoonotic infectious diseases that emerged in humans, proportions that will likely increase as agriculture expands and intensifies [15].
This article focuses specifically on influenza viruses, since these are the ones that have had the greatest impact on human life to date. Of course, there are many other zoonotic infectious diseases, both viral and bacterial, which have the potential to become pandemics.
Genetic drift and reassortment
Influenza is an acute respiratory infection caused by a negative-strand RNA virus of the Orthomyxoviridae family [16]. There are four recognized types of influenza viruses (A, B, C, and D), with influenza D virus (IDV) the most recently discovered [17].
Types A, B, and C are defined on the basis of variation in the nucleoprotein antigen. In types A and B, the hemagglutinin and neuraminidase antigens undergo genetic variation, which is the basis for the emergence of new strains; type C is antigenically stable [18].
Influenza A viruses (IAVs) cause the most morbidity in both humans and animals. They infect multiple species, including humans, swine, equines, and birds. They are more susceptible to antigenic variation and hence, are the cause of major pandemics [19].
Influenza viruses are spherical or filamentous enveloped particles 80 to 120 nm in diameter. The helically symmetric nucleocapsid consists of a nucleoprotein and a multipartite genome of single-stranded antisense RNA in seven or eight segments. The envelope carries a hemagglutinin attachment protein and a neuraminidase [18].
There are 18 recognized hemagglutinin (HA) and 11 neuraminidase (NA) glycoproteins by which IAVs are subtyped. IAVs change through mutation, recombination, and reassortment, frequently challenging the immune systems of their human and animal hosts [20].
As segmented RNA viruses, IAVs can exchange the gene segments through reassortment during co-infection. Specifically, when two or more IAVs infect the same cell, a hybrid virus can be produced by assembling the gene segments of the parental viruses into a nascent virion [21]. Reassortment and mutation are both the main driving forces for the evolution of IAVs. However, the effect caused by mutations needs to be accumulated for a long time, while the reassortment is often a leapfrog evolution.
Transmission amongst and between animals
The ability of IAVs to rapidly cross interspecies barriers and circulate in a variety of avian and mammalian species of wildlife and livestock creates a breeding ground for zoonotic strains with pandemic potential.
Aquatic birds specifically often carry IAVs without getting sick themselves. They can spread the virus broadly into animal agriculture, since the transmission route of IAVs in aquatic birds is fecal-oral, and feces can contain highly concentrated amounts of the virus [22,23].
Over the past 70 years, food animal production in much of the world has been transformed from traditional small-scale methods and entrepreneurial organization to industrial-scale operations and vertically integrated management, in which most if not all aspects of production (breeding, supply of young animals, feeds, animal husbandry) are controlled by a single entity [24,25].
Intensive animal farming creates conditions for the emergence and amplification of epidemics because of the physical and genetic proximity of the billions of animals, often in frail health, that are raised indoors every year [26]. As intensification of livestock production increases, especially pigs and poultry, disease transmission is facilitated by the increasing population size and density of the animals [27-29].
Intensification also requires greater frequency of movement of people and vehicles on and off farms, which further increases the risk of pathogen transmission since animal workers and other animal species may introduce new IAV strains to the flocks or herds [30].
Swine and poultry are two key reservoirs of IAVs and both are in rapidly growing livestock industries. As livestock industry farms are often home to several thousand birds or pigs, sometimes harboring multiple subtypes of IAVs, they are considered highrisk areas for novel IAV generation [20]. Thousands of animals can be infected within a few days.
The selection of the most profitable species of farmed animals in intensive farms leads to a high level of genetic similarity, facilitating the spread of the pathogens as all animals within the farms are immunologically naïve hosts. Frequent introductions of more immunologically naïve animals help maintain IAVs circulating within a livestock population, increasing the chance of catastrophic epidemics on the farm [28,31,32].
In particular, because swine are susceptible to infection with both avian and human influenza viruses, novel reassortant influenza viruses can be generated in this mammalian species by reassortment of influenza viral segments, leading to the “mixing vessel” theory that novel viruses, both infectious to and transmissible by humans, can be created among intensively farmed swine [33]. While much attention has been placed on the role of pigs as “mixing vessels”, the potential importance of chickens for the evolution of humanized influenza viruses should not be overlooked and, as such, warrants further study [34].
In summary, one of the key factors in the pathogenesis of zoonotic diseases is that there have been profound changes between natural and man-made ecosystems in recent decades, resulting in increased risk of novel diseases with pandemic potential (Table 1) [35].

Transmission to and amongst humans
Zoonotic influenza viruses that acquire the ability to transmit efficiently among humans via the air, through mutation, reassortment or both, are at the origin of emerging influenza viruses with pandemic potential [13].
Poultry and pigs are the major sources of human infections with IAVs. Farmworkers who work with livestock such as swine and poultry are potentially at risk for exposure to IAVs that originate in birds, pigs, or other species, are novel to humans, and may pose a pandemic threat [36-39]. In particular, occupational exposure to pigs has been shown to increase the risk of swine influenza virus infection in humans [40,41].
The portal of entry for human infections is mostly through the conjunctiva (e.g., rubbing the eye), nasal and mucosal membranes (e.g., inhalation of dust, droplets), or probably swimming in contaminated pools [42-45].
Many factory farm workers are provided with little or no protective clothing or opportunities for personal hygiene or decontamination on-site. A study of poultry-house workers in Maryland indicate that workers take their clothes home for washing [46]. Thus, it is not surprising that increased risks of pathogen exposure and infections, both bacterial and viral, have been reported among farmers, their families, and farm workers at poultry and swine operations [41,47-49,50-55].
These same animal workers may serve as a bridging population in moving IAVs circulating among livestock to other humans [56]. The primary mode of transmission between humans is via inhalation of infectious respiratory particles (large droplet transmission) when an infected person coughs or sneezes. There is also evidence of airborne (small particles transmitted by talking or exhalation) and fomite transmission [57,58]. The typical incubation period is 24 to 48 hours. Patients are infectious one to two days before symptom onset and for five to seven days afterward. Children and immunosuppressed people may exhibit prolonged viral shedding [59,60].
Amplification occurs if the size of the epidemic in humans is increased due to transmission of the influenza virus into the intensively farmed animal species which leads to an epidemic in that species, and subsequent transmission back to the local human population [41].
In developing countries, humans often live close to their livestock, leading to greater likelihood of transmission of influenza viruses from animals to humans [61-63]. Once these diseases are transmitted into human populations, they are transmitted to densely human populated areas in the world, where they can have a considerable public health impact [64].
Increased globalization is a significant factor in the spread of human influenza viruses that spillover from poultry and swine. [65,66] Modern methods of transportation lead to a more rapid spread of such viruses to different parts of the world, through the transportation of both humans and live animals.
Seasonal influenza occurs primarily in the colder months in temperate regions. Seasonal influenza A viruses (IAVs) circulate throughout the year in East and Southeast Asia, which are considered the source regions of the H3N2 strains that cause winter epidemics in the northern and southern hemispheres [67]. Pandemic influenza A viruses circulate around the world in several waves, eventually replacing an existing seasonal influenza A virus [13].
Vaccination efficacy
In the US, the Centers for Disease Control and Prevention’s (CDC’s) Advisory Committee on Immunization Practices (ACIP) and the American Academy of Family Physicians (AAFP) recommend annual influenza vaccination for all people six months and older who do not have contraindications, with an emphasis on those at higher risk of developing complications such as the very young (<5), older adults (≥65), pregnant women, and individuals with certain health conditions [68,69]. Multiple formulations of the influenza vaccine are available [68].
The composition of influenza strains in the vaccine is updated biannually based upon recommendations from the World Health Organization (WHO), which tracks clinical data on current and emerging strains during both the northern and southern hemisphere influenza seasons. This allows the production of a targeted vaccine, although further genetic alterations of the prevalent viral strains can result in a mismatch between circulating and vaccine strains, leading to poor vaccine effectiveness by the time vaccines are deployed. An example would be the 2017/18 trivalent vaccine which had a low effectiveness of ∼25% in the UK due to mismatching of the predominant influenza A strain and lacking the circulating Yamagata strain [70].
Following influenza vaccination, antibody titers to influenza antigens may persist for months. However, the changing nature of influenza viruses, particularly the influenza A type (antigenic drift) [71], warrants reformulation of vaccine each influenza season in an attempt to match vaccine with the circulating virus strains [72].
The immune response to vaccination among elderly persons is reduced compared with younger adults. [73,74] A review published in 2017 found the vaccine effectiveness against any type of influenza to be 51% for working-age adults and 37% for older adults [75,76].
The US influenza vaccine market was $3.82 billion in 2021 [77], to which can be added the cost of administering the program. Yet many Americans don’t get vaccinated. Only 59.0% of children 6 months to 17 years old and 43.3% of adults over 18 years old were vaccinated in the U.S. in the 2016–17 influenza season [78]. So, the effectiveness of this program is limited by both the efficacy of the vaccine and the degree of public access to the vaccine, resulting in the 25-50 million documented influenza cases per year, and 20,000 deaths.
Scope and economic cost of factory farming
Intensive livestock farming is increasing worldwide, encouraged by market demands including urbanization and expanding global populations, which have changed the way in which food is produced and supplied [79].
Increased human consumption of meat, more efficient animal husbandry practices and the resulting profit potential have led an increased number of farmers in the developing world to forego traditional practices, such as free-range or grazing, in favor of small-scale intensive models. The Council for Agricultural Science and Technology (1999) notes that these more intensive farms are replacing traditional models at a rate of 4.3 percent per year, especially in South America, Africa, and Asia, with poultry and swine farms outpacing any other livestock subsector [80,81].
This increase is largely related to the expansion of the integrated or industrial model of production [82] led by both national and multinational corporations for expanding markets of increasingly urban consumer populations within these countries as well as exports [81]. Concerns have been raised over the relatively weak veterinary and public health infrastructure in some of these countries [83].
In the U.S., this change began in the 1930s and now more than 90% of broiler chickens and turkeys are produced in houses in which between 15,000 and 50,000 birds are confined throughout their lifespan. For swine, this transformation occurred more recently and more rapidly: from 1994 to 2001, the market share of hogs produced in industrial food animal production increased from 10% to 72% of total U.S. production [27].
The occurrence of a zoonotic disease can lead to large economic losses in the agricultural sector [84-91]. At times of heightened infectious risk, livestock and wildlife are often culled as a means of restricting their movements and limiting their interactions with other animals and humans. Indeed, culling --- or ‘stamping out’ ---remains the major strategy used to control emergent disease events in animal populations [64].
Killing chickens to curb influenza outbreaks has significant costs. Fifteen years after its emergence, the direct economic costs of the ongoing H5N1 HPAI outbreak ---including destroying more than 250 million birds ---were estimated by the World Bank (2010) at more than US$10 billion [92].
Public health considerations
Public health is the science of protecting and improving the health of people and their communities. This work is achieved by promoting healthy lifestyles, researching disease and injury prevention, and detecting, preventing and responding to infectious diseases. Prevention strategies and interventions can be aimed at the environment, human behavior, or medical care practices.
The primary concern of infectious disease control in public health, whether in developing or industrialized countries, should be the reduction, elimination, or even eradication of infectious disease. This is accomplished by directing control measures to the agent, the routes of transmission, or the host. Such control measures include: (1) identifying and then reducing or eliminating infectious agents at their sources and reservoirs, (2) breaking or interfering with the routes of transmission of infectious agents, and (3) identifying susceptible populations and then reducing or eliminating their susceptibility [93].
The chart below shows how primary prevention of influenza viruses needs to occur at the Pathogen Spillover point to prevent spillover into humans, which then can result in influenza pandemics (Figure 2) [94].

Leaders in public health and many prominent policymakers have promoted plans that argue that the best ways to address future pandemic catastrophes should entail “detecting and containing emerging zoonotic threats.” [95] In other words, we should take actions only after humans get sick. Despite the extraordinary successes generated by immunizations, pharmaceuticals, and evidence-based public health interventions, the spread of infectious diseases remains a critical issue, so such approaches are insufficient.
Much of medical practice is based on a disease/treatment model rather than a prevention model, where the predominant focus is on treating existing symptoms and conditions. While few would argue this approach is necessary for acute conditions, this has not proved to be an efficient or effective way of delivering preventive care. Impressive evidence supports the value of preventive medicine. Primary prevention activities deter the occurrence of a disease in the first place [96].
The risk of complications from influenza, including lower respiratory tract infection, admission to hospital, and death vary depending on factors such as age and the type of comorbidity that may be present. Heart disease, hypertension, diabetes mellitus, obesity, asthma and chronic kidney disease increase the risk of complications of influenza [97]. A plant-based diet can reduce the risk of all these pathologies and can be a treatment or adjunct for them as well [98-101].
Discussion
The worldwide costs of the circulation of continually changing influenza viruses is massive, in terms of ill-health and lost work days of 9 percent of the world’s population annually; the costs of vaccinating the general public every year in those countries that can afford to do it, due to the evolving nature of the virus; and the ultimate death of 300-500,000 people every year that we know of.
Intensive farming of animals is an essential link in the development of influenza. Without these intensive, or factory farming, operations influenza could not emerge and spread. Variants would also be much less likely to develop.
The growth of intensive animal agriculture exacerbates this risk, due to an increase in the size of the reservoir pools among swine and chickens, an increase in the number and genetic variabilities of circulating IAVs, and the resulting risk of novel viruses causing future pandemics.
From a public health standpoint, preventing a disease from ever occurring in the first place is primary prevention, whereas seasonal vaccination, which is only moderately effective, is a form of secondary prevention. Relying on vaccination as a strategy to prevent the spread and impact of these diseases, is a costly strategy that has not proven sufficient to stop these viruses from spreading and damaging public health.
The only way to reduce the risk of such viruses emerging is to stop the crowding of billions of animals together in intensive factory farms, thus limiting the size of the viral reservoirs, the potential for genetic drift and reassortment, and the number of farm workers who come into contact with these animals and can transmit them into the human population.
However, there is insufficient farmland to enable the raising of this number of animals in any other way, so this reduction will only happen if there is a significant reduction in the demand for animal products for human food.
The most effective public health prophylaxis would therefore be to encourage less consumption of animal products, thus reducing the demand and therefore the need for intensive animal agriculture. This will cut the link in the chain of emergence of influenza viruses into the human population.
While this article focused on influenza, a plant-based diet can reduce the risk of many other pathologies such as coronary artery disease, type II diabetes, chronic kidney disease, asthma, and more. It is often an efficacious treatment for those pathologies as well.
While many years ago a plant-based diet might have been considered fringe that’s no longer the case. One indication of this is the soaring sales of meat and dairy substitutes. Since reducing the consumption of animal products will also hugely benefit both human health and the health of our planet, public health authorities should look into ways to encourage and facilitate plant-based eating among their populations with due haste.
References
- Lambert L, Fauci AS, Linda C (2010) Influenza vaccines for the future. N Engl J Med 363:2036-2044.
- Girard M, Cherian T, Pervikov Y, Kieny M (2005) A review of vaccine research and development: Human acute respiratory infections. Vaccine 23(50): 5708-5724.
- Thompson M, Shay D, Zhou H, Bridges C, Cheng P et al. (2010) Estimates of deaths associated with seasonal influenza - United States, 1976–2007. MMVR Morb Mortal Wkly Rep 59(33): 1057-1062.
- Hannoun C, Megas F, Piercy J (2004) Immunogenicity and protective efficacy of influenza vaccination. Virus Res 103(1-2): 133-138.
- Simonsen L, Fukuda K, Schonberger L, Cox NJ (2000) Impact of influenza epidemics on hospitalizations. J Infect Dis 181(3): 831-837.
- Simonsen L, Clarke MJ, Williamson GD, Stroup DF, Arden NH, et al. (1997) Impact of influenza epidemics on mortality: Introducing a severity index. Am J Public Health 87(12): 1944-1950.
- Thompson WW, Shay DK, Weintraub E, Brammer L, Bridges CB et al. (2004) Influenza-associated hospitalizations in the United States. JAMA 292(11): 1333-1340.
- Putri W, Muscatello D, Stockwell M, Newall A (2018) Economic burden of seasonal influenza in the United States. Vaccine 36(27): 3960-3966.
- Taubenberger JK, Kash JC (2010) Influenza virus evolution, host adaptation, and pandemic formation. Cell Host Microbe 7(6): 440-451.
- Monto AS, Webster RG (2013) Influenza pandemics: History and lessons learned. In: Webster RG, Monto AS, Braciale TJ, Lamb RA (eds.) Textbook of Influenza. 2nd (ed). Hoboken: John Wiley & Sons, Ltd.
- Webster RG, Bean WJ, Gorman OT, Chambers TM, Kawaoka Y (1992) Evolution and ecology of influenza A viruses. Microbiol Rev 56(1): 152-179.
- Taubenberger J, Morens D (2006) 1918 influenza: the mother of all pandemics. Emerg Infect Dis 12(1): 15-22.
- Reperant L, Moesker F, Osterhaus A (2016) Influenza: from zoonosis to pandemic. ERJ Open Res 2(1): 00013-2016.
- Mostafa A, Abdelwhab EM, Mettenleiter TC, Pleschka S (2018) Zoonotic potential of influenza A viruses: A comprehensive overview. Viruses 10(9): 497.
- Rohr J, Barrett C, Civitello D, Craft M, Delius B et al. (2019) Emerging human infectious diseases and the links to global food production. Nat Sustain 2(6): 445-456.
- Taubenberger J, Morens D (2010) Influenza: the once and future pandemic. Public Health Rep 125(suppl 3): 16-26.
- Hause BM, Ducatez M, Collin EA, Ran Z, Liu R et al. (2013) Isolation of a novel swine influenza virus from Oklahoma in 2011 which is distantly related to human influenza C viruses. PLoS Pathog 9(2): e1003176.
- Baron S (1996) Medical Microbiology. 4th (ed): Galveston (TX): University of Texas Medical Branch at Galveston, USA.
- Barberis I, Myles P, Ault Sk, Bragazzi Nl, Martini M (2016) History and evolution of influenza control through vaccination: from the first monovalent vaccine to universal vaccines. J Prev Med Hyg 57(3): E115-E120.
- Borkenhagen L, Salman M, Ma M, Gray G (2019) Animal influenza virus infections in humans: A commentary. Int J Inf Dis 88: 113-119.
- McDonald SM, Nelson MI, Turner PE, Patton JT (2016) Reassortment in segmented RNA viruses: mechanisms and outcomes. Nat Rev Microbiol 14(7): 448-460.
- Van Reeth K (2007) Avian and swine influenza viruses: our current understanding of the zoonotic risk. Vet Res 38(2): 243-260.
- Wertheim J (2009) When pigs fly: the avian origin of a 'swine flu'. Environ Microbiol 11(9): 2191-2192.
- Burkart M (2007) Diffuse pollution from intensive agriculture: sustainability, challenges, and opportunities. Water Sci Technol 55(3): 17-23.
- S. Department of Agriculture (2006) Trends in U.S. agriculture. National Agricultural Statistics Service. USDA.
- Coker R, Rushton J, Mounier-Jack S, Karimuribo E, Lutumba P et al (2011) Towards a conceptual framework to support one-health research for policy on emerging zoonoses. Lancet Infect Dis 11(4): 326-331.
- Graham JP, Leibler JH, Price L, Otte JM, Pfeiffer DU et al. (2008) The animal–human interface and infectious disease in industrial food animal production: rethinking biosecurity and biocontainment. Public Health Rep 123(3): 282-299.
- Drew T (2011) The emergence and evolution of swine viral diseases: To what extent have husbandry systems and global trade contributed to their distribution and diversity? Rev Sci Tech 30(1): 95-106.
- Cutler SJ, Fooks AR, Van der Poel WH (2010) Public health threat of new, reemerging, and neglected zoonoses in the industrialized world. Emerg Infect Dis 16(1): 1-7.
- Leibler JH, Carone M, Silbergeld EK (2010) Contribution of company affiliation and social contacts to risk estimates of between-farm transmission of avian influenza. PLoS ONE 5(3): e9888.
- Springbett AJ, MacKenzie K, Woolliams JA, Bishop SC (2003) The contribution of genetic diversity to the spread of infectious diseases in livestock populations. Genetics 165(3): 1465-1474.
- Rajao DS, Vincent AL, Perez DR (2018) Adaptation of human influenza viruses to swine. Front Vet Sci 5: 347.
- Ma W, Kahn RE, Richt JA (2008) The pig as a mixing vessel for influenza viruses: Human and veterinary implications. J Mol Genet Med 3(1): 158-166.
- Kuchipudi S, Nelli R, White G, Bain M, Chang KC et al. (2009) Differences in influenza virus receptors in chickens and ducks: Implications for interspecies transmission. J Mol Genet Med 3(1): 143-151.
- National Research Council (US) Committee on Achieving Sustainable Global Capacity for Surveillance and Response to Emerging Diseases of Zoonotic Origin. (2009) Sustaining Global Surveillance and Response to Emerging Zoonotic Diseases. Washington: National Academies Press, USA.
- Steege A, Baron S, Davis S, Torres-Kilgore J, Sweeney M (2009) Pandemic influenza and farmworkers: the effects of employment, social, and economic factors. Am J Public Health 99 Suppl 2(Suppl 2): S308-S315.
- Puzelli S, Trani LD, Fabiani C, Campitelli L, De Marco MA et al. (2005) Serological analysis of serum samples from humans exposed to avian H7 influenza viruses in Italy between 1999 and 2003. J Infect Dis 192(8): 1318-1322.
- Bridges CB, Lim W, Hu-Primmer J, Sims L, Fukuda K et al. (2002) Risk of influenza A (H5N1) infection among poultry workers, Hong Kong, 1997–1998. J Infect Dis 185(8): 1005-1010.
- Gray G, Baker W (2007) The importance of including swine and poultry workers in influenza vaccination programs. Clin Pharmacol Ther. 82(6): 638-641.
- Myers KP, Olsen CW, Setterquist SF, Capuano AW, Donham KJ at al. (2006) Are swine workers in the United States at increased risk of infection with zoonotic influenza virus? Clin Inf Dis 42(1): 14-20.
- Olsen CW, Brammer L, Easterday BC, Arden N, Belay E et al. (2002) Serologic evidence of H1 swine influenza virus infectionin swine farm residents and employees. Emerg Infect Dis 8(8): 814-819.
- Lai S, Qin Y, Cowling BJ, Ren X, Wardrop NA et al. (2016) Global epidemiology of avian influenza A H5N1 virus infection in humans, 1997-2015: A systematic review of individual case data. Lancet Infect Dis 16(7): e108-e118.
- Vong S, Ly S, Van Kerkhove MD, Achenbach J, Holl D et al. (2009) Risk factors associated with subclinical human infection with avian influenza A (H5N1) virus--Cambodia, 2006 J Infect Dis 199(12): 1744-1752.
- Wang X, Jiang H, Wu P, Uyeki TM, Feng L et al. (2017) Epidemiology of avian influenza A H7N9 virus in human beings across five epidemics in mainland China, 2013–2017: An epidemiological study of laboratory-confirmed case series. Lancet Infect Dis 17(8): 822-832.
- Belser JA, Lash RR, Garg S, Tumpey TM, Maines TR (2018) The eyes have it: Influenza virus infection beyond the respiratory tract. Lancet Infect Dis 18(7): e220-e227.
- Price L, Roess A, Graham J, Baqar S, Vailes R et al. (2007) Neurologic symptoms and neuropathologic antibodies in poultry workers exposed to Campylobacter jejuni. J Occup Environ Med 49(7): 748-755.
- Akwar T, Poppe C, Wilson J, Reid-Smith RJ, Dyck M et al. (2007) Risk factors for antimicrobial resistance among fecal Escherichia coli from residents on forty-three swine farms. Microb Drug Resist 13(1): 69-76.
- Withers MR, Correa MT, Morrow ME, Stebbins ME, Seriwatana J et al. (2002) Antibody levels to hepatitis E virus in North Carolina swine workers, non-swine workers, swine, and murids. Am J Trop Med Hyg 66(4): 384-388.
- Olsen B, Axelsson-Olsson D, Thelin A, Weiland O (2006) Unexpected high prevalence of IgG-antibodies to hepatitis E virus in Swedish pig farmers and controls. Scand J Infect Dis 38(1): 55-58.
- Fey PD, Safranek TJ, Rupp ME, Dunne EF, Ribot E et al. (2000) Ceftriaxone-resistant salmonella infection acquired by a child from cattle. N Engl J Med 342(17): 1242-1249.
- Levy S, FitzGerald G, Macone A (1976) Spread of antibiotic-resistant plasmids from chicken to chicken and from chicken to man. Nature 260(5546): 40-42.
- Kariuki S, Gilks C, Kimari J, Obanda A, Muyodi J et al. (1999) Genotype analysis of Escherichia coli strains isolated from children and chickens living in close contact. Appl Environ Microbiol 65(2): 472-476.
- Van den Bogaard A, London N, Driessen C, Stobberingh E (2001) Antibiotic resistance of faecal Escherichia coli in poultry poultry farmers and poultry slaughterers. J Antimicrob Chemother 47(6): 763-771.
- van den Bogaard A, Willems R, London N, Top J, Stobberingh E (2002) Antibiotic resistance of faecal enterococci in poultry, poultry farmers and poultry slaughterers. J Antimicrob Chemother 49(3): 497-505.
- Al-Ghamdi M, El-Morsy F, Al-Musafa Z, Al-Ramadhan M, Hanif M (1999) Antibiotic resistance of Escherichia coli isolated from poultry workers, patients and chickens in the eastern province of Saudi Arabia. Trop Med Int Health 4(4): 278-283.
- Ma MJ, Wang GL, Anderson BD, Bi ZQ, Lu B, et al. (2018) Evidence for cross-species influenza A virus transmission within swine farms, China: a one health, prospective cohort study. Clin Infect Dis 66(4): 533-540.
- Xu X, Blanton L, Elal AIA, Alabi N, Barnes J et al. (2019) Update: influenza activity in the United States during the 2018–19 season and composition of the 2019–20 influenza vaccine. MMWR Morb Mortal Wkly Rep 68(24): 544-551.
- Moser MR, Bender TR, Margolis HS, Noble GR, Kendal AP et al. (1979) An outbreak of influenza aboard a commercial airliner. Am J Epidemiol 110(1):1-6.
- Influenza (flu): clinical signs and symptoms of influenza. Centers for Disease Control and Prevention. 2019.
- Leekha S, Zitterkopf NL, Espy MJ, Smith TF, Thompson RL et al. (2007) Duration of influenza A virus shedding in hospitalized patients and implications for infection control. Infect Control Hosp Epidemiol 28(9): 1071-1076.
- Liu T (2008) Custom, taste and science: raising chickens in the Pearl River Delta region, South China. Anthropol Med 15(1): 7-18.
- Lohiniva AL, Dueger E, Talaat M, Refaey S, Zaki A et al. (2013) Poultry rearing and slaughtering practices in rural Egypt: an exploration of risk factors for H5N1 virus human transmission. Influenza Other Respir Viruses 7(6): 1251-1259.
- Helmy YA, Krücken J, Nöckler K, Samson-Himmelstjerna G, Zessin KH (2013) Molecular epidemiology of Cryptosporidium in livestock animals and humans in the Ismailia province of Egypt. Vet Parasitol 193(1-3): 15-24.
- Jones KE, Patel NG, Levy MA, Storeygard A, Balk D et al. (2008) Global trends in emerging infectious diseases. Nature 451(7181): 990-993.
- Hosseini P, Sokolow S, Vandegrift K, Kilpatrick A, Daszak P (2010) Predictive power of air travel and socio-economic data for early pandemic spread. PLoS ONE 5(9): e12763.
- Vandegrift KJ, Sokolow SH, Daszak P, Kilpatrick AM (2010) Ecology of avian influenza viruses in a changing world in The Year in Ecology and Conservation Biology: Annals of the New York Academy of Sciences
- Russell CA, Jones TC, Barr IG, Cox NJ, Garten RJ et al. (2008) The global circulation of seasonal influenza A (H3N2) viruses. Science 320(5874): 340-346.
- Grohskopf LA, Alyanak E, Broder KR, Walter EB, Fry AM et al. (2019) Prevention and control of seasonal influenza with vaccines: recommendations of the Advisory Committee on Immunization Practices—United States, 2019–20 influenza season. MMWR Recomm Rep 68(3): 1-21.
- Seasonal Influenza Prevention & Control. 2022-2023 Influenza Season. American Academy of Family Physicians. Accessed Sept 6, 2022. AAFP
- Rondy M, Kissling E, Emborg H, Gherasim A, Pebody R, et al. (2018) Interim 2017/18 influenza seasonal vaccine effectiveness: combined results from five European studies. Euro Surveill 23(9): 18-00086.
- Boni M (2008) Vaccination and antigenic drift in influenza. Vaccine 26(Suppl 3): C8-C14.
- Petrie JG, Ohmit SE, Johnson E, Truscon R, Monto AS (2015) Persistence of antibodies to influenza hemagglutinin and neuraminidase following one or two years of influenza vaccination. J Infect Dis 212(12): 1914-1922.
- Demicheli V, Jefferson T, Di Pietrantonj C, Ferroni E, Thorning S et al. (2018) Vaccines for preventing influenza in the elderly. Cochrane Database Syst Rev 2(2): CD004876.
- Belongia E, Simpson M, King J, Sundaram ME, Kelley N et al. (2016) Variable influenza vaccine effectiveness by subtype: a systematic review and meta-analysis of test-negative design studies. Lancet Infect Dis 16(8): 942-951.
- Rondy M, El Omeiri N, Thompson M, Levêque A, Moren A et al. (2017) Effectiveness of influenza vaccines in preventing severe influenza illness among adults: a systematic review and meta-analysis of test-negative design case-control studies. J Infect 75(5): 381-394.
- Darvishian M, Bijlsma M, Hak E, van den Heuvel E (2014) Effectiveness of seasonal influenza vaccine in community-dwelling elderly people: a meta-analysis of test-negative design case-control studies. Lancet Infect Dis 14(12): 1228-1239.
- Research and Markets (2022) United States Influenza Vaccines Market Size, Share, Trends, Analysis, Demand, Opportunity, and Forecast, 2022 - 2030.
- Dabestani N, Leidner A, Seiber E, Kim H, Graitcer S et al. (2019) A review of the cost-effectiveness of adult influenza vaccination and other preventive services. Prev Med 126: 105734
- Rushton J (2017) Improving the use of economics in animal health - Challenges in research, policy and education. Prev Vet Med 137(Pt B): 130-139.
- Council for Agricultural Science and Technology (1999) Animal agriculture and global food supply. Task Force Report No. 135.
- Silverside D, Jones M (1992) Small-scale poultry processing. United Nations Food and Agriculture Organization p. 124.
- Nierenberg D (2005) Happier Meals Rethinking the global meat industry. World Watch Institute, Washington, USA.
- Schelling E, Wyss K, Bechir M, Moto D, Zinsstag J (2005) Synergy between public health and veterinary services to deliver human and animal health interventions in rural low-income settings. BMJ 331(7527): 1264-1267.
- Verbeke W, Velhuis AG (2003) Consumer perception of food safety: role and influencing factors. New Approaches to Food-safety Econ. Kluwer Academic, pp21-26 Univ of Gent.
- Fitzgerald JR (2012) Livestock-associated Staphylococcus aureus: origin, evolution and public health threat. Trends Microbiol 20(4): 192-198.
- Chatard-Pannetier A, Rousset S, Bonin D, Guillaume S, Droit-Volet S (2004) Nutritional knowledge and concerns about meat of elderly French people in the aftermath of the crises over BSE and foot-and-mouth. Appetite 42(2): 175-183.
- Ogoshi K, Yasunaga H, Obana N, Ogawa T, Imamura T (2010) Consumer reactions to risk information on bovine spongiform encephalopathy in Japan. Environ Health Prev Med 15(5): 311-318.
- Bennett R, Christiansen K, Clifton-Hadley R (1999) Preliminary estimates of the direct costs associated with endemic diseases of livestock in Great Britain. Prev Vet Med 39(3): 155-171.
- Christou L (2011) The global burden of bacterial and viral zoonotic infections. Clin Microbiol Infect 17(3): 326-330.
- Leibler J, Otte J, Silbergeld E (2008) Zoonotic disease risks and socioeconomic structure of industrial poultry production: review of the US experience with contract growing. Research Reports 1-24.
- Torgerson PR, Macpherson CNL (2011) The socioeconomic burden of parasitic zoonoses: global trends. Vet Parasitol 182(1): 79-95.
- Degeling C, Lederman Z, Rock M (2016) Culling and the common good: re-evaluating harms and benefits under the one health paradigm. Public Health Ethics 9(3): 244-254.
- Detels R, Gulliford M, Karim QA, Tan CC (eds) Oxford Textbook of Global Public Health. 6 (ed): Oxford University Press,
- Berstein AS, Ando AW, Loch-Temzelides T, Vale MM, Li BV et al. (2022) The costs and benefits of primary prevention of zoonotic pandemics. Sci Adv 8(5): eabl4183.
- Elias C, Nkengasong JN, Qadri F (2021) Emerging infectious diseases-Learning from the past and looking to the future. N Engl J Med 384(13): 1181-1184.
- Hensrud D (2000) Clinical preventive medicine in primary care: background and practice: 1. Rationale and current preventive practices. Mayo Clin Proc 75(2): 165-172.
- Mertz D, Kim TH, Johnstone J, Lam PP, Science M et al. (2013) Populations at risk for severe or complicated influenza illness: systematic review and meta-analysis. BMJ 347: f5061.
- Rose S, Strombom A (2018) A comprehensive review of the prevention and treatment of heart disease with a plant-based diet. J Cardiol & Cardiovas Ther 12(5): 555847.
- Strombom A, Rose S (2017) The prevention and treatment of Type II Diabetes Mellitus with a plant-based diet. Endocrin Metab Int J 5(5): 00138.
- Rose S, Strombom A (2019) A plant-based diet prevents and treats chronic kidney disease. JOJ Uro & Nephron 6(3): 555687.
- Rose S, Strombom A (2022) Asthma – prevention and treatment with a plant-based diet. Int J Pul & Res Sci 5(5): 555672.






























