Utilizing Nanomolecular Silicon Dioxide to Help Eliminate and Reduce the Risks of Surgical Site Infections: MicroSURETM, A Novel Technology
Hamid Khan A1*, Erwin Lo, Arshad Khan A3 and Hassan Chahadeh4
1American Microsurgical Orthoplastic Society, Foot and Ankle Deformity Correction Center, Chicago, USA
2 Department of Neurosurgery, Spine Tech. Houston, USA
3 Department of Lower Extremity Peripheral Nerve Surgery, Spine Tech. Houston, TX, USA
4 Department of Interventional Pain Management. Spine Tech. Houston, TX, USA
Submission: July 30, 2020;Published: August 11, 2020
*Corresponding author: Hamid Khan A, American Microsurgical Orthoplastic Society, Foot and Ankle Deformity Correction Center, Chicago, USA
How to cite this article: Hamid Khan A, Erwin L, Arshad K A, Hassan C. Utilizing Nanomolecular Silicon Dioxide to Help Eliminate and Reduce the Risks of Surgical Site Infections: MicroSURETM, A Novel Technology. JOJ Orthoped Ortho Surg. 2020; 2(4): 555595. DOI: 10.19080/JOJOOS.2020.02.555595
Abstract
There are a number of complications which have the potential to arise post-operatively and depending on the type of surgery being performed, the location and size of the incision sites, past medical history of the patient involved and patient compliance following the performed surgery, each of these factors play a major role in the post-surgical outcome. Of the many potential complications possible, infection at or around the incision site is not only common and costly but can lead to several devastating obstacles that are extremely strenuous on the patient, the surgeon and the patient’s primary care physician. microSURETM wound care is an antimicrobial antiseptic solution that utilizes nano molecular silicon dioxide to prevent such complications and this novel technology is continuing to be a promising one based on the outcomes observed thus far. This short literature will discuss our experience with using microSURETM wound care, review the technical information as it relates to nanomolecular silicon dioxide, and also explain the benefits of its use over some of the more commonly implemented treatment or preventative measures taken to help reduce the risk of surgical site infections.
Keywords: Surgical site infection; Antimicrobial resistance; Microsure; Silicon dioxide; Diabetic; Microsure wound care
Abbreviations: SSI: Surgical Site Infection; CDC: Centers for Disease Control and Prevention
Introduction
Surgical site infections (SSIs) are one of the most encountered complications that arises after an operation, they account for 20 percent of all nosocomial infections and are responsible for 38 percent of nosocomial infections amongst surgical patients as a subset.1,2 There have been a number of recommended measures set forth by the Centers for Disease Control and Prevention (CDC) and the Surgical Improvement Project that were created in an attempt to try and control these post-operative infection rates [1], however with an estimated SSI present in 2 to 5 percent of patients undergoing inpatient surgery and an estimated annual incidence of SSIs in the United States ranging from 160,000 to 300,000, further improvements and recommendations must be investigated. [1-3]. By definition, a SSI includes infections related to an operative procedure that occurs at or near the surgical incision within 30 days of the procedure or within 90 days if prosthetic material is implanted at the time of surgery.[4] This means that fduring the mentioned postoperative time periods, there is a high potential and vulnerability for contamination to occur at or around the surgical site and based on a patient’s underlying medical issues, past medical history, lack of compliance, or absence of surgical site monitoring, the risks for infection tend to increase considerably [2]. Often times, patients suffer from a SSI soon after in patient surgery, research has demonstrated that this increases the length of hospital stays by an average of 9.7 days, resulting in an estimated annual cost that ranges between $3.5 billion to $10 billion as it relates to SSIs in the United States. [1,2].
A number of guidelines including but not limited to the cessation of smoking, maintenance of blood sugar, use of antibiotic sutures and showering have all been recommended to help reduce the risks for a SSI to occur.[3,5-7] Unfortunately, once patients are released from the hospital and surgical site care is out of the nurses or physicians’ control, patient compliance becomes vital [3]. It is really at this point in time that the concern for a SSI to occur is at its peak and why there is a need for additional options to help decrease this risk. Studies and advancements in the field of Nanotechnology, has led to the creation and understanding of a ‘mechanical kill’, where in which nanomolecular particles rely on a physical mechanism of action to destroy or kill harmful pathogens. This novel technology is one that is being utilized more frequently as studies continue to be published and is a subject matter that deserves more recognition.
Discussion
Aside from the typical prophylactic antibiotics prescribed to help protect against potential bacterial infection, recommendations involving the use of antiseptics is often made [4-6]. Antiseptics, such as hydrogen peroxide, rubbing alcohol and iodine have been utilized to deter bacterial growth, as these have all proven to weaken or decelerate the growth of various microorganisms. Each of these common antiseptics however have certain undesirable attributes related to them, hydrogen peroxide is often too harsh of a treatment selection for many patients, alcohol tends to burn and dry out skin, and the occurrence of iodine related contact dermatitis and anaphylaxis has been well documented throughout a large number of patients [6-8]. Apart from these issues, these are modalities that have been employed for a long period of time, yet the SSI incidence rate is still exceedingly high and continues to be problematic.
Today, there are several strains of bacteria capable of withstanding the effects of common antibiotics, leading to infections associated to antimicrobial resistance as well as biofilm development.8 Recently, the use of nanoparticles containing antibiotics has gained a great deal of attention, especially as it relates to using silica nanoparticles to deliver drugs such as antibiotics. Silica nanoparticles contain a high surface area, are generally considered safe, and are understood as being very chemically and thermally stable, therefore, its use for improving pharmacokinetics is becoming much more common.8-10. Although nanomolecular silica for the delivery of antibiotics is widely accepted and understood, the use of colloidal silicon dioxide nanoparticles containing antimicrobial characteristics has yet to be examined in depth. There is a limited amount of literature that focuses directly on colloidal silicon dioxide as an effective option for the reduction of bacterial contamination. However, from the data that is available, there are obvious associated biocidal properties present with the use of this novel technology. As it relates to microSURETM wound care specifically, the colloidal amorphous silica is re-engineered and formulated to be 4-6 nanometers in diameter, as opposed to most microbial matter, which is larger in size. The material has been developed in such a way that it creates “Crystalline- like structures” (Figure 1) once it has dried and creates a surface barrier that penetrates the cellular membranes or outer ‘shells’ of the trespassing pathogens. These structures will disrupt the microbial organism, thus killing the cell (bacteria) or destroying its proteins (Viruses) and rendering that microorganism harmless.
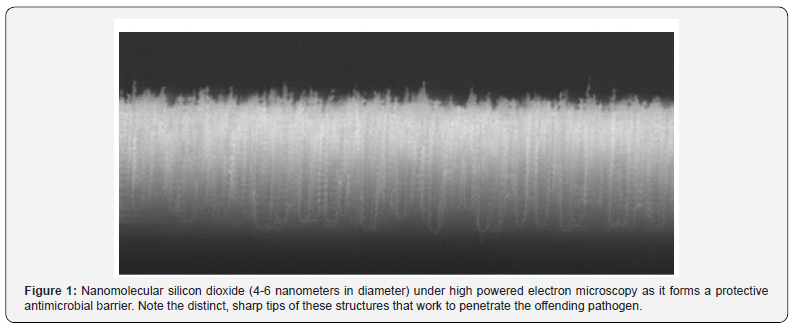
While the biocidal activity of these nanomaterials has been documented, their practical applications and usage have been limited largely because of the complexity and cost of synthesizing these compounds [9-10]. Since silica is commonly used in the operating room and because of the widely accepted safety around its use, the decision to start utilizing microSURETM wound care, a formulated solution consisting of deionized water, a hydrated silica complex and minimal amounts of benzalkonium chloride (BZK) on surgical incision sites after operations to help reduce the risks of a SSI was implemented on patients who were in agreement. The outcomes witnessed after the use of microSURETM wound care have been astonishing and the rate of SSIs encountered in those patients using the nanomolecular silicon dioxide formulation has been significantly reduced [11- 12]. There have been several clinical trials performed with the utilization of microSURETM wound care both domestically and internationally. The data present supports that aside from our own personal findings, surgeons who have used this method for reducing SSIs have seen incredibly positive results. Not only were the incidences of SSI after operation essentially nonexistent when the product was applied accordingly, but it appears as though the rate at which the incision sites healed occurred at a far more rapid speed than normally encountered or expected. This solution has also been approved for use with diabetic wounds, and has helped to restore chronic, non-healing ulcers that would have otherwise led to possible infection and or amputation. This is one especially important characteristic of this novel technology as diabetics are already at an increased susceptibility for infection following surgery, as opposed to a healthy individual with no underlying medical issues.
Conclusion
Although this short piece of literature is not intended to represent specific case by case results of patient outcomes and does not go into detail regarding the application process or suggested protocol, it is clear that there are obvious benefits to using nanomolecular silicon dioxide to help decrease the rate of SSIs. Since the start of using this novel technology, there have not been any negative effects documented or witnessed. This option for reducing the risk of a SSI is particularly useful in those patients who are allergic to other antiseptics or are seeking an alternative to the traditional antiseptics commonly used. As physicians and surgeons, patient safety and well-being is always our number one concern, and by implementing the use of nanomolecular colloidal silicon dioxide as an option to help eliminate and reduce the risks of SSIs, we strongly feel as though this is the ideal alternative to consider utilizing. By avoiding SSIs and promoting advanced healing of surgical incision sites, patient safety rapidly improves, nosocomial infection rates drastically decrease, and the healthcare system saves a bundle on costs. It is for these very reasons that we as medical professionals recommend the use of microSURETM wound care and encourage the use of colloidal nanomolecular silicon dioxide post operatively.
Conflict of Interests
The Authors did not receive any specific grant from any funding agency in the public, commercial or non-for-profit sectors. Dr. Erwin Lo (Co Author), is an inventor and owner of the IP technology utilized throughout the microSURE antimicrobial product line. Dr. Hamid A. Khan (corresponding author) serves as the Chief of Research and Compliance at Strategia Project Management Inc., the company that renders the microSURE brand.
References
- (2017) Surgical site infections are the most common and costly of hospital infections: Guidelines for preventing surgical site infections are updated. Loyola University Health System.
- Kristen Ban A, Joseph Minei P, Christine Laronga, Brian Harbrecht G, Eric Jensen H (2017) Therese M. Duane. American College of Surgeons and Surgical Infection Society: Surgical Site Infection Guidelines, 2016 Update. Journal of the American College of Surgeons 224(1): 59-74.
- Global guidelines for the prevention of surgical site infection. World Health Organization 2016.
- Horan TC, Gaynes RP, Martone WJ et al. (1992) CDC definitions of nosocomial surgical site infections, 1992: a modification of CDC definitions of surgical wound infections. Am J Infect Control 13(10): 606-608.
- Anderson DJ, Podgorny K, Berríos-Torres SI, et al. (2014) Strategies to prevent surgical site infections in acute care hospitals: 2014 update. Infect Control Hosp Epidemiol 35(6): 608-627.
- Ban KA, Minei JP, Laronga C, et al. (2016) American College of Surgeons and Surgical Infection Society: Surgical Site Infection Guidelines, 2016 Update. J Am Coll Surg 2017; 224(1): 59-74.
- Allahverdiyev AM, Kon KV, Abamor ES, Bagirova M, Rafailovich M (2011) Coping with antibiotic resistance: combining nanoparticles with antibiotics and other antimicrobial agents. Expert Review of Anti-infective Therapy 9(11):1035-1052.
- Campoccia D, Montanaro L, Speziale P, Arciola CR (2010) Antibiotic-loaded biomaterials and the risks for the spread of antibiotic
- resistance following their prophylactic and therapeutic clinical use. Biomaterials 31(25): 6363-6377.
- Huh AJ, Kwon YJ (2011) Nanoantibiotics: A new paradigm for treating infectious diseases using nanomaterials in the antibiotics
- resistant era. Journal of Controlled Release 156(2): 128-145.
- Hetrick EM, Shin JH, Paul HS, Schoenfisch MH (2009) Anti-biofilm efficacy of nitric oxide-releasing silica nanoparticles. Biomaterials 30: 2782-2789.






























