Abstract
Episodic ataxia type 2 (EA2) is a rare genetic disorder associated with episodic nystagmus. In this review, we discuss the history and pathophysiology of EA2, including the role of CACNA1A gene mutations in the disorder. We will also provide an overview of the demographic associations, symptoms and disease course of EA2. Finally, we will review the differential diagnoses and expected prognosis. Case reports are summarized in two tables that provide representative examples of patients with EA2. Lastly, we highlight management of EA2 through pharmacologic treatment and lifestyle changes. Though it is rare, EA2 is an important disease as patients may present initially to pediatric ophthalmology with intermittent or constant nystagmus of varying intensity or to adult ophthalmology describing oscillopsia from similar symptoms.
Keywords: CACNA1A mutation; Downbeat Nystagmus; Episodic Ataxia; Episodic Ataxia type 2; Gaze Evoked Nystagmus
Abbreviations: FHM: Familial Hemiplegic Migraine-1; SCA6: Spinocerebellar Ataxia Type 6; AZM: Acetazolamide; EA2: Episodic Ataxia Type 2
Materials and Methods
The method of literature search was through the MEDLINE database. We obtained articles listed in the references of articles identified by our initial literature search with the keywords “episodic ataxia”, “episodic ataxia type 2”, “CACNA1A mutation”, and “episodic ataxia and nystagmus”. All years were included and searched. We included case reports only if they contributed new information on the presentation, treatment, and associations of episodic ataxia type 2. Non-English articles were included in our literature search and included when they contributed clinically significant information. We included select articles published prior to 2005 but focused our overview on articles from the past two decades.
Disease Entity
EA2, also known as acetazolamide-responsive cerebellar ataxia and hereditary paroxysmal cerebellar ataxia, is a rare autosomal dominant disorder characterized by spells of imbalance, ataxia, and nystagmus [1,2]. H.L. Parker first reported EA2 in 1946 [3], and in 1996 Ophoff et al identified that mutations in the calcium channel gene CACNA1A caused EA2 [2,4]. Although EA2 is uncommon with an estimated incidence of 1 in 100,000, it is the most frequent subtype of the eight episodic ataxias [2,5]. EA2 has high phenotypic variability and there is a recognized clinical overlap with spinocerebellar ataxia type 6 and familial hemiplegic migraine type 1 [1].
Etiology
The most commonly reported cause of EA2 is loss of function mutations in the CACNA1A gene, located at chromosome 19p13 [6]. The CACNA1A gene encodes the ɑ1A subunit of the P/Q type voltage-gated calcium channel Cav2.1 in Purkinje cells and granule cells in the cerebellum [7]. The ɑ1A subunit of Cav2.1 directs the activity of the multiunit calcium channel [7]. P/Q voltage-gated channels control pace making in Purkinje cells [1]. More than 80 mutations relating to EA2 have been identified, including nonsense, frameshift, and missense mutations [2]. These mutations may lead to a complete loss of function in calcium channels [8]. One potential cause is when a CACNA1A mutation encodes a premature stop codon, forming truncated peptides [3]. CACNA1A mutations in EA2 lead to a decrease in the flow of Ca2+ into Cav2.1 channels [1].
Pathophysiology
The proposed mechanism of EA2 involves CACNA1A mutations inhibiting the activity of Purkinje cells, leading to motor dysfunction [9]. Such mutations cause improper amino acid sequences in peptides that make up Ca2+ channel subunits, resulting in a partial or complete loss of function in Ca2+ channels [3-10].The Cav2.1 subunit is the predominant channel for Purkinje cells, and P/Q currents in this channel play a crucial role in neurotransmitter release [11,12]. Tara et al. [13] reports CACNA1A mutations in tottering mice models reduce the P/Qtype calcium current of the Cav2.1 channel. Reduced calcium flow lowers Purkinje cell coordination and leads to erratic firing, which is linked to motor attacks in mice. Lack of precision in Purkinje cells likely contributes to EA2 symptoms during and in between attacks [1,14]. A lower number of functioning calcium channels corresponding to the cerebellum may also play a role in bouts of ataxia [7]. Since EA2 is an autosomal dominant disorder, wild-type Cav2.1 channels may still be expressed. However, it is possible that mutant Cav2.1 channels interfere with and decrease the function of wild-type Cav2.1 channels, further contributing to EA2 symptoms [15]. While a lower density of calcium channels likely corresponds to ataxia and other symptoms in EA2 patients, no definite mechanism has been identified.
Demographics and Associations
EA2 presents most commonly between the ages of 5 and 20 and has a prevalence of three to five people in 100,000 [4,16]. From the fourteen case reports we analyzed, the mean age at which patients presented symptoms of EA2 was 11.5 years (Table 1). The youngest age a patient presented was 2 years, and the oldest age was 26 years. Nevertheless, there are cases of EA2 presenting in late adulthood [5]. Symptoms of EA2 may continue into or begin in later decades [17]. While there is a typical age at which EA2 presents, there are few known factors other than genetics that predispose one to this disease. Since EA2 is an autosomal dominant disorder, it affects males and females equally, and it remains unknown if certain racial groups are more predisposed to this disease. Studies conducted by Li et al. [18], Niu et al. [19], Rajakulendran et al. [20], and Wong-Spracklen et al. [21] link CACNA1A variations with epilepsy, indicating EA2 patients may be at a greater risk of epilepsy than the general population.
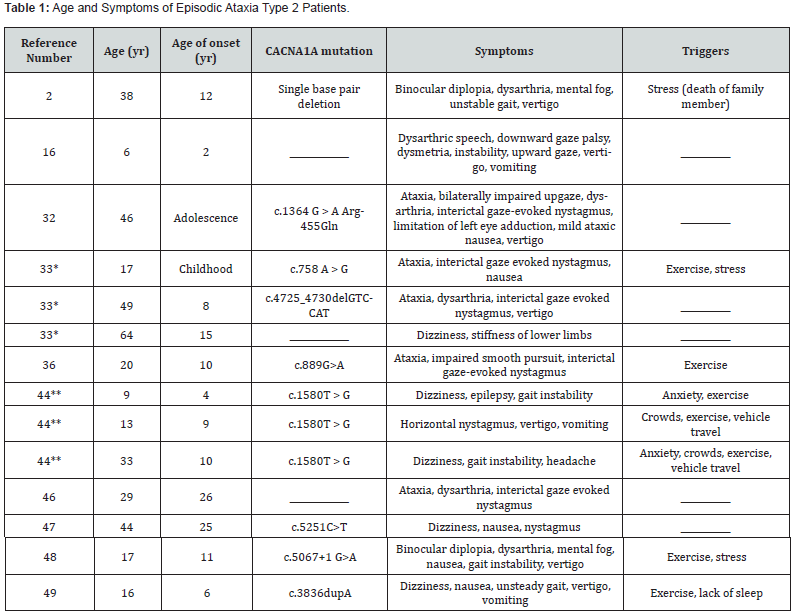
*Patients related to each other, familial EA2 **Patients related to each other, familial EA2
CACNA1A mutations also cause the disorders familial hemiplegic migraine-1 (FHM1) and spinocerebellar ataxia type 6 (SCA6), and like EA2, are associated with progressive ataxia [4]. Further similarities lie in early-stage symptoms. Both EA2 and SCA6 patients experience imbalance, loss of coordination, and stumbling. Symptoms of FHM1 may include seizures, nystagmus, and ataxia, like EA2 [22]. Nardello et al reports different phenotypes corresponding to these three disorders from a common CACNA1A mutation [23]. FHM1 is characterized by severe migraines with aura [24]. Symptoms of aura include temporary loss of sensation, vision, and motor control. A notable symptom during the aura phase is hemiparesis [25]. The effects of hemiplegic migraine are typically temporary, but cases such as those reported by T.J. Schwedt et al. [26] and Obendorfer et al. [27] involve patients who experience permanent symptoms. Medications such as sodium valproate and acetazolamide have been prescribed as treatment for FHM1, often reducing attack duration and frequency [28]. Thus, while FHM1 is not a fatal disorder, patients may experience lifelong effects that may be mitigated with medication and lifestyle changes. SCA6 is distinguishable from its allelic disorders in that it has an adult age of onset, whereas EA2 typically involves a childhood onset [5,29]. SCA6 is characterized by late onset progressive ataxia. Patients with SCA6 may display EA2 symptoms such as nystagmus and dysarthria due to the similarity of the disorders [29]. SCA6 is not typically a fatal disorder, but quality of life may be hindered by severity of symptoms. Symptoms of SCA6 may be managed through medications such as rovatirelin and acetazolamide, as well as physical therapy [30].
Management
Symptoms
The symptoms of EA2 can have a wide range between patients. Table 1 illustrates the symptoms that have been reported in various case reports of EA2 patients. Symptoms include gaze-evoked nystagmus, rebound nystagmus, primary position downbeat nystagmus, headaches, vertigo, and imbalance [1,17]. Episodes of ataxia may be triggered by bouts of emotional or physical stress, exercise, and alcohol, with duration varying from seconds to days [31]. The frequency of these spells varies from several times a week to once or twice a year [32,33]. Patients are generally asymptomatic between attacks, although they may develop constant nystagmus, progressive ataxia, and cerebellar atrophy [4]. While it is an autosomal dominant disorder, individuals may not report a family history of either EA2 or FHM1 [8]. EA2’s effect on Purkinje cell activity may influence the behavior the cerebellum regulates, including motor function, problem-solving, and emotion regulation [34]. EA2-induced cerebellar atrophy may cause cognitive deficiencies [34]. These include impairments of verbal fluency, figural memory, visuoconstructive abilities, and psychomotor delays and are common in these patients during childhood. Many adult EA2 patients have deficiencies in attention, memory, visuoconstructive abilities, and cognitive control [35].
Clinical Diagnosis
Physicians may diagnose EA2 through physical examination, assessment of family history, and neurological examination, including analysis of coordination, motor function, and sensory function [5]. Tests such as computed tomography and magnetic resonance imaging provide insight into any cerebellar atrophy [8]. In our review of case reports on EA2, 42% of patients who underwent brain imaging had some form of cerebellar atrophy (Table 2). Cerebellar atrophy may aid in the diagnosis of EA2 but is not present in all patients. Between EA2 attacks, the brain often functions normally, but atrophy of the cerebellum may occur as a long-term effect of the disease [5]. Genetic testing is necessary when diagnosing EA2 in a patient, as it may identify mutations in the CACNA1A gene. Whole exome sequencing and next generation sequencing are often used to diagnose ataxic disorders [5]. Mutation in the CACNA1A gene may signify EA2, but it may also indicate SCA6 or FHM1 [2,4]. Therefore, a referral to both genetics and neurology is needed. A full history of the condition of the patient should be established, including onset age, duration and frequency of attacks, cognitive impairments, events that affected childhood development, and family history of the past three generations [5]. A video of the patient’s attacks and symptoms between episodes may provide insight into the telltale signs of ataxia and nystagmus [5]. EA2 may be overlooked due to its wide range of phenotypes and genetic variability. Misdiagnoses include migraine, vestibular disorders, seizures, transient ischemic attack, and anxiety [5].
Prognosis
EA2 is a lifelong condition that does not have a cure, yet symptoms can be mitigated with the use of medications and lifestyle changes. In some cases, symptoms cease entirely in patients who have been treated with medication [15,36]. It does not shorten lifespan and can be managed with treatments such as 4-aminopyridine or acetazolamide [24].These medications can be used as long as patients experience EA2 episodes, which can occur for the entirety of one’s life [14].
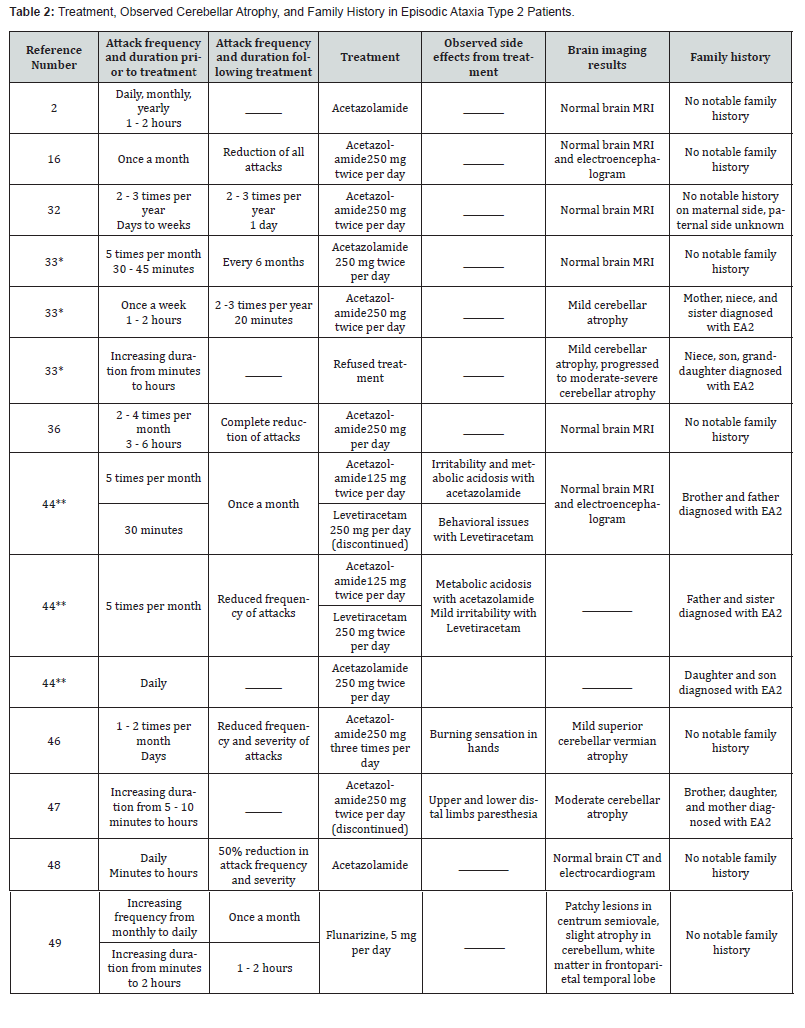
*Patients related to each other, familial EA2 **Patients related to each other, familial EA2
Treatment
4-aminopyridine (4-AP) and acetazolamide (AZM) are mainstays in the treatment of this condition. They may reduce the duration and frequency of attacks in patients [34]. 4-AP operates by blocking potassium channels, mainly the Kv1.5 subunit, increasing and extending action potential [14,37]. This has demonstrated improvement in the precision of pace making in Purkinje cells in tottering mice, which in turn increases the motor function of the animals [38]. Typical doses of 4-AP are between 10 to 20 mg/day [38,39]. Reported side effects of 4-AP include paresthesia, fatigue, dizziness, and insomnia [39,40]. AZM functions by inhibiting carbonic anhydrase, lowering lactate and pyruvate levels in the brain and decreasing pH. Lower pH reduces potassium flow, resetting firing potential of Purkinje cells [19]. The typical dosage of AZM is 250 to 1000 mg/day [41]. Reported side effects include kidney stones, nephrocalcinosis, paresthesia, dysgeusia, fatigue, hyperhidrosis, and gastrointestinal disturbances [41,42]. 4-AP and AZM have demonstrated their abilities to mitigate symptoms in EA2 attacks through numerous studies, including a doubleblind crossover trial with 30 EA2 patients. Concluding the 12- week period of the study, 4-AP reduced the number of attacks to 63%, while AZM reduced attack frequency to 52% [39]. Levetiracetam is another medication that may reduce symptoms in EA2 patients. Levetiracetam likely functions by intracellularly inhibiting presynaptic calcium channels. This may prevent the release of neurotransmitters and potentially mitigate ataxic episodes [43]. Combined treatment of levetiracetam and AZM has worked to reduce severity of symptoms in specifi cases. Patients who have previously been treated with AZM and experienced little to no change or an eventual increase in attacks have responded well to taking both medications [44]. To best manage EA2, one may make lifestyle changes to avoid triggers such as stress or lack of sleep. Maintaining one’s health through exercise, a balanced diet, and stress-relieving or managing practices may aid in reducing EA2 attacks. Several studies have demonstrated that patients with degenerative ataxia improved motor skills with consistent physical therapy [41,45]. While EA2 is not typically degenerative, patients may still experience improvements in gait and coordination with consistent physical therapy [46-49].
Conclusion
EA2 involves interictal nystagmus and possibly triggerinduced episodes of ataxia and vertigo. The frequency of attacks ranges widely between patients but typically present in childhood and persist into late adulthood. EA2 may be managed through the administration of 4-aminopyridine, AZM, or levetiracetam, and the avoidance of triggers. Potential areas of future study include further research into the mechanism between CACNA1A mutations and ataxic episodes, long-term effects of medications used to treat EA2, and risk factors to EA2.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest..
References
- Choi KD, Choi JH (2016) Episodic ataxias: Clinical and genetic features. J Mov Disord 9(3): 129-135.
- Guterman EL, Yurgionas B, Nelson AB (2016) Pearls & Oy-Sters: Episodic ataxia type 2: Neurology 86(23): 239-241.
- Ophoff RA, Terwindt GM, Vergouwe MN, Van Eijk R, Oefner PJ, et al. (1996) Familial hemiplegic migraine and episodic ataxia type-2 are caused by mutations in the Ca2+ channel gene CACNL1A4. Cell 87(3): 543-552.
- Kipfer S, Strupp M (2014) The clinical spectrum of autosomal‐dominant episodic ataxias. Mov Disord Clin Pract 1(4): 285-290.
- Hassan A (2023) Episodic ataxias: Primary and secondary etiologies, treatment, and classification approaches. Tremor Other Hyperinet Mov 13(1): 9.
- Maksemous N, Roy B, Smith RA, Griffiths LR (2016) Next‐generation sequencing identifies novel CACNA1A gene mutations in episodic ataxia type 2. Mol Genet Genomic Med 4(2): 211-222.
- Rajakulendran S, Schorge S, Kullmann DM, Hanna MG (2010) Dysfunction of the CaV2.1 calcium channel in cerebellar ataxias. F1000 Biology Reports 2: 4.
- Nilantha de Silva R, Vallortigara J, Greenfield J, Hunt B, Giunti P, et al. (2019) Diagnosis and management of progressive ataxia in adults. Pract Neurol 19(3): 196-207.
- Sintas C, Carreño O, Fernàndez-Castillo N, Corominas R, Vila-Pueyo M, et al. (2017) Mutation spectrum in the CACNA1A gene in 49 patients with episodic ataxia. Sci Rep 7(1): 2514.
- Guida S, Trettel F, Pagnutti S, Mantuano E, Tottene A, et al. (2001) Complete loss of P/Q calcium channel activity caused by a CACNA1A missense mutation carried by patients with episodic ataxia type 2. Am J Hum Genet 68(3): 759-764.
- Alvina K, Khodakhah K (2010) KCa channels as therapeutic targets in episodic ataxia type-2. J Neurosci 30(21): 7249-7257.
- Grosso BJ, Kramer AA, Tyagi S, Bennett DF, Tifft CJ, et al. (2022) Complex effects on Cav2.1 channel gating caused by a CACNA1A variant associated with a severe neurodevelopmental disorder. Sci Rep 12(1): 9186.
- Tara E, Vitenzon A, Hess E, Khodakhah K (2018) Aberrant cerebellar Purkinje cell activity as the cause of motor attacks in a mouse model of episodic ataxia type 2. Dis Model Mech 11(9): 34181.
- Alcalá-Torres J, Pérez-de la Fuente R, Cárdenas-Del Carre A, Arteche-López A, Posada-Rodriguez J, et al. (2023) Episodic ataxia type 2: A clinical, genetic and radiological study of 10 patients. Rev Neurol 76(10): 321-321.
- Mezghrani A, Monteil A, Watschinger K, Sinnegger-Brauns MJ, Barrère C, et al. (2008) A destructive interaction mechanism accounts for dominant-negative effects of misfolded mutants of voltage-gated calcium channels. J Neurosci 28(17): 4501-4511.
- Bady RS, Niksirat A (2013) Downward vertical gaze palsy as a prominent manifestation of episodic ataxia type 2: A case report. Iran J Child Neurol 7(4): 58-60.
- Strupp M, Zwergal A, Brandt T (2007) Episodic ataxia type 2. Neurotherapeutics 4(2): 267-273.
- Li XL, Li ZJ, Liang XY (2022) CACNA1A mutations associated with epilepsies and their molecular sub-regional implications. Front Neurosci 15: 860662.
- Niu X, Yang Y, Chen Y, Cheng M, Liu M, et al. (2022) Genotype-phenotype correlation of CACNA1A variants in children with epilepsy. Dev Med Child Neurol 64(1): 105-111.
- Rajakulendran S, Graves TD, Labrum RW, Kotzadimitriou D, Eunson L, et al. (2010) Genetic and functional characterisation of the P/Q calcium channel in episodic ataxia with epilepsy. J Physio 588(11): 1905-1913.
- Wong-Spracklen VMY, Kolesnik A, Eck J, Sabanathan S, Spasic-Boskovic O, et al. (2022) Biallelic CACNA1A variants: Review of literature and report of a child with drug‐resistant epilepsy and developmental delay. Am J Med Genet 188(11): 3306-3311.
- Garza-López E, Sandoval A, González-Ramírez R, Gandini MA, Van den Maagdenburg A, et al. (2012) Familial hemiplegic migraine type 1 mutations W1684R and V1696I alter G protein-mediated regulation of CaV2.1 voltage-gated calcium channels. Biochim Biophys Acta 1822(8): 1238-1246.
- Nardello R, Plicato G, Mangano GD, Gennaro E, Mangano S, et al. (2020) Two distinct phenotypes, hemiplegic migraine and episodic ataxia type 2, caused by a novel common CACNA1A variant. BMC Neurol 20(1): 155.
- Strupp M, Kalla R, Claassen J, Adrion C, Mansmann U, et al. (2011) A randomized trial of 4-aminopyridine in episodic ataxia type 2 and related familial episodic ataxias. Neurology 77(3): 269-275.
- Stefano VD, Rispoli MG, Pellegrino N, Graziosi A, Rotondo E, et al. (2020) Diagnostic and therapeutic aspects of hemiplegic migraine. J Neurol Neurosurg Psychiatry 91(7): 764-771.
- Schwedt TJ, Zhou J, Dodick DW (2014) Sporadic hemiplegic migraine with permanent neurological deficits. Headache: The Journal of Head and Face Pain 54(1): 163-166.
- Oberndorfer S, Wöber C, Nasel C, Asenbaum S, Lahrmann H, et al. (2004) Familial hemiplegic migraine: Follow-up findings of diffusion-weighted magnetic resonance imaging (MRI), perfusion-MRI and [99mTc] HMPAO-SPECT in a patient with prolonged hemiplegic aura. Cephalalgia 24(7): 533-539.
- Pelzer N, Stam AH, Carpay JA, de Vries B, van den Maagdenberg AM, et al. (2014) Familial hemiplegic migraine treated by sodium valproate and lamotrigine. Cephalalgia 34(9): 708-711.
- Sinke RJ, Ippel EF, Diepstraten CM, Beemer FA, Wokke JH, et al. (2001) Clinical and molecular correlations in spinocerebellar ataxia type 6. Arch Neurol 58(11): 1839-1844.
- Ghanekar SD, Kuo SH, Staffetti JS, Zesiewicz TA (2022) Current and emerging treatment modalities for spinocerebellar ataxias. Expert Rev Neurother 22(2): 101-114.
- Jen J, Kim GW, Baloh RW (2004) Clinical spectrum of episodic ataxia type 2. Neurology 62(1): 17-22.
- Isaacs DA, Bradshaw MJ, Brown K, Hedera P (2017) Case report of novel CACNA1A gene mutation causing episodic ataxia type 2. Sage Open Med Case Rep pp. 5.
- Verriello L, Carrera P, Pauletto G, Bernardini A, Valente M, et al. (2021) Case report and ten-year follow-up of episodic ataxia type 2 due to a novel variant in CACNA1A. eNeurologicalSci 23: 100334-100334.
- Koziol LF, Budding D, Andreasen N, D’Arrigo S, Bulgheroni S, et al. (2014) Consensus paper: The cerebellum’s role in movement and cognition. Cerebellum 13(1): 151-177.
- Ahmed S, Brennan L, Eppig J, Price CC, Lamar M, et al. (2016) Vasoconstriction impairment in subtypes of mild cognitive impairment. Appl Neuropsychol Adult 23(1): 43-52.
- Orsucci D, Raglione LM, Mazzoni M, Vista M (2019) Therapy of episodic ataxias: Case report and review of the literature. Drugs Context 8: 212576.
- Choquet D, Korn H (1992) Mechanism of 4-aminopyridine action on voltage-gated potassium channels in lymphocytes. J Gen Physiol 99(2): 217-240.
- Strupp M, Teufel J, Zwergal A, Schniepp R, Khodakhah K, et al. (2017) Aminopyridines for the treatment of neurologic disorders. Neurol Clin Pract 7(1): 65-76.
- Muth C, Teufel J, Schöls L, Synofzik M, Franke C, et al. (2021) Fampridine and acetazolamide in episodic ataxia type 2 and related familial EA. Neurol Clin Pract 11(4): e438-e446.
- King AM, Menke NB, Katz KD, Pizon AF (2012) 4-aminopyridine toxicity: A case report and review of the literature. J Med Toxicol 8(3): 314-321.
- Synofzik M, Ilg W (2014) Motor training in degenerative spinocerebellar disease: Ataxia-specific improvements by intensive physiotherapy and exergames. BioMed Res Int 2014: 583507.
- Schmickl CN, Owens RL, Orr JE, Edwards BA, Malhotra A, et al. (2020) Side effects of acetazolamide: A systematic review and meta-analysis assessing overall risk and dose dependence. BMJ Open Respir Res 7(1): e000557.
- Shimazaki H (2025) Effects of levetiracetam on episodic ataxia type 2 and spinocerebellar ataxia type 6 with episodic ataxic symptoms: A case series. Gene 16(3): 335-335.
- Reyes-Fernández A, Miles-Acuña J, González-del Valle JM, Ávila-Smirnow (2024) Episodic ataxia type 2 in a Latin American family 95(6): 786-793.
- Miyai I, Ito M, Hattori N, Mihara M, Hatakenaka M, et al. (2012) Cerebellar ataxia rehabilitation trial in degenerative cerebellar diseases. Neurorehabil Neural Repair 26(5): 515-522.
- Singhvi JP, Prabhakar S, Singh P (2000) Episodic ataxia: A case report and review of literature. Neurol India 48(1): 78-80.
- Verriello L, Pez S, Pauletto G, Valente M (2025) Efficacy and safety of 4-aminopyridine in episodic ataxia type 2: A case series. J Neurol 272(3): 205.
- Wu HJ, Lau WL, Chan TYC, Chen SPL, Ko CH, et al. (2020) Differentiating episodic ataxia type 2 from migraine: a case report. Hong Kong Med J 26(6): 526-527.
- Yuan X, Zheng Y, Gao F, Sun W, Wang Z, et al. (2022) Case report: A novel CACNA1A mutation caused flunarizine-responsive type 2 episodic ataxia and hemiplegic migraine with abnormal MRI of cerebral white matter. Front Neurol 13: 899813.






























