Abstract
Purpose: To evaluate the effectiveness of gonioscopy-assisted transluminal trabeculotomy (GATT), alone or combined with phacoemulsification, in patients with primary open-angle glaucoma (POAG), pseudoexfoliative glaucoma (PEXG), and primary angle-closure glaucoma (PACG) in a three-month prospective follow-up.
Methods: Prospective clinical study conducted at “Instituto de la Visión” between 2023 and 2024. Twenty-seven eyes were included: POAG (n=13), PEXG (n=9), and PACG (n=5), treated with GATT alone or GATT + phacoemulsification. Outcomes included intraocular pressure (IOP), number of hypotensive medications, best-corrected visual acuity (BCVA), and visual field mean deviation (MD). Paired t-tests were used for comparison; p<0.05 was considered significant.
Results: A significant IOP reduction was observed in POAG and PEXG groups (-37.9% and -58.5%, p<0.05), with a decrease in medication use of 64.7% and 66.7%, respectively. In the PACG group, IOP reduction was not statistically significant. The greatest IOP reduction occurred in the GATT-only group (-58.2%). BCVA remained stable. The most frequent complication was mild, self-limited hyphema.
Conclusions: GATT, either standalone or combined with phacoemulsification, is a safe and effective surgical option for patients with primary glaucomas, particularly in POAG and PEXG, allowing for significant pressure reduction and medication burden relief.
Keywords: Open-Angle Glaucoma; Exfoliation Syndrome; Angle-Closure Glaucoma; Trabeculotomy; Trabeculotomy
Abbreviations: POAG: Primary Open-Angle Glaucoma; PEXG: Pseudoexfoliative Glaucoma; IOP: Intraocular Pressure; GATT: Gonioscopy-Assisted Transluminal Trabeculotomy; BCVA: Best-Corrected Visual Acuity; MIGS: Minimally Invasive Glaucoma Surgery; PEXG: Pseudoexfoliative Glaucoma; PAS: Peripheral Anterior Synechiae
Introduction
Glaucoma blindness is a major public health problem worldwide. It is estimated that by 2040 approximately 112 million people will suffer from glaucoma, consolidating this disease among the main causes of irreversible blindness [1]. Intraocular pressure (IOP) reduction is the only effective strategy to slow glaucomatous progression. In advanced stages, more aggressive IOP control (maintaining IOP <18 mmHg, ideally <14 mmHg) is associated with lower risk of visual field deterioration [1,2] so clinical guidelines recommend early surgical intervention in advanced glaucomas to prevent visual loss [2,3].
Trabeculectomy has been the standard surgery for decades due to its efficacy in lowering IOP; however, it carries potentially severe postoperative complications (hypotony, retinal detachment, endophthalmitis), a risk that is accentuated in patients with advanced glaucoma [2-4]. Consequently, there has been increased interest in minimally invasive glaucoma surgery (MIGS), which avoids the formation of a filtration bleb and offers a more favorable safety profile compared to traditional filtering surgery [5,6].
Within MIGS, gonioscopy-assisted transluminal trabeculotomy (GATT), described by Grover et al. in 20147, stands out. GATT is an ab interno procedure that preserves the conjunctiva and eliminates resistance to aqueous humor drainage at the level of the trabecular meshwork and the inner wall of Schlemm’s canal [7,8]. Numerous prospective studies and case series have reported encouraging results with GATT in the medium term [9]. In primary open angle glaucoma (POAG), for example, an average IOP reduction of 35-40% has been observed 1 to 2 years post-surgery, with success rates (blood pressure control with medication reduction) exceeding 75% [10,11]. These results are comparable to those obtained with trabeculectomy in eyes with GPAA, but with fewer complications related to the filtering procedure [12,13].
In fact, GATT has been proposed as a safe and effective alternative to trabeculectomy even in advanced glaucoma, achieving significant IOP reductions with low incidence of serious complications [14] because it does not require the creation of a filtering bleb. The efficacy of GATT extends to secondary glaucomas and aggressive forms of the disease. In pseudoexfoliative glaucoma (PEXG), recent studies have shown that GATT achieves a significant decrease in IOP and reduction in the number of drugs required, with a low complication profile and without the need for conjunctival bleb [15].
Some findings further suggest that 360° trabeculotomy may exert an even greater effect in pseudoexfoliative glaucomas than in primary glaucomas, given that the drainage obstruction in exfoliative syndrome is predominantly located at the trabecular level [16]. This would support the ability of GATT to directly remove obstructive material deposited in the trabecular meshwork, explaining the remarkable IOP reduction observed in GPEX. On the other hand, in primary narrow-angle glaucoma (PACG) surgical management presents additional challenges.
Phacoemulsification of the lens alone can widen the iridocorneal angle and contribute to lower IOP - as demonstrated by the EAGLE clinical trial in primary angle-closure [17], but many patients require steeper IOP decreases. In this context, combining MIGS surgery with lens extraction is an attractive strategy. A recent randomized clinical trial in patients with GPAE and cataract showed that adding GATT to phacoemulsification achieved greater IOP reduction, lower drug dependence and a higher surgical success rate compared to performing phacoemulsification alone [3]. This result suggests a synergistic effect when simultaneously addressing the angular closure component, through lensectomy, and trabecular resistance, through ab interno trabeculotomy.
The option of combining cataract surgery with an antiglaucomatous intervention is not new; historically it has attracted interest due to the frequent coincidence of both pathologies in older patients [18]. A single combined procedure avoids the trauma of two separate surgeries and, in addition, prevents the cataract progression that often follows glaucoma filtering surgery [18]. Multiple variants of combined glaucoma surgery have been devel developed in recent years. In the specific case of GATT combined with phacoemulsification (PHACO-GATT), prospective series have reported favorable results in terms of blood pressure control and reduction of medical treatment in patients with glaucoma and cataract [17,18]. In a 24-month follow-up, for example, success rates greater than 80-85% were achieved when combining GATT with phacoemulsification, with average IOP decreases to ranges of 14- 16 mmHg and no vision-threatening complications [16-18]. These preliminary data support the safety and efficacy of combined surgery, although long-term evidence is still limited [18].
Despite the promising results mentioned above, much of the available evidence on GATT comes from retrospective studies or single case series, which may introduce biases. There is a need to confirm these findings under rigorous prospective designs. In fact, in recent years, randomized controlled trials focusing on MIGS techniques, comparing trabecular shunt devices [17] or evaluating internal trabeculotomy variants [16,17] have begun to be published, reflecting the search for level I evidence in glaucoma surgery. However, knowledge gaps persist regarding the optimal role of GATT, particularly in subtypes such as GPEX and narrow-angle glaucoma. Therefore, we have designed a prospective clinical study to evaluate the efficacy and safety of GATT, alone and in combination with phacoemulsification, in patients with primary open-angle glaucoma (POAG), pseudoexfoliative glaucoma (PEXG) and narrow-angle glaucoma (PACG). This study follows the CONSORT 2010 guidelines for clinical trial reporting [19], ensuring transparency in the conduct and reporting of each phase.
Materials and Methods
Study design
A prospective, longitudinal, single-center clinical trial was conducted to evaluate the efficacy of gonioscopy-assisted transluminal trabeculotomy (GATT), alone or combined with phacoemulsification, in the control of intraocular pressure (IOP) in patients with primary open-angle glaucoma (POAG), pseudoexfoliative glaucoma (PEXG), and primary narrow-angle glaucoma (PACG). The study included two parallel intervention groups: one with isolated GATT surgery and the other with combined phacoemulsification plus GATT surgery in the same surgical procedure. Assignment to combined surgery depended on the presence of significant cataract; no masking was performed since the type of intervention was evident to surgeons and patients. All patients were followed prospectively for 12 months, with preoperative baseline assessments and postoperative follow-ups scheduled at 1 week, 1, and 3 months. This prospective and longitudinal design allowed the collection of data in a standardized manner over time, constituting a methodological strength for the reliability of the results.
Participants
The study was conducted at the “Instituto de la Visión” (IOI) and included consecutive adult patients seen between October 2022 and June 2024 who met the inclusion criteria. All participants had a confirmed diagnosis of POAG, PEXG or PACG in moderate to advanced stages, with indication for antiglaucomatous surgery according to clinical evaluation. Patients with other forms of secondary glaucoma were excluded (Table 1) to obtain a homogeneous sample.

POAG: Primary Open-Angle Glaucoma; PEXG: Pseudoexfoliative Glaucoma; PACG: Primary Angle-Closure Glaucoma; IOP: Intraocular Pressure; GATT: Gonioscopy-Assisted Transluminal Trabeculotomy.
Inclusion criteria consisted of patients who received a series of at least two intravitreal corticosteroids for the treatment of posterior segment pathology, including diabetic macular edema, central or branch retinal vein occlusion with macular edema, pseudophakic cystoid macular edema, macular or retinal edema, uveitis, diabetic retinopathy, and neovascular macular degeneration. Exclusion criteria included concurrent use of multiple intravitreal steroid formulations, incomplete IOP data, or follow-up consisting of less than 2 injections.
Sample Size: 27 eyes of 24 consecutive patients who met the established selection criteria were included. Of these, 5 eyes were assigned to isolated GATT intervention, while 22 eyes were treated by combined phacoemulsification and GATT surgery (PHACO- GATT). All patients signed written informed consent before entering the study, according to institutional ethical guidelines and international clinical research regulations.
Intervention
Surgical procedure: All surgeries were performed by the same glaucoma expert surgeon, under peribulbar anesthesia and under ambulatory surgery conditions. The GATT technique was performed following the original description by Grover et al. using an ab-internal approach [20]. In general terms, the procedure consists of performing a goniotomy in the nasal quadrant through an incision in the trabecular meshwork, introducing a microcatheter or a 5-0 polypropylene suture along 360° of Schlemm’s canal, and finally exerting external traction on the end of the thread to achieve a complete 360° circumferential trabeculotomy, without the need for scleral or conjunctival incisions [20].
Combined Surgery: In cases assigned to combined surgery, phacoemulsification with intraocular lens (IOL) implantation followed by the GATT procedure was performed in the same surgical procedure. Specifically, after removal of the opaque lens by phacoemulsification, we proceeded to inject viscoelastic and perform transluminal trabeculotomy as described above, taking advantage of the larger anterior chamber width after phacoemulsification. This sequential approach (phacoemulsification before GATT) sought to optimize gonioscopic visualization and safety during catheterization of Schlemm’s canal [21]. In patients without significant cataract, only GATT was performed, preserving the lens.
Postoperative Care: After surgery, all patients received the same postoperative regimen consisting of antibiotic eye drops and intensive topical corticosteroids during the first week, gradually reducing according to inflammation. Hypotensive medication was continued or readjusted according to IOP at each control. Intraoperative and postoperative complications were recorded, providing treatment according to clinical practice.
Variables and Measurement Instruments
Specified clinical outcome variables were defined and standardized
instruments were used for their measurement:
Intraocular Pressure (IOP): primary efficacy variable. Measured
in mmHg by Goldmann tonometry at each visit (preoperative
and postoperative controls at 1 week, 1 and 3 months). IOP
reduction was calculated as the absolute and percentage difference
from baseline for each eye.
Automated Visual Field (VF): Evaluated by computerized
campimetry with SITA-FAST protocol in the Humphrey analyzer
(Carl Zeiss Meditec®), using the 24-2 program. The mean deviation
(MD) value was recorded as a continuous measurement in
decibels (dB), both preoperatively and at 3 months postoperatively.
The objective was to detect functional progression of glaucomatous
damage. Progression was defined as a sustained worsening
of MD greater than 1 dB, confirmed in at least two consecutive
fields. The values were used as a secondary measure of efficacy
and correlated with IOP variations.
Surgical Success Rate: complete success was defined as
achieving an IOP ≤ 18 mmHg and a reduction ≥ 20% from baseline
without antihypertensive medication; qualified success if these
goals were achieved with medication; and failure if IOP goals
were not achieved or if additional surgery was necessary to con
trol glaucoma. This variable was assessed at 12-month follow-up
and for survival analysis.
Number of Antihypertensive Medications: Counted the
number of topical antiglaucomatous drugs that each patient required
before surgery and at each control during follow-up, adjusting
for clinical need.
Best Corrected Visual Acuity (BCVA): Measured in each eye
by Snellen chart (converted to log MAR) preoperatively and at the
end of follow-up, to monitor visual changes associated with surgery.
Biomicroscopy: Performed at all pre- and postoperative visits
to evaluate anterior segment integrity, inflammation (anterior
chamber swelling), presence of hyphema, corneal opacity or anterior
synechiae.
Gonioscopy: The iridocorneal angle was evaluated by means
of a four-mirror Sussman-type slit-lamp lens. The degree of angular
opening according to Shaffer’s classification (0-4) and the
presence of peripheral anterior synechiae (PAS) were recorded.
This evaluation was especially relevant in patients with PAS to
document angular reopening or postsurgical synechiae.
Postoperative Complications: Systematically recorded at
each visit. In particular, the occurrence of hyphema and corneal
edema, transient hypertension, synechiae, infections or any adverse
event was monitored. Spontaneous resolution of hyphema
and other complications was documented until discharge.
All evaluations were performed by team ophthalmologists, standardizing measurements to minimize bias. Data was recorded on specific forms and then entered an electronic database for analysis.
Statistical Analysis
The data collected were analyzed with IBM SPSS Statistics software. Prior to inferential analysis, the distribution of continuous variables was checked using the Shapiro-Wilk test. Variables with approximately normal distribution were summarized as mean ± standard deviation and compared using parametric tests: paired Student’s t-test to compare IOP and other pre- vs. postoperative values within each group, and unpaired Student’s t-test to compare differences between the GATT alone vs. combined group at each time point. In case of non-normal distribution, equivalent non-parametric tests were used: Wilcoxon test for paired comparisons and Mann-Whitney U for comparisons between groups. Categorical variables (e.g., proportion of successful eyes, incidences of complications) were expressed as percentages and analyzed using the chi-square test or Fisher’s exact test, as appropriate.
A bilateral significance level of p < 0.05 was used in all hypothesis contrasts. Per-protocol analyses were performed; in addition, an intention-to-treat sensitivity analysis was performed considering as failures the possible patients lost to follow-up. No adjustment for multiplicity was made since the primary objective was a single IOP reduction, but secondary findings were interpreted in an exploratory manner.
Ethical Procedures: This study was conducted following the fundamental ethical principles for clinical research, guaranteeing respect for the rights of the participants. The research complied with regulations on personal data protection, informed consent and patient welfare.
Results
Twenty-seven eyes of 24 patients were included, with a mean age of 71.6 ± 8.6 years (range: 56-91). Of the total, 58.3% were male (n = 14) and 41.7% female (n = 10). Regarding diagnosis, 13 eyes (48.1%) corresponded to primary open-angle glaucoma (POAG), 9 eyes (33.3%) to pseudoexfoliative glaucoma (PEXG) and 5 eyes (18.5%) to primary narrow-angle glaucoma (PACG). Regarding the intervention, 22 eyes (81.5%) underwent combined phacoemulsification + GATT (PHACO-GATT) surgery, and 5 eyes (18.5%) underwent GATT alone. Preoperatively, mean intraocular pressure (IOP) was 22.3 ± 6.9 mmHg, mean number of antiglaucomatous medications was 3.4 ± 0.7, and mean best corrected visual acuity (BCVA) was 0.19 logMAR (Table 2).

Values are expressed as mean ± standard deviation unless otherwise specified. POAG: Primary Open-Angle Glaucoma; PEXG: Pseudoexfoliative Glaucoma; PACG: Primary Angle-Closure Glaucoma; IOP: Intraocular Pressure.
Changes in Intraocular Pressure (IOP)
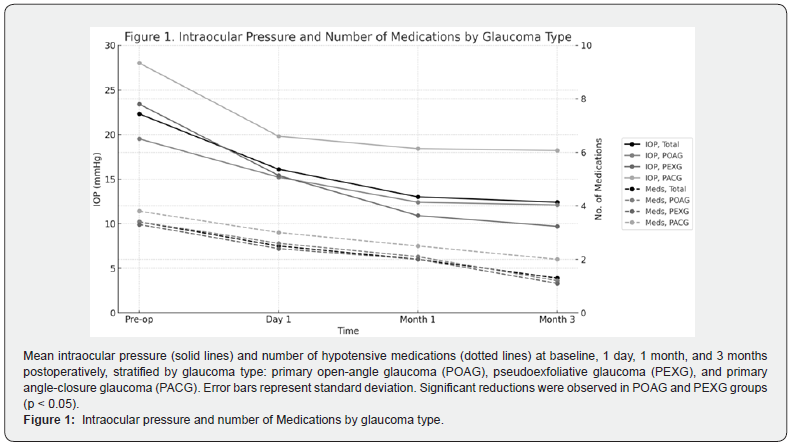
At three months postoperatively, mean IOP decreased to 12.4 ± 8.3 mmHg, representing an overall reduction of 44.3%. This decrease was statistically significant in the POAG (-37.9%; p < 0.05) and PEXG (-58.5%; p < 0.05) subgroups. In contrast, the decrease in the PACG group (-33.9%) did not reach statistical significance, probably due to the smaller sample size and greater intra-individual variability (Table 3).

Data are expressed as mean ± standard deviation or n (%). IOP: Intraocular Pressure; BCVA: Best-Corrected Visual Acuity; MD: Mean Deviation; Pre-Op: Preoperative; Post-Op: Postoperative.
Reduction in the Number of Drugs
The number of antihypertensive drugs was significantly reduced in all subgroups. In POAG, the mean decreased from 3.4 to 1.2 medications (-64.7%; p < 0.05); in PEXG, from 3.3 to 1.1 (-66.7%; p < 0.05); and in PACG, from 3.8 to 2.0 (-47.4%; p < 0.05). These results reflect a significant decrease in the post-surgical pharmacologic burden, especially in open-angle glaucoma (Table 3).
Visual Field
Regarding visual field analysis, the preoperative Mean Deviation (MD) was -20.1 ± 9.9 dB in the entire cohort. When broken down by diagnostic subgroups, the values were similar: POAG -20.5 ± 7.9 dB, PEXG -19.3 ± 13.4 dB and PACG -20.5 ± 9.4 dB. Since postoperative measurements at 3 months are not yet available, it was not possible to evaluate significant changes in this functional variable in the present analysis. Its inclusion in longer-term follow- up is contemplated (Table 4).

Mean Deviation (MD) is expressed in decibels (dB) and represents the baseline visual field loss measured by automated perimetry.
No postoperative MD data were available at 3 months.
POAG: Primary Open-Angle Glaucoma; PEXG: Pseudoexfoliative Glaucoma; PACG: Primary Angle-Closure Glaucoma.
Analysis According to Type of Intervention
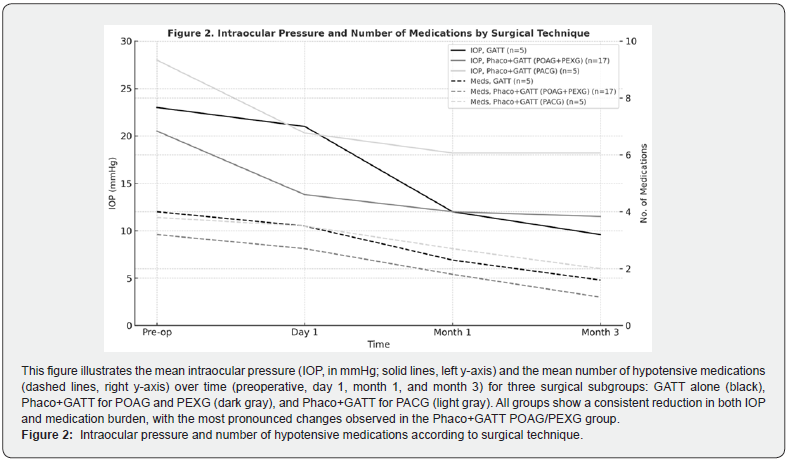
To identify differences related to the type of procedure, a comparative analysis was performed between three groups: GATT alone (n = 5), PHACO-GATT in POAG + PEXG (n = 17), PHACO-GATT in PACG (n = 5). The greatest IOP reduction was observed in the GATT alone group, with a 58.2% decrease (from 23.0 ± 8.8 to 9.6 ± 3.2 mmHg; p < 0.05). The PHACO-GATT group in POAG + PEXG showed a 43.9% reduction (from 20.5 ± 4.9 to 11.5 ± 3.3 mmHg; p < 0.05). In the PHACO-GATT group in PACG, IOP was reduced by 33.9% (from 28.0 ± 9.3 to 18.2 ± 18.4 mmHg), but without statistical significance (Table 5). Regarding the hypotensive drug load, all three groups showed statistically significant reductions: PHACO-GATT in POAG + PEXG: from 3.2 ± 0.4 to 1.0 ± 1.2 drugs (-68.8%); GATT alone: from 4.0 ± 0.7 to 1.6 ± 1.5 drugs (-60.0%); PHACO-GATT in PACG: from 3.8 ± 0.8 to 2.0 ± 1.6 drugs (-47.4%) (Table 6).

Values are expressed as mean ± standard deviation (SD). POAG: Primary Open-Angle Glaucoma; PEXG: Pseudoexfoliative Glaucoma;
PACG: Primary Angle-Closure Glaucoma.
*Statistically significant reduction compared to baseline (p < 0.05)

Values expressed as mean ± Standard Deviation (SD). IOP: Intraocular Pressure; GATT: Gonioscopy-Assisted Transluminal Trabeculotomy;
Phaco: Phacoemulsification; POAG: Primary Open-Angle Glaucoma; PEXG: Pseudoexfoliative Glaucoma; PACG: Primary
Angle-Closure Glaucoma.
*Statistically significant reduction compared to preoperative values (p < 0.05).
Changes in Visual Acuity
Global AVMC improved discreetly from 0.19 to 0.14 log MAR at 3 months postoperatively. No statistically significant differences were found in any of the subgroups, although it is important to consider that the visual improvement in the combined group could be related to lens extraction, and not exclusively to antiglaucomatous surgery.
Complications
The complications observed were generally mild and transient. The most frequent was hyphema, present in 63% of eyes (n = 17), mostly mild (<1 mm) and resolved spontaneously at follow-up. Only one patient presented a 3-mm hyphema, with no subsequent sequelae. Five patients (18.5%) developed hypertensive elevations greater than 30 mmHg within the first 24 hours postoperatively, the highest reaching 35 mmHg. All were controlled with medical treatment without visual complications.
Discussion
This study evaluated the effectiveness of the GATT procedure and GATT combined with phacoemulsification (PHACO-GATT) in three subtypes of glaucoma: primary open-angle glaucoma (POAG), pseudoexfoliative glaucoma (PEXG), and primary narrow- angle glaucoma (PACG). The best results were observed in the PEXG subgroup, where the reduction in intraocular pressure (IOP) and the number of hypotensive medications was statistically significant, which is consistent with previous studies highlighting the high response to GATT in glaucomas with dysfunctional trabecular meshwork as in PEXG (Figure 1). Our results confirm that GATT represents an effective alternative in patient’s refractory to medical treatment who seek to avoid the complications of traditional filtering surgeries. During the follow-up period, no patient with either POAG or PEXG required a new surgical intervention, even considering that the sample included advanced glaucomas, mean preoperative mean deviation (MD) of -20.1 dB, which reinforces the potential of GATT as a useful technique even in severe stages [21].
In the PACG subgroup, the IOP reduction did not reach statistical significance, probably due to the low sample size and to a particular case operated in the context of acute glaucoma, which biased the averages. However, a significant reduction in the number of drugs was observed. These findings suggest that studies with a larger sample size and stratification according to the evolutionary phase of glaucoma could better clarify the role of GATT in PACG. Furthermore, it would be interesting to compare PHACO versus PHACO-GATT in this subgroup to assess whether there is a clinically relevant synergistic effect [22,23] (Figure 2). Regarding the safety of the procedure, GATT presented a favorable profile, the most common complication was hyphema, usually mild (≤1 mm) and self-limited. Only one case presented a 3-mm hyphema, which also did not require further intervention. Immediate hypertensive peaks (postoperative day 1) were rare and managed conservatively, with no recurrences at follow-up. These results coincide with those reported by Grover et al. who highlighted the low rate of major complications in surgeries with GATT7.
Conclusion
GATT proved to be an effective and safe technique for the treatment of glaucoma, especially in patients with POAG and PEXG who do not respond adequately to medical therapy. At 3-month follow- up, a significant reduction in IOP and number of hypotensive medications was achieved, being more pronounced in the PEXG subgroup, suggesting that this condition could particularly benefit from the procedure. In the PACG subgroup, although the reduction in IOP was not statistically significant, a relevant decrease in the number of medications was observed, which warrants future research with larger sample size and direct comparisons with PHACO alone, to evaluate the possible additional advantage of GATT.
Funding and Conflict of Interest
The authors declare no conflict of interest. The present research has not received any specific grants from agencies in the public, commercial, or for-profit sectors.
References
- Soyugelen G, Güvenç U, Burcu A (2025) Outcomes of Gonioscopy-Assisted Transluminal Transluminal Trabeculotomy (GATT) in Advanced Glaucoma: A Retrospective Analysis. Medicine (Kaunas) 61(3): 444.
- Eduardo Akio Pereira I, Cláudia Gomide Vilela de S Franco, Ana Cláudia Alves Pereira, Bruno Teno, Francisco Lucena-Neto, et al. (2024) Real-world outcomes and predictors of failure of gonioscopy-assisted transluminal trabeculotomy in a large glaucoma cohort: a multicenter study. Scientific Reports 14(1): 30934.
- Sayed YME, Mettias NM, Elghonemy HME, Mostafa YSE (2023) Phacoemulsification with gonioscopy-assisted transluminal trabeculotomy versus phacoemulsification alone in primary angle closure glaucoma: A randomized controlled study. Acta Ophthalmologica 102(2).
- Dar N, Haim LNB, Yehezkeli V, Sharon T, Belkin A (2023) Gonioscopy-assisted transluminal trabeculotomy in patients with advanced glaucoma. Indian Journal of Ophthalmology 71(8): 3024-3030.
- Wang Y, Zhang W, Xin C, Sang J, Sun Y, et al. (2023) Gonioscopy-assisted transluminal trabeculotomy for open-angle glaucoma with failed incisional glaucoma surgery: two-year results. BMC Ophthalmology 23(1): 89.
- Habash AAA, Alrushoud M, Abdulsalam OA, Somali AIA, Aljindan M, et al. (2020) <p>Combined Gonioscopy-Assisted Transluminal Transluminal Trabeculotomy (GATT) with Ab Interno Canaloplasty (ABiC) in Conjunction with Phacoemulsification: 12-Month Outcomes</p> Clinical Ophthalmology 14: 2491-2496.
- Grover DS, Godfrey DG, Smith O, Feuer WJ, Montes de Oca I, et al. (2014) Gonioscopy-assisted transluminal trabeculotomy, ab interno trabeculotomy: Technique report and preliminary results. Ophthalmology 121(4): 855-861.
- Aktas Z, Ucgul AY, Bektas C, Sahin Karamert S (2019) Surgical outcomes of prolene gonioscopy-assisted transluminal trabeculotomy in patients with moderate to advanced open-angle glaucoma. J Glaucoma 28(10): 884-888.
- Ćwiklińska-Haszcz A, Żarnowski T, Wróbel-Dudzińska D, Kosior-Jarecka E (2023) The Efficacy and Safety of the GATT Procedure in Open-Angle Glaucoma-6-Month Results. International Journal of Environmental Research and Public Health 20(3): 2759.
- Sharkawi E, Lindegger DJ, Artes PH, Lehmann-Clarke L, Wardani ME, et al. (2020) Outcomes of gonioscopy-assisted transluminal trabeculotomy in pseudoexfoliative glaucoma: 24-month follow-up. British Journal of Ophthalmology 105(7): 977-982.
- Rahmatnejad K, Pruzan NL, Amanullah S, Shaukat BA, Resende AF, et al. (2017) Surgical outcomes of gonioscopy-assisted transluminal trabeculotomy (GATT) in patients with open-angle glaucoma. J Glaucoma 26(12): 1137-1143.
- Panigrahi A, Kumar A, Gupta S, Grover DS, Gupta V (2025) Outcomes of gonioscopy-assisted transluminal trabeculotomy in advanced pigmentary glaucoma. Br J Ophthalmol 109(3): 340-346.
- Aktas Z, Ozmen MC, Atalay HT, Ucgul AY (2018) Evaluation of episcleral venous fluid wave during gonioscopy-assisted transluminal trabeculotomy in patients with advanced glaucoma. Eye 33(4): 668-673.
- Cakir I, Balci AS, Alagoz N, Cakir GY, Altan C, et al. (2024) Efficacy of gonioscopy-assisted transluminal trabeculotomy and trabeculectomy in patients with primary open-angle glaucoma and pseudoexfoliative glaucoma: A single surgeon's experience. Indian J Ophthalmol 72(Suppl 5): S821-S826.
- Eamon Sharkawi, Daniel Josef Lindegger, Paul H Artes, Lydia Lehmann-Clarke, Mohamad El Wardani, et al. (2021) Outcomes of gonioscopy-assisted transluminal transluminal trabeculotomy in pseudoexfoliative glaucoma: 24-month follow-up British Journal of Ophthalmology 105(7): 977-982.
- Aktas Z, Zeydanli EO, Uysal BS, Yigiter A (2022) Outcomes of Prolene Gonioscopy Assisted Transluminal Trabeculotomy in Primary Open Angle Glaucoma and Pseudoexfoliation Glaucoma: A Comparative Study. Journal Of Glaucoma 31(9): 751-756.
- Naftali Ben Haim L, Yehezkeli V, Kratz A, Dar N, Sharon T, et al. (2025) Surgical Outcomes of Gonioscopy-Assisted Transluminal Trabeculotomy (GATT) in Primary and Secondary Open- and Closed-Angle Glaucoma. Diagnostics 15(10): 1226.
- Yue Wan, Kai Cao, Jin Wang, Yunxiao Sun, Rong Du, et al. (2023) Gonioscopy-assisted Transluminal Trabeculotomy (GATT) combined phacoemulsification surgery: Outcomes at a 2-year follow-up. Eye 37(6): 1258-1263.
- Liu X, Cruz Rivera S, Moher D, Calvert MJ, Denniston AK (2023) SPIRIT-AI and CONSORT-AI Working Group; SPIRIT-AI AND CONSORT-AI STEERING GROUP; SPIRIT-AI and CONSORT-AI Consensus Group. Directrices para presentación de informes de ensayos clínicos sobre intervenciones con inteligencia artificial: extensión CONSORT-AI [Reporting guidelines for clinical trial reports for interventions involving artificial intelligence: the CONSORT-AI extensionDiretrizes para relatórios de ensaios clínicos com intervenções que utilizam inteligência artificial: a extensão CONSORT-AI]. Rev Panam Salud Publica 48: e13.
- GATT: Novel 360oab interno glaucoma procedure (2020) Ophthalmology Times.
- Quera JR (2024) Disruptive procedures: gonioscopy-assisted transluminal trabeculotomy. Annals d'Oftalmologia 32(4): 181-193.
- Yunsheng Qiao, Chen Tan, Xueli Chen, Xinghuai Sun, Junyi Chen (2021) Gonioscopy-assisted transluminal trabeculotomy versus goniotomy with Kahook dual blade in patients with uncontrolled juvenile open-angle glaucoma: a retrospective study. BMC Ophthalmol 21: 395.
- Bussel II, Kaplowitz K, Schuman JS, Loewen NA (2015) Trabectome Study Group. Outcomes of ab interno trabeculectomy with the trabectome by degree of angle opening. Br J Ophthalmol 99(7): 914-919.






























