Abstract
Objective: To evaluate the anatomical and functional results of the use of lyophilized human amniotic membrane (lhAM) as a surgical graft in the treatment of large and/or refractory full-thickness macular holes (FTMH).
Methods: A case series was performed in 13 eyes of 13 patients surgically operated on at the Fundación Oftalmológica Los Andes (FOLA) in Chile, between 2020 and 2024. Patients with FTMH ≥400 μm or a history of failed surgery were included. All were treated by pars plana vitrectomy (PPV) with lhAM placement and tamponade with C3F8 or silicone. Anatomical closure by OCT and best corrected visual acuity (BCVA) were evaluated pre and postoperatively at 6 months. Statistical analysis was applied with significance p<0.05.
Results: The mean age was 61.4 years and 84.6% of the patients were women. Anatomical closure was achieved in 84.6% of cases. AVMC improved from 0.06 to 0.13 (decimal scale), a statistically significant difference (p=0.007). No relevant differences were found in closure rates according to type of tamponade or presence of high myopia.
Conclusion: lhAM is a safe and effective complementary surgical technique for the management of complex FTMH, particularly useful in cases of large FTMH or previous failed surgeries. Its application represents a valuable alternative when internal limiting membrane (ILM) is not available.
Keywords: Macular hole; Amniotic membrane; Pars plana vitrectomy; Visual acuity
LHAM: Lyophilized Human Amniotic Membrane; FOLA: Fundación Oftalmológica Los Andes; FTMH: Full-Thickness Macular Holes; PPV: Pars Plana Vitrectomy; BCVA: Best Corrected Visual Acuity; ILM: Internal Limiting Membrane; RPE: Retinal Pigment Epithelium; OCT: Optical Coherence Tomography; IVTS: International Vitreomacular Traction Study; OLM: Outer Limiting Membrane; EPZ: Ellipsoid Zone; RD: Retinal Detachment
Introduction
Full-thickness macular hole (FTMH) corresponds to a usually foveal lesion, characterized by an interruption and discontinuity of all retinal layers, from the internal limiting membrane (ILM) to the retinal pigment epithelium (RPE), evolving in a deterioration of best corrected visual acuity (BCVA) [1]. Its pathogenesis, historically described by Gass [2] and later redefined through the use of optical coherence tomography (OCT), involves anteroposterior and tangential traction forces on the vitreoretinal interface, progressively altering the foveal architecture1. The estimated annual incidence is approximately 8.7 per 100,000 inhabitants, predominantly in women over 60 years of age [1]. The most widely accepted classification for FTMH is that proposed by the International Vitreomacular Traction Study (IVTS) Group, which divides holes into small (<250 µm), medium (250-400 µm) and large (>400 µm), according to their minimum diameter measured by OCT [3]. More recent studies have proposed additional subclassifications for large FTMH, incorporating XL (550-800 µm), XXL (800-1000 µm) and giant (>1000 µm) categories, due to the progressive reduction in the rate of anatomical closure as size increases [4]. Currently, pars plana vitrectomy (PPV) with ILM peeling and gas tamponade is the standard treatment for most FTMH, achieving anatomic closure rates above 90% in small and medium-sized holes4. However, large, persistent or recurrent holes, especially in patients with high myopia, remain a surgical challenge due to lower anatomic and functional success rates [4,5].
In response to this, complementary surgical techniques have been developed such as the ILM flap, the neurosensory retinal autograft and, more recently, the use of freeze-dried human amniotic membrane (hAM) as a graft [6-8]. The reverse ILM flap, described by Michalewska et al. [8], significantly improves closure rates in large or highly myopic FTMH by acting as a scaffold for glial cell proliferation [7]. However, in cases where it is not possible to perform the flap due to the absence of available ILM, lhAM has emerged as a promising alternative [8,9]. lhAM is an avascular structure derived from the placenta with anti-inflammatory, antifibrotic, antiangiogenic and cell regeneration-promoting properties. Its application in vitreoretinal surgery has been described to treat retinal tears, myopic macular detachment, and more recently, for the management of difficult-to-manage FTMH [9-11]. The ability of AM to induce reorganization of the outer retinal layers has been documented in several studies, where restoration of the ellipsoid complex and postoperative visual improvement is achieved [9,11]. Several studies have demonstrated anatomic closure rates around 70 to 90% in patients with large and refractory MH treated with AM grafts, including cases with a history of high myopia, previous frustrating surgeries or giant holes [9-13]. This suggests that its use represents a valuable therapeutic option in cases where conventional techniques are not sufficient. The present study aims to describe the anatomical and functional results obtained after the use of lhAM in the surgery of large and/or refractory FTMH treated at Fundación Oftalmológica Los Andes (FOLA) between 2020 and 2024, and to explore possible factors associated with surgical success in our population.
Materials and Methods
A case series study was carried out at the Fundación Oftalmológica Los Andes (FOLA), where clinical records of patients who underwent surgery between January 2020 and September 2024, with a diagnosis of full-thickness macular hole (FTMH), classified as refractory (persistent without anatomical closure after previous surgery) and/or large (greater than 400 µm in baseline diameter according to OCT), were reviewed; in addition to the follow-up for 6 months after surgery. Therefore, the main objective was to evaluate the anatomical and functional results of the use of lhAM as a complementary technique in the surgery of patients diagnosed with large AMEC with size (≥400 µm) and/or refractory to previous vitreoretinal surgery, operated by pars plana vitrectomy between January 2020 and September 2024; by means of OCT (Spectralis, from Heidelberg) and BCVA. For this purpose, thirteen eyes of thirteen patients, who met the inclusion criteria and underwent PPV 23 or 25G, with placement of intraocular lhAM graft (AmbioDry 2®, IOP Ophthalmics, USA), and tamponade with 14% C3F8 expandable gas or 1000 cSt silicone oil (SO), according to surgical indication, were entered into the study. The surgeries were performed by four different expert retinal surgeons from the same center. The inclusion and exclusion criteria are detailed in Table 1. Ethical considerations, this study was approved by the Ethics Committee of the Fundación Oftalmológica Los Andes (FOLA). The confidentiality of the data and anonymity of the participants were guaranteed, complying with the principles of the Declaration of Helsinki. No external financing was used and there were no conflicts of interest declared by the authors.
Regarding the surgical procedure, it should be noted that the surgeries were performed by four retinal surgeons with experience in advanced vitreoretinal surgery, following a common protocol. The surgical technique is detailed step by step in Table 2. Anatomical success was defined as complete closure of the macular hole, as evidenced by horizontal and vertical macular OCT. Functional improvement was assessed by the change in BCVA from immediate preoperative to the last control recorded at 6 months postoperatively. Regarding statistical analysis, data were initially consolidated in Microsoft Excel 365 and subsequently imported into Jamovi v2.6.2612, an interface that operates on the R v4.3.113 statistical engine (R Core Team, Vienna, Austria), in order to ensure a reproducible analytical flow. Continuous variables were described as mean ± standard deviation when they met normality criteria according to the Shapiro-Wilk test; otherwise, they were expressed as median and interquartile range. Categorical variables were summarized as absolute frequencies and proportions. To contrast anatomic closure rates between subgroups-myopia, tamponade type, and autologous blood patch use-the Pearson's χ² test was used; whenever a cell showed expected counts less than five, the contrast was replaced by Fisher's exact test. Functional variation in best-corrected visual acuity (BCVA, LogMAR scale) between preoperative and 6-month follow-up was assessed with Student's t test for paired samples; if the distribution of differences violated normality, the nonparametric Wilcoxon test was used. In order to explore independent factors associated with visual improvement, a generalized linear model was constructed that included final BCVA as the dependent variable and the baseline diameter of the hole, age, number of previous surgeries and type of tamponade as predictors. The absence of significant collinearity was tested by the variance inflation factor (VIF < 2). The β coefficients were presented with their respective 95% confidence intervals and p values. All contrasts were considered statistically significant when p < 0.05 (bilateral).

Results
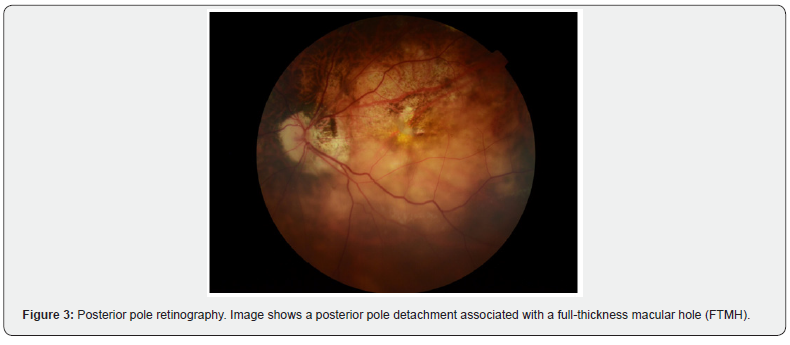
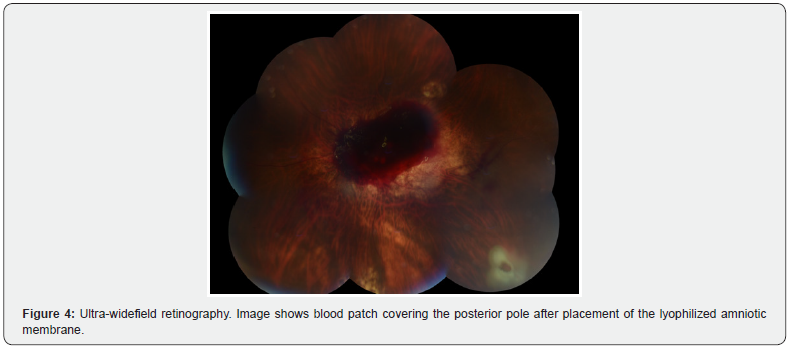
The records of 13 patients with a diagnosis of CMA, operated by pars plana vitrectomy (PPV) with lhAM placement and tamponade with expandable gas or silicone oil, were analyzed. Regarding demographic and clinical characteristics, the mean age of the patients was 61.4 years (range 40 - 76 years). Most of the patients were female (84.6%). As for the diagnosis of high myopia (defined as >6 diopters or axial length >26.5 mm), 61.5% of the patients were diagnosed. Of the patients, 84.6% had a history of at least one previous surgery for FTMH. The average macular hole size was 946.8 µm (Figure 1), with all cases considered large holes based on OCT measurements (≥400 µm) (Figure 2). Regarding the intervened eye, 9 procedures were performed in the left eye and 4 in the right eye. The proportion of phakic patients was 23%. The surgical procedure, regarding the type of intraocular tamponade used, 69.2% of the patients received C3F8 gas and 30.8% silicone oil 1000 cSt. 84.6% of the cases included the placement of autologous blood patch (Figure 3 and 4), as a coadjuvant maneuver to maintain the amniotic membrane in position and favor the closure of the hole. Results are summarized in Table 3.
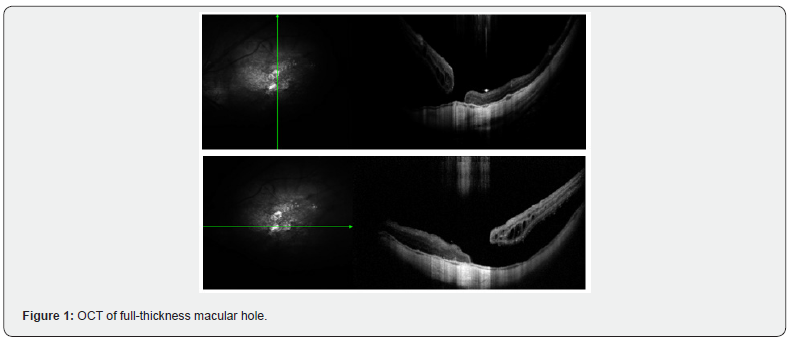

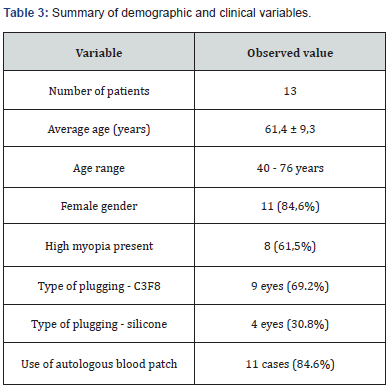
The results show that anatomical success, defined as complete closure of the macular hole as evidenced by horizontal and vertical optical coherence tomography (OCT), was achieved in 11 of the 13 cases (84.6%). In the remaining two cases (15.4%), the hole remained open at 6 months postoperatively. Functionally, best-corrected visual acuity (BCVA), measured on a decimal scale, showed an overall improvement at follow-up. The mean preoperative BCVA was 0.06, while the mean postoperative BCVA at 6 months was 0.13, representing an average functional improvement of more than double. This difference was statistically significant when applying Student's t-test for paired samples (p < 0.05) (Figure 5). The dispersion between baseline macular hole diameter and visual gain (BCVA, decimal scale) at 6 months. No obvious linear relationship was identified, the regression slope is practically horizontal, and the Pearson correlation coefficient was r = -0.02 (p = 0.95). This indicates that, within the range analyzed (400 - 1800 µm), the initial size of the foramen was not significantly associated with the visual improvement obtained after intervention with freeze-dried amniotic membrane (Figure 6). Regarding the associations between the measured variables, anatomic closure was achieved in 88.9% eyes with high myopia and in 80% of eyes without high myopia, with no statistically significant differences (p > 0.05, chi-square test). There was no significant difference in closure rate between cases with and without blood patch use (p > 0.05), although most successful cases did use it as an adjunct. There was also no significant difference in anatomic success between patients with C3F8 tamponade (88.9%) versus silicone (75%). Table 4 summarizes our case series. Optical coherence tomography (OCT) shows a full-thickness foveal defect with thinned and slightly everted retinal borders in the shape of "mouse ears". Hyperreflective intraretinal cysts and slight elevation of the inner retina are seen at both margins of the hole, indicating residual centrifugal traction. The base of the hole has an estimated diameter > 400 µm. In the vitreous space there is hyporeflective shadowing suggesting complete posterior vitreous detachment, without epiretinal membrane.
Discussion
In our case series, it provides locally valuable evidence regarding the use of freeze-dried human anti-membrane (hAM) as a complementary technique in the treatment of difficult-to-manage FTMH. The observed anatomical success rate of 84.6% agrees with that reported by Caporossi et al. who described 100% closure in an initial series of cases treated with lhAM after conventional surgical failure. It supports the efficacy of this technique in real-world settings, with a heterogeneous population including complex, refractory and/or high myopic cases [13-16]. The average size of the treated foramen was 946.8 µm, which is significantly higher than in international series. The literature has reported that FTMHs larger than 400 µm have a poorer prognosis for closure when treated with ILM peeling alone [15,16]. In this context, techniques such as ILM "reverse flap", described by Michalewska et al. [8], have demonstrated closures of up to 98% in large holes, but require an intact ILM, which is not always possible in recurrent surgeries. The lhAM, acting as a bioactive substrate, provides a biological scaffold with regenerative properties that favor the reconstitution of outer retinal layers, including the outer limiting membrane (OLM) and the ellipsoid zone (EPZ) [13,17,18] which can make up for this structural failure. Functionally, our patients experienced a statistically significant visual improvement from a mean BVCA of 0.06 to 0.13 (p = 0.007). This result is consistent with the findings of Qiao et al, who documented functional improvement in 88% of highly myopic eyes treated with lhAM and associated retinal detachment (RD) [19]. Although no significant differences were observed in the rate of anatomic closure according to the type of tamponade (C3F8 or silicone) or the presence of high myopia, the highest proportion of closures was achieved in eyes treated with C3F8, as has also been suggested by other studies [20]. The choice of tamponade could influence graft stabilization and closure kinetics, but this hypothesis requires validation with controlled studies. Likewise, the use of autologous blood patch, present in 84.6% of the cases, could have facilitated the initial adhesion of the lhAM, although its direct influence on the anatomical results was not statistically confirmed. Its use has been documented as a safe adjuvant technique in other publications on macular hole closure [18-20]. From a safety point of view, no complications related to lhAM implantation were reported, which supports its high biocompatibility, as already widely described in studies on both ocular surface and intraocular applications [15,16]. The limitations of this study include its retrospective design, the limited sample size, the absence of a control group and the surgical variability between operators. However, these findings constitute a relevant contribution to support the use of lhAM in complex clinical scenarios with difficult surgical resolution.
Conclusion
Lyophilized human amniotic membrane (lhAM) represents a safe, effective and promising surgical alternative for the treatment of large and/or refractory macular holes, especially in contexts of high complexity and limited availability of ILM. In our series, a high rate of anatomic closure and significant functional improvement was achieved. Although prospective and controlled studies are required to confirm these findings, lhAM should be considered among the therapeutic alternatives for vitreoretinal surgeons in challenging cases.
References
- Ezra E (2001) Idiopathic full-thickness macular hole: natural history and pathogenesis. Br J Ophthalmol 85(1): 102-108.
- Gass JDM (1995) Reappraisal of biomicroscopic classification of stages of development of a macular hole. Am J Ophthalmol 119(6): 752-759.
- Duker JS, Kaiser PK, Binder S, de Smet MD, Gaudric A, et al. (2013) The International Vitreomacular Traction Study Group classification of vitreomacular adhesion, traction, and macular hole. Ophthalmology 120(12): 2611-2619.
- Rezende FA, Ferreira BG, Rampakakis E, Steel DH, Koss MJ, et al. (2023) Surgical classification for large macular hole: based on different surgical techniques results: the CLOSE study group. Int J Retina Vitreous 9(1): 4.
- Laura Liu, Ijilmurun Enkh-Amgalan, Nan-Kai Wang, Lan-Hsin Chuang, Yen-Po Chen, et al. (2018) Results of macular hole surgery. Retina 38(5): 900-906.
- Soon Wai Ch'ng, Niall Patton, Mahmoud Ahmed, Tsveta Ivanova, Carmen Baumann, et al. (2018) The Manchester large macular hole study: is it time to reclassify large macular holes? Am J Ophthalmol 195: 36-42.
- Pradhan D, Agarwal L, Joshi I, Kushwaha A, Aditya K, et al. (2022) Internal limiting membrane peeling in macular hole surgery. Ger Med Sci 2: 20.
- Michalewska Z, Michalewski J, Adelman RA, Nawrocki J (2010) Inverted internal limiting membrane flap technique for large macular holes. Ophthalmology 117(10): 2018-2025.
- Sharma YR, Sudan R, Gaur A, Reddy RR (2002) Macular hole. JK Science 4(2): 57-70.
- Nawrocki J, Michalewska Z, Dziegielewski K, et al. (2022) Use of human amniotic membrane for the treatment of recurrent and large macular holes. Retina 42(3): 458-465.
- Li X, Meng X, Wang Y, Xu G (2021) Human amniotic membrane plug for macular hole repair. Medicine (Baltimore) 100(31): e26908.
- The jamovi project (2024) jamovi.
- R Core Team (2024) R: A language and environment for statistical computing.
- Morescalchi F, Duse S, Gambicorti E, Romano MR, Costagliola C, et al. (2018) Amniotic membrane use in ophthalmology. Curr Opin Ophthalmol 29(3): 248-254.
- Caporossi T, Franco F, Finocchio L, et al. (2021) Human amniotic membrane plugs to treat recurrent and large macular hole: anatomical and functional outcomes. Graefes Arch Clin Exp Ophthalmol 259(4): 905-912.
- Caporossi T, et al. (2020) Human amniotic membrane plug to promote anatomical recovery in macular hole surgery. Retina 40(7): 1337-1342.
- Kelly NE, Wendel RT (1991) Vitreous surgery for idiopathic macular holes. Arch Ophthalmol 109(5): 654-659.
- Christensen UC, la Cour M (2012) Visual outcome of surgery for idiopathic macular hole: implications for clinical practice. Br J Ophthalmol 96(10): 1325-1330.
- Qiao Y, et al. (2022) Human amniotic membrane transplantation in high myopia-related macular holes with or without retinal detachment. BMC Ophthalmol 22: 183.
- Rossi T, et al. (2022) Autologous plasma as biological glue for surgical treatment of recurrent macular holes. Int Ophthalmol 42(5): 1467-1473.






























