Abstract
In MOGAD, the age of onset exhibits a biphasic distribution, typically occurring between 5 to 10 years and 20 to 45 years, with a median age of onset ranging from 20 to 30 years. Our patient experienced onset in the sixth decade of life, which is unusual and infrequently reported, and presented with painful blurred vision in both eyes. The fundus exam revealed bilateral optic disc edema accompanied by pyramidal signs. MRI revealed diffused thickening of bilateral optic nerve up to apex with T2 hyper intensity, with focal T2 FLAIR hyper intensities in frontal and parietal white matter, CSF analysis showed marked pleocytosis (2730 cells), 58% polymorph nuclear cells, 42% mononuclear cells, nil-RBC, with increased protein. Given the patient’s age and the involvement of the optic nerves and pyramidal tract, along with a cerebrospinal fluid profile that does not showcase lymphocyte predominance, there is a suspicion of NMOSD. However, since the patient tested negative for aquaporin 4, we moved forward with testing for MOG antibodies, which turned out to be positive.
Conclusion: MOG optic neuritis is infrequent in the sixth decade of life and may manifest similarly to an opticospinal disorder, affecting both the optic nerve and the brain.
Keywords: MOGAD; Bilateral Optic Neuritis; Late Onset MOGAD; Perineuritis
Abbreviations: MOGAD: Myelin Oligodentrocyte Glycoprotein Antibody -Associated Disease; NMOSD: Neuromyelitis Optica Spectrum Disorders; ADEM: Acute Disseminated Encephalomyelitis; CSF: Cerebrospinal Fluid; IVIG: Intravenous Immunoglobulin; IgG: Immunoglobulin G; ON: Optic Neuritis; AQP: Aquaporin; MRI: Magnetic Resonance Imaging; FLAIR: FLuid Attenuation Inversion Recovery; VEP: Visually Evolked Potential; RNFL: Retinal Nerve Fibre Layer; OCT: Optic Coherence Tomography
Introduction
MOG antibody associated disease (MOGAD) is a distinct CNS inflammatory disease with symptoms and imaging findings that overlap other neuro inflammatory disorders [1], with the spectrum of optic neuritis, transverse myelitis, acute disseminated encephalomyelitis (ADEM) and MOG- encephalitis. MOG Optic neuritis frequently occurs in adults, while ADEM is the most common manifestation in children. It is due to the production of IgG autoantibodies that are targeted at myelin oligodendrocyte glycoprotein (MOG), a minor transmembrane protein located on the outermost layers of oligodendrocytes within the myelin sheath of the central nervous system [2]. Approximately 50% of patients with multiple sclerosis (MS) experience typical optic neuritis, which often results in spontaneous recovery. In contrast, atypical optic neuritis associated with MOG antibody disease (MOG-AD) and neuromyelitis optica spectrum disorder (NMOSD) is less likely to resolve on its own. Optic neuritis in MOG-AD may lead to a more favourable visual outcome compared to NMOSD. Recognizing the characteristics of optic neuritis in MS, MOG-AD, and NMOSD is crucial, as the visual prognosis and treatment strategies differ [3-10].
Case Report
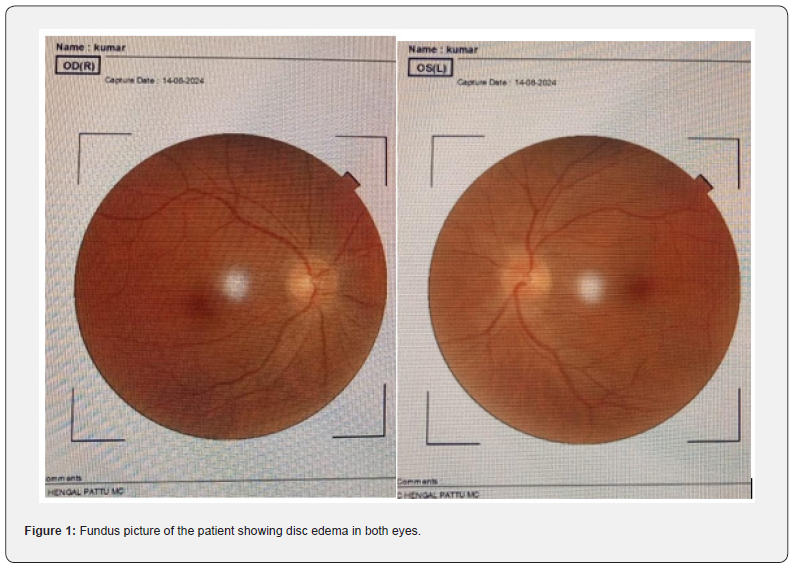
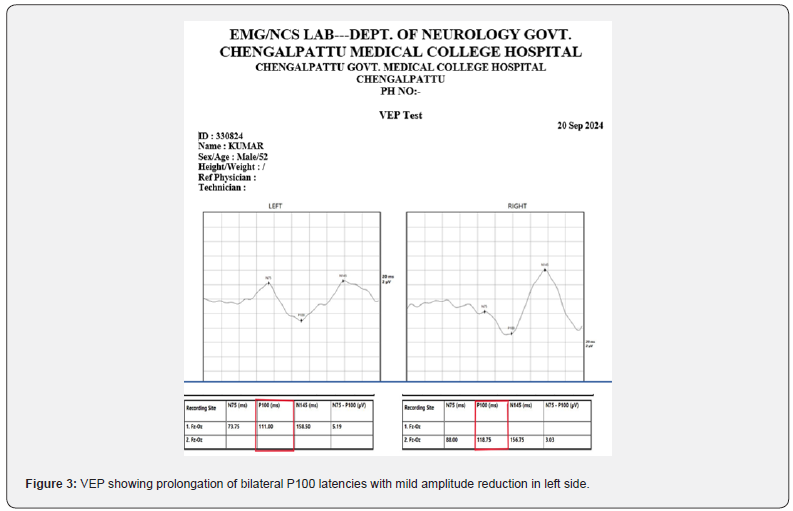
A 52-year-old male came in with blurred vision in his right eye that had lasted for 8 days, followed by similar issues in his left eye. He experienced pain when moving his eyes sideways, which began suddenly and was not accompanied by drooping eyelids, double vision, bulging eyes, or tearing. He has no known pre-existing co -morbidities or risk factors. Examination showed sluggishly reacting pupils, drop in the visual acuity RE-6/36 LE- 6/60, dyschromatopsia for red, green with Normal extra ocular movements. In addition, he had spasticity and brisk reflexes of all four limbs. With pupils responding sluggishly, a decline in visual acuity, dyschromatopsia, and disc swelling, the patient was clinically diagnosed with bilateral optic neuritis. Further evaluation with OCT revealed swelling of the optic nerve head in both eyes along with significant RNFL thickening. Both eyes’ VEP P100 latencies were prolonged, 118.75 ms for the right eye and 111 ms for the left, with the right eye’s amplitude significantly decreasing by 3.03 microvolts. Significant pleocytosis (2730), 58% polymorph nuclear cells, 42% mononuclear cells, nil-RBC, and elevated protein were all seen in the CSF study.
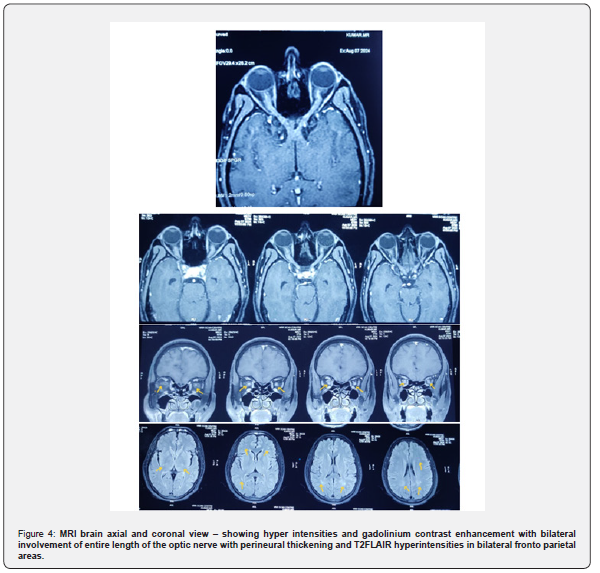
He was not exposed to drugs, toxins; doesn’t have constitutional symptoms, no immuno compromised status with that toxic and infectious causes are ruled out. Suspected for demyelinating or paraneoplastic etiology. Patient had bilateral optic nerve disease with marked papilledema which is unlikely for multiple sclerosis and NMOSD, because multiple sclerosis will have patchy involvement of optic nerve and NMOSD involves pre chiasmatic posterior end of optic nerve [11-22]. Though his age profile, bilateral optic nerve disease with marked disc edema and associated pyramidal features were suggestive of NMOSD, MRI revealed bilateral optic nerve long segment involvement with perineuritis with bilateral fronto parietal hyperintensities, features were suggestive of MOGAD and the same was confirmed with cell-based assay. Patient diagnosed as a case of late onset MOGAD optic neuritis
Treated with injection methyl prednisolone 1g/day for 5 days tapered with oral steroids symptomatically improved and on follow up (Figure1-4).
Discussion
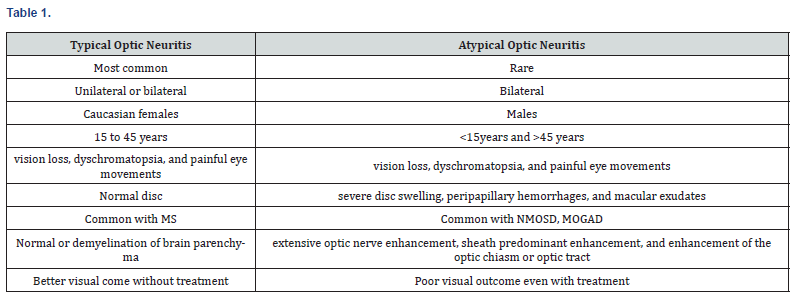
Patient presented in 6th decade with bilateral atypical optic neuritis with pyramidal signs, clinically suspected for NMOSD, but imaging suggestive of MOGAD-optic neuritis, serologically confirmed as MOGAD by cell-based assay. Treated with injection methyl prednisolone 15-20mg/kg/day for five days and tapered with oral steroids for eleven days. Patient visual acuity improved by two grades. MOGAD is a disease with bimodal distribution, observed in less than 45years of age, recently MOGAD optic neuritis and transverse myelitis are being reported more frequently, particularly after COVID19 on observation they also had milder and monophasic course with better outcome, as that of usual MOGAD presentation [23,24]. Features of typical and atypical optic neuritis were described in table 1 and characteristics of MOGAD, NMOSD and MS were described in table 2.

Laboratory Diagnosis MOG-IgG Antibody
The panel strongly recommends testing serum from patients suspected of having MOGAD using cell-based assays that use whole human MOG to detect MOG-IgG. Serum is the preferred specimen type for MOG-IgG testing. While clotting factors in plasma may influence results, studies have highlighted the low sensitivity of MOG-IgG testing in CSF alone. CSF testing for MOG IgG may be used in selected circumstances to confirm clinical and MRI findings supporting the diagnosis of MOGAD in seronegative patients with MOG-IgG [2]. ELISA is not recommended for MOGIgG measurement owing to low sensitivity and specificity. Patients are most likely to be MOG-IgG seropositive if tested during a clinical episode. Ideally, testing should be performed before administration of corticosteroids, immunoglobulins, or apheresis, as these treatments may reduce detection of serum MOG-IgG, as observed with the AQP4 IgG assay [2].
Cerebro Spinal Fluid
CSF pleocytosis is more likely during attack than during remission and is more common in patients with ADEM or transverse myelitis than in patients with optic neuritis. CSF protein levels are elevated in 30% of patients with a first demyelinating attack and MOG-IgG, and do not distinguish MOG-IgG-associated demyelination from other neuroinflammatory disorders [2].

Diagnosis of MOGAD by International MOGAD Panel Proposed Criteria [25] Optic Neuritis
Optic neuritis is characterized by unilateral or bilateral reduced visual acuity that develops over hours to days and is often associated with retro bulbar orbital pain that is typically exacerbated with eye movement and accompanied by color vision and visual field loss. Diagnosis of optic neuritis can be supported by the presence of a T2-hyperintense signal in the optic nerve or chiasm, by enhancement of the optic nerve or chiasm with gadolinium, and by exclusion of clinical or radio graphical evidence of an alternative compressive, infiltrative, or vascular process impacting the optic nerve or retina.
MOGAD is evolving day by day, there is change in recent trend of autoimmune neuro inflammatory diseases, particularly for MOGAD after COVID pandemic. Incidence is increasing with post infectious MOGAD and even during acute infective phase of SARS COV 2 [15-17]. When comparing NMOSD and MOGAD patients in ON cases, we found that MOGAD patients had severe visual impairment during the acute phase, with some patients reaching a maximum visual functional system score (VFSS) of 6. However, during the follow-up period, most patients showed obvious improvement in VA, and no patient achieved a maximum VFSS score of 6. JARIUS’s multi -center experience gave a similar result used differences in Expanded Disability Status Scale (EDSS) scores to assess recovery in MOGAD patients and showed that EDSS scores were significantly reduced in MOGAD patients compared with AQP-4 antibody-positive patients [3].
Treatment with IVIG in addition to oral corticosteroids significantly reduced relapse rates with few side effects. Rituximab, mycophenolate mofetil, and azathioprine have also shown good efficacy and tolerability and can be used as suitable alternatives. Disease-modifying therapies should be avoided, but intravenous immunoglobulin and oral corticosteroids may be appropriate firstline treatment for patients with MOGAD [18], visual outcomes at hospital discharge and at 3-month follow-up were significantly better in those patients treated with plasmapheresis [19].
Red Flags Against a Diagnosis of Myelin Oligodendrocyte Glycoprotein Antibody-Associated Disease [2]
• Progressive neurological impairment in the absence of
attacks
• Rapid worsening of clinical deficits from onset to nadir
within minutes to hours
• No improvement following treatment with high-dose
corticosteroids for an acute attack
• MRI findings of well circumscribed T2-hyperintense
lesions in a pattern meeting dissemination in space criteria
for multiple sclerosis, especially when accompanied by CSF
oligoclonal bands and by the accrual over time of new silent T2-
hyperintense focal lesions and retention of most previous T2-
hyperintense lesions
• Lesion contrast enhancement that persists for 6 months
or more.
Conclusion
Although a small proportion of MOGAD patients with extensive optic nerve damage experienced severe and irreversible visual impairment, the long-term visual outcomes of MOGAD patients after 5 years of ON were generally comparable to those of MS patients and significantly better than those of AQP4-positive NMOSD patients [2,20-22]. MODAD optic neuritis is a severe neuro inflammatory disease with good visual outcome with prompt immunosuppressive therapy, atypical neuritis in atypical presentations to be suspected, diagnosed and to be treated early for the best outcome.
References
- Shahriari M, Sotirchos ES, Newsome SD, Yousem DM (2021) MOGAD: How It Differs from and Resembles Other Neuroinflammatory Disorders. Am J Roentgenol 216(4): 1031-1039.
- Banwell B, Bennett JL, Marignier R, Kim HJ, Brilot F, et al. (2023) Diagnosis of myelin oligodendrocyte glycoprotein antibody-associated disease: International MOGAD Panel proposed criteria. Lancet Neurol 22(3): 268-282.
- Li Y, Liu X, Wang J, Pan C, Tang Z (2022) Clinical Features and Imaging Findings of Myelin Oligodendrocyte Glycoprotein-IgG-Associated Disorder (MOGAD). Front Aging Neurosci 14: 850743.
- https://fyra.io. Practical Neurology. Bryn Mawr Communications; The Evolution of Myelin Oligodendrocyte Glycoprotein Antibody-Associated Disease.
- Lebar R, Baudrimont M, Vincent C (1989) Chronic experimental autoimmune encephalomyelitis in the guinea pig. Presence of anti-M2 antibodies in central nervous system tissue and the possible role of M2 autoantigen in the induction of the disease. J Autoimmun 2(2): 115-132.
- Hor JY, Fujihara K (2023) Epidemiology of myelin oligodendrocyte glycoprotein antibody-associated disease: a review of prevalence and incidence worldwide. Front Neurol 14: 1260358.
- Bruijstens AL, Wong YYM, Pelt DE van, Linden PJ van der, Haasnoot GW, et al. (2020) HLA association in MOG-IgG- and AQP4-IgG-related disorders of the CNS in the Dutch population. Neurol Neuroimmunol Neuroinflammation 7(3): e702.
- Grant-Peters M, Passos GRD, Yeung HY, Jacob A, Huda S, et al. (2021) No strong HLA association with MOG antibody disease in the UK population. Ann Clin Transl Neurol 8(7): 1502-1507.
- Sun X, Qiu W, Wang J, Wang S, Wang Y, et al. (2020) Myelin oligodendrocyte glycoprotein-associated disorders are associated with HLA subtypes in a Chinese paediatric-onset cohort. J Neurol Neurosurg Psychiatry 91(7): 733-739.
- Brill L, Ganelin-Cohen E, Dabby R, Rabinowicz S, Zohar-Dayan E, et al. (2021) Age-Related Clinical Presentation of MOG-IgG Seropositivity in Israel. Front Neurol 11: 612304.
- Jeyakumar N, Lerch M, Dale RC, Ramanathan S (2024) MOG antibody-associated optic neuritis. Eye 38(12): 2289-2301.
- Hassan MB, Stern C, Flanagan EP, Pittock SJ, Kunchok A, et al. (2020) Population-Based Incidence of Optic Neuritis in the Era of Aquaporin-4 and Myelin Oligodendrocyte Glycoprotein Antibodies. Am J Ophthalmol 220: 110-114.
- (2020) American Academy of Ophthalmology. How common is MOG-positive optic neuritis among adults?
- Ambika S, Durgapriyadarshini S, Padmalakshmi K, Noronha V, Arjundas D (2022) Clinical profile, imaging features and short-term visual outcomes of Indian optic neuritis patients with and without seromarkers for myelin oligodendrocyte glycoprotein and neuromyelitis optica. Indian J Ophthalmol 70(1): 194-200.
- Bhardwaj A, Mishra HP, Goel A, Gupta A (2023) COVID-19 - a potential trigger for MOGAD-associated optic neuritis: a case report and literature review. Ther Adv Ophthalmol 15: 25158414231199541.
- Chakraborty U, Chaudhuri J, Datta AK, Mukherjee A, Pandit A, et al. (2023) COVID-19 and optic neuritis: a series of three cases and a critical review. Egypt J Neurol Psychiatry Neurosurg 59(1): 172.
- Graciano G, Ghatali M (2023) COVID-19 Infection Linked to MOG Antibody-Associated Disease (MOGAD): Case Report (P10-5.023). Neurology 100(17_supplement_2): 2358.
- Wang X, Kong L, Zhao Z, Shi Z, Chen H, et al. (2022) Effectiveness and tolerability of different therapies in preventive treatment of MOG-IgG-associated disorder: A network meta-analysis. Front Immunol 13: 953993.
- Herman M, Ngo S, Shi H, Winkel D, Ma T, Hutto S (2024) Plasmapheresis Improves Visual Outcomes in Attacks of Optic Neuritis in MOGAD (P9-14.002). Neurology 102(17_supplement_1): 6726.
- Akaishi T, Himori N, Takeshita T, Misu T, Takahashi T, et al. (2021) Five-year visual outcomes after optic neuritis in anti-MOG antibody-associated disease. Mult Scler Relat Disord 56: 103222.
- Nurul-Ain M, Kamal ZNK, Hitam WHW, Munaaim MA, Zaki FM (2021) Myelin Oligodendrocyte Glycoprotein (MOG) Optic Neuritis: A Case Series. Cureus 13(4): e14452.
- Chen JJ, Flanagan EP, Bhatti MT, Tisavipat N, Jamali S, et al. (2021) Details and outcomes of a large cohort of MOG-IgG associated optic neuritis. Mult Scler Relat Disord 68: 104237.
- Huang Y, Luo W, Cheng X, Sun X, Wang Y, et al. (2024) Clinical and imaging features of patients with late-onset myelin oligodendrocyte glycoprotein antibody-associated disease. Mult Scler Relat Disord 82: 105405.
- Kim KH, Cho J, Shin HY, Kim SW (2021) A Case of Anti-Myelin Oligodendrocyte Glycoprotein Antibody-Positive Late-Onset Acute Disseminated Encephalomyelitis. J Clin Neurol 17(2): 330-332.
- Banwell B, Bennett JL, Marignier R, Kim HJ, Brilot F, et al. (2023) Diagnosis of myelin oligodendrocyte glycoprotein antibody-associated disease: International MOGAD Panel proposed criteria. The Lancet Neurology 22(3): 268-282.






























