Polypoidal Choroidal Vasculopathy: Subretinal Hemorrhage Tracks
Fubin Wang*
Shanghai Bright Eye Hospital, 899 Maotai Road, Changning District, Shanghai 200336, Shanghai, China
Submission: April 04, 2023;Published: April 10, 2023
*Corresponding author: Fubin Wang, Shanghai Bright Eye Hospital, 899 Maotai Road, Changning District, Shanghai 200336, Shanghai, China
How to cite this article: Fubin W. Polypoidal Choroidal Vasculopathy: Subretinal Hemorrhage Tracks. JOJ Ophthalmol. 2023; 9(5): 555773. DOI: 10.19080/JOJO.2023.09.555773
Abstract
Aim: To describe the subretinal hemorrhage tracks of PCV.
Methods: Among all patients of PCV (22 cases,27 eyes), 6 cases (8 eyes) with the thick subretinal hemorrhage tracks were observed by Clarus 500 (Carl Zeiss Meditec, Inc), Panoramic Ophthalmoscope and FAF (Daytona P200T), SD-OCT and OCTA ( Cirrus HD-OCT 5000, Germany; Spectralis OCT, Heidelberg Engineering, Heidelberg, Germany).
Results: All patients with thick subretinal hemorrhages, subretinal hemorrhage tracks were observed. In the early period hemorrhage of macular PCV, because of gravity, the blood flow tracks were toward the lower retina originating in the macula, which was subretinal hemorrhage it appears as a band. FAF showed hypofluorescence. In the later period hemorrhage of macular PCV, the banded lesions appear yellowish-white and FAF showed hypofluorescence. However, most of the bleeding has been absorbed. FAF of peripapillary PCV showed intermixed hyperfluorescence and hypofluorescence.
Conclusion: Subretinal hemorrhage tracks are directed towards the lower retina due to gravity. Presumably, these tracks are formed through the subretinal space.
Keywords: Polypoidal Choroidal Vasculopathy; Subretinal Hemorrhage Tracks; Subretinal Space; Fundus Autofluorescence; Reddish Orange Subretinal Nodules
Abbreviations: PCV: Polypoidal Choroidal Vasculopathy; OCTA: OCT Angiography; FAF: Fundus Autofluorescence; SRHTS: Subretinal Hemorrhage Track Signs; BNN: Branching Neovascular Network; PED: Pigment Epithelial Detachment; ICGA: Indocyanine Green Angiography; RD: Retinal Detachment; nAMD: Neovascular Age-Related Macular Degeneration; ILM: Sub-Internal Limiting Membrane; RAM: Retinal Artery Macroaneurysm; SRS: Subretinal Space; RPE: subretinal Space
Introduction
Polypoidal choroidal vasculopathy (PCV) is increasingly recognized as an important cause of exudative maculopathy. Prognosis depends on multiple factors such as the location and size of PCV lesion, presence or absence of polyp with residual AVN, amount of sub macular hemorrhage, presence or absence of leakage on fundus fluorescein angiography, visual acuity, and so on. The classical clinical finding of PCV is the presence of reddish orange subretinal nodules [1]. Apart from polyps, the clinical features more commonly seen include varying degree of serous or serosanguinous PEDs, subretinal hemorrhage, lipid deposition as well as neurosensory retinal detachment in the peripapillary or macular retina [2,3]. In a study by Kwok et al.,[4] the most common clinical feature noted was subretinal hemorrhage (63.6%) followed by retinal exudation (59.1%) and hemorrhagic PED (59.1%). In this study, the subretinal hemorrhage track signs were observed by multimodal imaging.
Subjects and Methods
This is retrospective research. Among all patients of PCV (22 cases,27 eyes), 6 cases (8 eyes) with the subretinal hemorrhage tracks were observed with thick subretinal hemorrhage by Clarus 500 (Carl Zeiss Meditec, Inc), Panoramic Ophthalmoscope and fundus autofluorescence(FAF) (Daytona P200T), SD-OCT and en-face OCT angiography (OCTA) (Cirrus HD-OCT 5000, Germany; Spectralis OCT, Heidelberg Engineering, Heidelberg, Germany). In all patients, there were 9 males and 13 females, and the age range was from 36 to 69 years old. Institutional review board approval and informed consent from patients was obtained.
Results
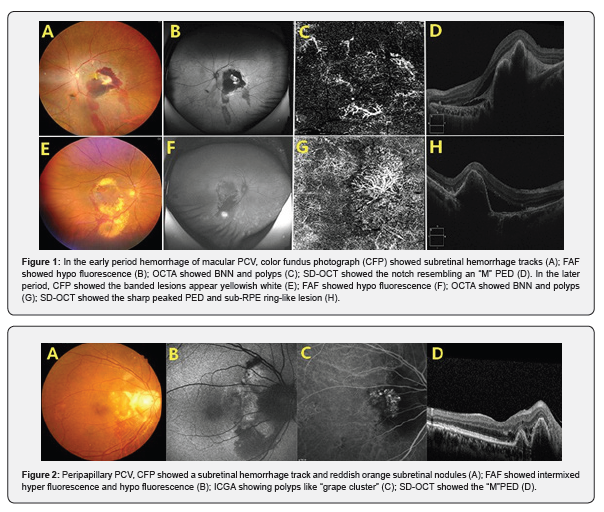
All patients with thick subretinal hemorrhages, subretinal hemorrhage tracks were observed, called subretinal hemorrhage track signs (SRHTS) in this paper. In the early period hemorrhage of macular polypoidal choroidal vasculopathy, because of gravity, the blood flow tracks were toward the lower retina originating in the macula, which was the subretinal hemorrhage it appears as a band. In fundus autofluorescence image, it showed hypofluorescence. The presence of reddish orange subretinal nodules were observed. OCT angiography images showed branching neovascular network(BNN) and polyps. SD-OCT images showed the sharp peaked pigment epithelial detachment (PED) with notch resembling an “M”.
Sometimes sub-RPE ring-like lesion can be seen. In the later period hemorrhage of macular PCV, the banded lesions appear yellowish-white and FAF showed hypofluorescence. However, most of the bleeding has been absorbed (Figure 1). For the peripapillary PCV like “grape cluster”, the reddish orange subretinal nodule tended to be larger, the blood flow track was toward the lower retina, the subretinal hemorrhage appears as a band. FAF showed intermixed hyperfluorescence and hypofluorescence and the notch resembling an “M” PED by SD-OCT. Indocyanine green angiography (ICGA) confirmed the presence of polyps (Figure 2).
Discussion
PCV is a vision-threatening disease characterized by polyp-like dilations of choroidal vessels. PCV may cause pigmented epithelial detachment (PED), serous retinal detachment (RD), massive subretinal hemorrhage and extensive macular atrophy. For many decades, PCV has been described as a subtype of neovascular age-related macular degeneration (nAMD) [5]. It is identified by the associated polypoidal dilatation and a branching network within the choroidal circulation resulting in recurrent hemorrhages and serous leakage clinically. PCV has previously been known as peripheral exudative hemorrhagic chorioretinopathy, multiple recurrent serosanguinous retinal pigment epithelial detachments, posterior uveal bleeding syndrome, idiopathic polypoidal vasculopathy [6].
Subretinal hemorrhage is different from preretinal hemorrhage, such as sub-internal limiting membrane (ILM), the common causes of sub-ILM hemorrhages include Valsalva retinopathy, Terson syndrome, retinal artery macroaneurysm (RAM), hematological disorders, trauma, or idiopathic. Although sub-ILM hemorrhages have been reported in PCV, PCV has not been confirmed as a cause of isolated sub-ILM hemorrhage [7]. But massive subretinal hemorrhage is one of the major images of PCV. If there is a large amount of subretinal bleeding, there is often a blood flow path, which is directed towards the lower retina due to gravity. However, no reports have proposed how these blood flow paths are generated. In this study, presumably, these tracks are formed through the subretinal space.
The subretinal space (SRS) is a potential area between the neurosensory retina and the RPE. It is a safer area as it is anatomically closed and immune privileged [8]. The retinal pigment epithelium (RPE) is separated from the photoreceptor outer segments by the subretinal space. While the actual volume of this space is minimal, the communication that occurs across this microenvironment is important to the visual process. The retinal pigment epithelium (RPE) lies between the outer segments of the photoreceptors and the choroidal blood supply [9]. The apical membrane of the RPE is separated from the plasma membrane of the outer segments by an extracellular space of only 10-20 nm [10]. Although small, this subretinal space contains a highly structured matrix which ensheathes the outer segments and extends to the RPE [11,12]. So far why there is such a potential subretinal space is not clear. However, with subretinal hemorrhage of PCV, it is highly possible that subretinal hemorrhage tracks form in the subretinal space.
References
- BV Priya, Ishank Gupta, B Poornachandra, Chaitra Jayadev, Arpitha Pereira, et al. (2021) Morphological features of focl choroidal excavation and its association with macular pathology in Asian Indian eyes. Indian J Ophthalmol 69(4): 886-889.
- Jampol LM, Shankle J, Schroeder R, Tornambe P, Spaide RF, et al. (2006) Diagnostic and therapeutic challenges. Retina 26(9): 1072-1076.
- Wakabayashi Y, Nishimura A, Higashide T, Ijiri S, Sugiyama K (2010) Unilateral choroidal excavation in the macula detected by spectral-domain optical coherence tomography. Acta Ophthalmol 88(3): e87-e91.
- Margolis R, Mukkamala SK, Jampol LM, Spaide RF, Ober MD, et al. (2011) The expanded spectrum of focal choroidal excavation. Arch Ophthalmol 129(10): 1320-1325.
- Samarth Mishra, Barun Garg, Deepak Senger, Anushree Kumar, Ashwin C, et al. (2020) Focal choroidal excavation and giant choroidal cavern in an eye with pachychoroid. Oman J Ophthalmol 13(3): 155-157.
- Park KA, Oh SY (2015) The absence of focal choroidal excavation in children and adolescents without retinal or choroidal disorders or ocular trauma. Eye 29(6): 841-842.
- Rahul M Dhodapkar, Jane Zhu Spadaro, Ron A Adelmanb (2022) A case of extrafoveal focal choroidal excavation. Am J Ophthalmol Case Rep 27: 101682.
- Pulak Agarwal, Shayeri Roy, Shorya V Azad, Vinod Kumar (2019) Juxtapapillary Focal Choroidal Excavation. Indian J Ophthalmol 67(3): 400-401.






























