Peripapillary Subretinal Space of the Blunt Traumatic Optic Neuropathy: RPE/Bruch’s Membrane Complex Micro-Rips - The Novel SD-OCT Findings
Fubin Wang*
Shanghai Bright Eye Hospital, 899 Maotai Road, Changning District, Shanghai 200336, Shanghai, China
Submission: February 01, 2023;Published: February 28, 2023
*Corresponding author: Fubin Wang, Shanghai Bright Eye Hospital, 899 Maotai Road, Changning District, Shanghai 200336, Shanghai, China
How to cite this article: Fubin Wang. Peripapillary Subretinal Space of the Blunt Traumatic Optic Neuropathy: RPE/Bruch’s Membrane Complex Micro- Rips - The Novel SD-OCT Findings. JOJ Ophthalmol. 2023; 9(4): 555769. DOI: 10.19080/JOJO.2023.09.555769
Abstract
Aim: To report the unusual SD-OCT findings in peripapillary subretinal space of the blunt traumatic optic neuropathy (TON).
Methods: All patients underwent a comprehensive eye examination included color fundus photography, ultrasonography, fundus fluorescein angiography, visual field, PVEP if patient’s vision was allowed to examine. An orbital CT scan was performed for all of patients and no orbital fracture was found. However, we did not assess them here and assessed the results of SD-OCT examination only.
Results: Peripapillary subretinal spaces of four contused eyes were abnormal that showed a flat, localized hyperreflection due to hemorrhages. It was adjacent to optic disc. The intensities of reflection could be nonuniform due to the blood concentration or contained serous liquid. It could be observed that RPE/Bruch’s membrane complex micro-rips, but ELM were normal that were not involved in the peripapillary subretinal space. Choroid rupture of macula was observed at times.
Conclusion: For TON patients, to examine the peripapillary subretinal space by SD-OCT at first time might have valuable for diagnosis. The results suggested that the hemorrhages of peripapillary subretinal space might come from the peripapillary choriocapillaris.
Keywords: Peripapillary Subretinal Space; RPE/Bruch’s Membrane Complex; Micro-Rips; Traumatic Optic Neuropathy; SD-OCT; Peripapillary Choriocapillaris
Abbreviations: TON: Traumatic Optic Neuropathy; BCVA: Best Corrected Visual Acuity; CFP: Color Fundus Photography; NLP: No Light Perception; ILM: Internal Limiting Membrane; NFL complex: Nerve Fiber Layer Complex; GCL: Ganglion Cell Layer; IPL: inner plexiform layer; INL: Inner Nuclear Layer; OPL: Outer Plexiform Layer; ONL: Outer Nuclear Layer; ELM: External Limiting Membrane; MZ: Myoid Zone; EZ: Ellipsoid Zone; IZ: Interdigitation Zone; RPE: Retinal Pigment Epithelium; PPRD: Peripapillary Retinal Detachment
Background:
Traumatic optic neuropathy (TON) is an important cause of severe visual loss following blunt for facial trauma. Although rare, it is a reported sequela of significant facial trauma. TON results from direct or indirect forces causing injury to the optic nerve. Causes of direct TON include transection, avulsion, orbital hemorrhage, orbital emphysema, and optic nerve sheath hemorrhage [1]. However, the unusual abnormalities were observed, a novel SD-OCT finding, in the peripapillary subretinal space. The interesting finding here is that, by the image presences of SD-OCT (Spectralis OCT system, Heidelberg Engineering GmbH, Germany), hemorrhages in the peripapillary subretinal space as if come from the peripapillary choriocapillaris. In this study, RPE/Bruch’s membrane micro-rips are reported of TON. In this study, we assessed the results of SD-OCT examination only. Although all patients had been conducted many ophthalmologic examinations, we did not assess them.
Subjects and Methods
Four eyes of four patients with TON were examined. All patients first visited to our outpatient service within one week after the blunt trauma. Age range of patients was 21 to 39 years. Best corrected visual acuity (BCVA) was respectively no light perception (NLP), 0.02, 0.04 and 0.25. All patients had a history of acute direct blunt trauma on unilateral eye by the fists or feet. Systemic, neurological and neuro-imaging findings were unremarkable in all patients. All patients underwent a comprehensive eye examination included color fundus photography (CFP), ultrasonography, fundus fluorescein angiography (FFA, HRA-2, Heidelberg Engineering, Germany), visual field (Humphrey 720i-6226, Germany), PVEP (Roland RETI port/scan 21) if patient’s vision was allowed to examine. An orbital CT scan was performed for all of 4 patients and no orbital fracture was found. However, we did not assess them here and we assessed the results of SD-OCT examination only.
Institutional review board approval and informed consent from patient was obtained.
Results
At first normal subjects were examined by SD-OCT as control group. OCT images showed internal limiting membrane/nerve fiber layer complex (ILM/NFL complex), ganglion cell layer (GCL), inner plexiform layer (IPL), inner nuclear layer (INL), outer plexiform layer (OPL), outer nuclear layer (ONL), external limiting membrane (ELM), myoid zone (MZ), ellipsoid zone (EZ), outer segnents of the photoreceptors, interdigitation zone (IZ), retinal pigment epithelium (RPE)/Bruch’s membrane complex, choriocapillaris, Sattler’s layer, Haller’s layer, choroid sclera junction and revealed the peripapillary subretinal space clearly (Figure 1A). Peripapillary subretinal space of all contused eyes were abnormal that showed a flat, localized hyperreflection due to hemorrhages between RPE/Bruch’s membrane complex and ELM. It was found that one eye’ s RPE/Bruch’s membrane complex micro-rips but ELM was intact (Figure 1B). If blood concentration was slightly low or contained serous liquid, in that way, the intensities of reflection could be not homogeneous. The changes of peripapillary subretinal space were reversible after treatment, however, the improvement of vision was uncertain (Figure 1 C1&C2).
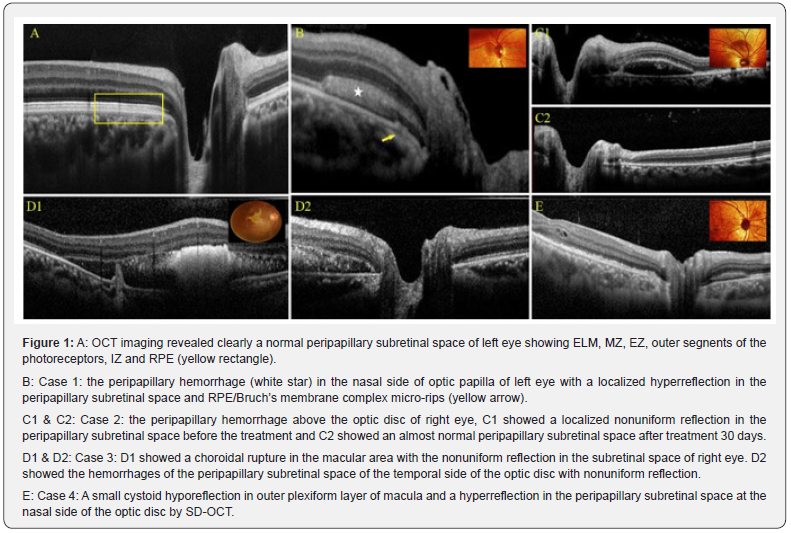
Choroidal rupture was observed at macular area of one eye with the macular thickness increase and peripapillary subretinal space showed nonuniform reflection (Figure 1 D1&D2). Another affected eye presented a small cystoid hyporeflection in outer plexiform layer of macula with the uneven ellipsoid zone and a higher reflection in the peripapillary subretinal space by SD-OCT (Figure 1E). FFA images showed the peripapillary blocked fluorescence under the retinal vessels that blocked the background fluorescence from choroid.
Discussion
TON is a form of optic nerve injury that occurs secondary to trauma and is etiologically associated with acute axonal loss with severe vision loss [2]. The mechanism of optic nerve damage secondary to trauma can be classified as primary or secondary. Primary damage occurs as a result of external forces at the moment of trauma, e.g., rupture of nerve fibers or of capillary vessels. Secondary damage may not be present initially but may occur later on and results from compromised blood supply to the optic nerve, e.g., following edema or angiospasm. Indirect traumatic optic neuropathy is the most common form of traumatic optic neuropathy [3]. However, there have been few reports of OCT imaging changes in the peripapillary subretinal space to date.
TON may result from a direct blunt injury; the exact biomechanical mechanisms of injury are not clear. For the direct blunt injury, the mechanism of the injury could be the direct mechanical compression to act on eyeball, the compression forces transmitted to the fundus cause the damages of optic nerve. Some results suggested that there are two principal mechanisms of injury: the rotation of the optic nerve relative to the globe and the increased IOP arising from the deformation of the globe [4]. When a direct blunt force acts on the eyeball, it may cause the damages of optic nerve involving the peripapillary subretinal space. In most cases the unusual changes of the peripapillary subretinal space were local, sometimes, connected to the subretinal space of macular area. There are two important spaces in the retina including the subretinal space and the sub- RPE space [5]. In this study the changes of peripapillary lesions involved in the peripapillary subretinal space and did not the sub-RPE space. In the images of SD-OCT, the subretinal space lies between ELM and RPE ending at edge of optic disc. Peripapillary subretinal space is a part of the subretinal space. Subretinal space is an important structure to the extent that so many reports referred to it such as central serous chorioretinopathy [6].
The cross-sectional anatomic configurations of the peripapillary atrophy were evaluated also by using SDOCT. It showed that the termination of the retinal layers and configurations of the scleral bed in the peripapillary area varied among normal subjects [7]. Peripapillary retinal detachment (PPRD) can occur in a variety of diseases, including pathologic myopia, peripapillary choroidal neovascularization (idiopathic or secondary to other conditions such as age-related macular degeneration) and polypoidal choroidal vasculopathy. When associated with pathologic myopia, PPRD or retinal schisis could be found by OCT [8]. However, the image changes of SD-OCT of peripapillary subretinal space of the traumatic optic neuropathy due to the direct blunt force were not paid attention to by previous reports.
In the present study, the hemorrhages or serous liquids in the peripapillary subretinal space were observed. These changes seemed different from the subretinal liquid due to other causes [9,10]. The interesting finding here is that RPE broken off and choroidal rupture occurred, maybe it is significant in diagnosis. Peripapillary hemorrhages in the peripapillary subretinal space could come from the peripapillary choriocapillaris. The blood could escape into the peripapillary subretinal space from choriocapillaris through RPE/Bruch’s membrane complex micro-rips. It was highly unlikely that hemorrhages were from the retinal capillaries system because the retinal layers around optic disc were normal including RNFL, GCL, IPL, INL and OPL, also, FFA demonstrated it. Peripapillary hemorrhages showed a localized higher reflection in the subretinal space, and it was adjacent to optic disc in the SD-OCT images. The hemorrhages of peripapillary subretinal space were reversible after treatment, but visual acuities improved variously in this study.
Usually, TON is treated by corticosteroids in our daily medical practice for a long time, but some controversial study results have been reported which claims that steroids provide nothing additional visual benefit. Some evidence also suggests a possible detrimental effect of steroids in TON and further studies are urgently needed to clarify this important issue [11]. Given human and animal data suggesting that treatment is harmful and the lack of demonstrated clinical efficacy, corticosteroids should not be used to treat traumatic optic neuropathy. The benefit of optic canal decompression is also unclear [12]. A study concluded also that neither retrobulbar administration of triamcinolone nor systemic administration of methylprednisolone has any neuroprotective effects in a rat model of optic nerve crush [13]. Erinacine A (EA), a natural neuroprotectant, is isolated from a Chinese herbal medicine, Hericium erinaceus. EA treatment has neuroprotective effects on an experimental model of traumatic optic neuropathy by suppressing apoptosis, neuroinflammation, and oxidative stress to protect the RGCs from death as well as preserving the visual function [14].
It is considered that wild-type erythropoietin (EPO) is promising for neuroprotection, but its therapeutic use is limited because it causes a systemic rise in hematocrit [15]. The mechanism of injury is poorly understood. Peripapillary hemorrhages in the peripapillary subretinal space could come from the peripapillary choriocapillaris, this is the first clinical observations, to our knowledge, using SD-OCT examining TON patients and the result showed the SD-OCT was a useful tool in diagnosis evaluation of TON patients especially within one week after injury. It is remains to be further proved through a larger sample study for this promising study result.
References
- Aria Ghahramani, Mona L Camacci, Rucha Borkhetaria, Anne Poulsen, Samuel Beckstead (2021) Traumatic Optic Nerve Sheath Hematoma. Case Rep Ophthalmol 12(2): 569-573.
- Won June Lee, Eun Hee Hong, Hae Min Park, Han Woong Lim (2019) Traumatic optic neuropathy-associated progressive thinning of the retinal nerve fiber layer and ganglion cell complex: two case reports. BMC Ophthalmol 19(1): 216.
- Seyed Ali Tabatabaei, Mohammad Soleimani, Mahdi Alizadeh, Morteza Movasat, Mohammad Reza Mansoori, et al. (2011) Predictive value of visual evoked potentials, relative afferent pupillary defect, and orbital fractures in patients with traumatic optic neuropathy. Clin Ophthalmol 5: 1021-1026.
- S Cirovic, RM Bhola, DR Hose, IC Howard, PV Lawford, et al. (2006) Computer modelling study of the mechanism of optic nerve injury in blunt trauma. Br J Ophthalmol 90(6): 778-783.
- Steven N Truong, Suhail Alam, Robert J Zawadzki, Stacey S Choi, David G Telander, et al. (2007) High-resolution Fourier-domain optical coherence tomography of retinal angiomatous proliferation. Retina 27(7): 915-925.
- Chrysanthos Symeonidis, Konstantinos Kaprinis, Kyriakos Manthos, Sofia Androudi, Konstantinos Anastassilakis, et al. (2011) Central Serous Chorioretinopathy with Subretinal Deposition of Fibrin-Like Material and Its Prompt Response to Ranibizumab Injections. Case Report Ophthalmol 2(1): 59-64.
- Kelvin Yoon Chiang Lee 1, Atsuo Tomidokoro, Rei Sakata, Shinsuke Konno, Chihiro Mayama, Hitomi Saito, et al. (2010) Cross-sectional Anatomic Configurations of Peripapillary Atrophy Evaluated with Spectral Domain-Optical Coherence Tomography. Invest Ophthalmol Vis Sci 51(2): 666-671.
- Michele Carbonelli, Giacomo Savini, Maurizio Zanini, Piero Barboni (2007) Peripapillary detachment in pathologic myopia: Unusual OCT findings. Clin Ophthalmol 1(3): 327-329.
- Thomas R Hedges, Laurel N Vuong, Alberto O Gonzalez-Garcia, Carlos E Mendoza-Santiesteban, Maria Luz Amaro-Quierza (2008) Subretinal Fluid from Anterior Ischemic Optic Neuropathy Demonstrated by Optical Coherence Tomography. Arch Ophthalmol 126(6): 812-815.
- María Moreno-López, Marta Pérez-López, Pilar Casas-Llera, Elena Jarrín, Francisco José Muñoz-Negrete (2011) Persistent subretinal fluid due to central serous chorioretinopathy after retinal detachment surgery. Clin Ophthalmol 5: 1465-1467.
- Yu-Wai-Man P, Griffiths PG (2011) Steroids for traumatic optic neuropathy. Cochrane Database Syst Rev 2013(1): CD006032.
- Steinsapir KD, Goldberg RA (2011) Traumatic optic neuropathy: an evolving understanding. Am J Ophthalmol 151(6): 928-933.
- Tzu Lun Huang, Chung Hsing Chang, Kung Hung Lin, Min Muh Sheu, Rong Kung Tsai (2011) Lack of protective effect of local administration of triamcinolone or systemic treatment with methylprednisolone against damages caused by optic nerve crush in rats. Exp Eye Res 92(2): 112-119.
- Chiao-Ling Hsu, Yao-Tseng Wen, Tzu-Chao Hsu, Chin-Chu Chen, Li-Ya Lee, et al. (2023) Neuroprotective Effects of Erinacine A on an Experimental Model of Traumatic Optic Neuropathy. Int J Mol Sci 24(2): 1504.
- CR DeJulius, A Bernardo-Colón, S Naguib, JR Backstrom, T Kavanaugh, et al. (2021) Microsphere antioxidant and sustained erythropoietin-R76E release functions cooperate to reduce traumatic optic neuropathy. J Control Release 329: 762-773.






























