RPE/Bruch’s Membrane Complex Micro-Rips in Central Serous Chorioretinopathy
Fubin Wang*
Shanghai Bright Eye Hospital, Changning District, Shanghai, China
Submission: November 15, 2022;Published: November 30, 2022
*Corresponding author: Fubin Wang, Shanghai Bright Eye Hospital, 899 Maotai Road, Changning District, Shanghai 200336, Shanghai, China
How to cite this article: Fubin W. RPE/Bruch’s Membrane Complex Micro-Rips in Central Serous Chorioretinopathy. JOJ Ophthalmol. 2022; 9(3): 555761. DOI: 10.19080/JOJO.2022.09.555761
Abstract
Aim: To describe the RPE/Bruch’s membrane complex micro-rips image of central serous chorioretinopathy (CSC) by OCT.
Methods: The novel OCT images were observed in patients with CSC. CSC diagnosis was based on findings from both clinical examination and multimodal images. Although the patients had been conducted many ophthalmologic examinations, such as fundus fluorescein angiography (FFA) and indocyanine green angiography (ICG), visual field, ultrasonography, pattern visual evoked potential (PVEP), we did not assess them and evaluate the results of SD-OCT and en-face OCT examination only.
Results: On the images of SD-OCT and en-face OCT, the fluid was blowout passing through RPE /Bruch’s membrane complex into sub-interdigitation zone space from choroid, it’s like a whale blow to be called “blowout sign” with the hyperreflection. RPE/Bruch’s membrane complex micro-rips, especially Bruch’s membrane micro-rips, Double-layer sign (DLS), pigment epithelial detachment ( PED ) and hyperreflective dots (HRDs) were observed.
Conclusion: The blowout sign can be found in patients with CSC, micro-RPE rips with Bruch’s membrane micro-rips, but it is random and not every patient can be seen. The liquid erupts into the sub-interdigitation zone space from choroid associated with hyperpermeable choroidal vessels and increased tissue hydrostatic pressure.
Keywords: Central Serous Chorioretinopathy; Micro-RPE Rips; Bruch’s Membrane Micro-Rips; OCT; Sub-Interdigitation Zone Space
Abbreviations: CSC: Central Serous Chorioretinopathy; FFA: Fundus Fluorescein Angiography; ICG: Indocyanine Green Angiography; PVEP: Pattern Visual Evoked Potential; DLS: Double-Layer Sign; PED: Pigment Epithelial Detachment; HRDs: Hyperreflective Dots; RPE: retinal pigment epithelium; CFP: Color Fundus Photography; SRF: Subretinal Fluid; PCN: Pachychoroid Neovasculopathy; PCV: Polypoidal Choroidal Vasculopathy
Introduction
Central serous chorioretinopathy (CSC) is a common disorder characterized by serous retinal detachment with or without serous pigment epithelial detachment (PED). It is an idiopathic ocular disease [1]. The pathogenesis of CSC remains poorly understood. However, some advancements have led to further understanding of the disease, choroidal abnormalities are believed to be the primary underlying pathophysiology. These abnormalities can include choroidal thickening and hyperpermeability, together with increased hydrostatic pressure, which has been hypothesized to induce detachment of the retinal pigment epithelium (RPE). These points of RPE detachment can remain isolated, but breakdown of the outer blood-retina barrier can also cause leakage of fluid into the subretinal space, resulting in active CSC [2].
In the present study, the novel image change with CSC was observed by SD-OCT and en-face OCT (Spectralis is OCT, Heidelberg Engineering, Heidelberg, Germany. Cirrus HD-OCT 5000, Germany), the fluid is blowout passing through RPE/ Bruch’s membrane complex into sub-interdigitation zone space from choroid and micro-RPE rips and Bruch’s membrane micro-rips were observed.
Subjects and Methods
The eight eyes of eight male patients with CSC were retrospectively evaluated. All patients underwent a comprehensive eye examination. Ages range was from 35 to 50 years. CSC diagnosis was based on findings from both clinical examination and multimodal images. The findings included the presence of serous macular detachment, thick choroid with congested choroidal vasculature, color fundus photography (CFP), SD-OCT and en-face OCT, fundus fluorescein angiography (FFA) and indocyanine green angiography (ICGA) and so on. The patients were defined acute CSC as that occurring within 6 months of symptom onset and chronic CSC as that occurring more than 6 months after symptom onset [3].
Patients were diagnosed in our hospital and all patients provided informed consent. This study adhered to the tenets of the Declaration of Helsinki. In this study, we assessed the results of SD-OCT and en-face OCT examination only. Although the patients had been conducted many ophthalmologic examinations, such as FFA, ICGA, visual field, ultrasonography, pattern visual evoked potential (PVEP), we did not assess them.
Results
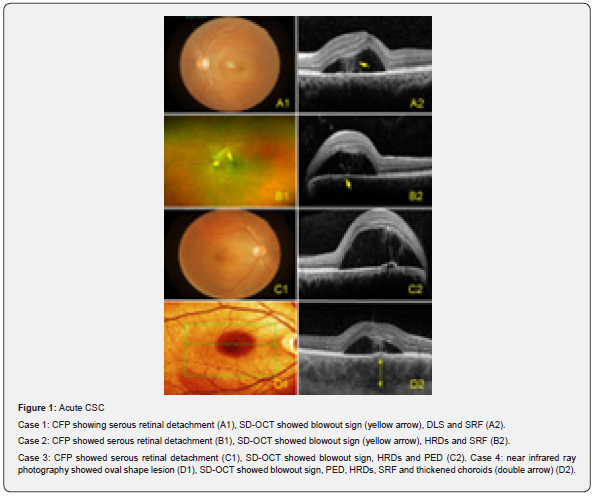
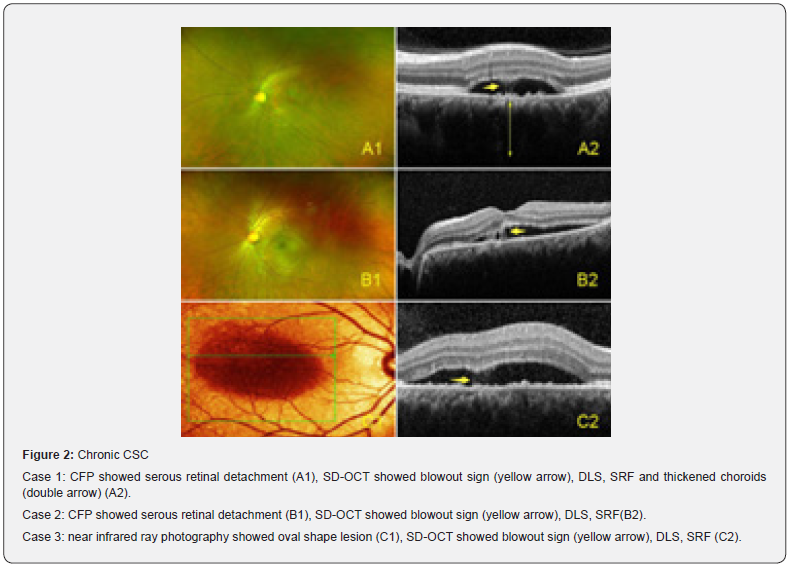
The unusual images were observed with acute or chronic CSC by B-scan images of SD-OCT and en-face OCT. Both acute and chronic CSC, the fluid spout passing through RPE /Bruch’s membrane complex into sub-interdigitation zone space from choroid with a hyperreflection, it’s like the whale blows to be called as “blowout sign” in this paper. Blowout sign occurs in subinterdigitation zone space associated with PED and double-layer sign (DLS) that describes the shallow and irregular elevation of the retinal pigment epithelium from the underlying intact Bruch’s membrane visualized on OCT. On SD-OCT, the eyes with CSC had thickened choroids as well as an increase in the diameter of choroidal vessels, particularly of the larger veins in Haller’s layer. Hyperreflective dots (HRDs) were observed.
In acute CSC, well-defined serous retinal detachment with or without serous PED is typically confined to the macula. In chronic CSC, the retinal detachment is usually shallow and broad, with attenuation of outer retinal layers related to chronic serous detachment (Figures 1, 2). In chronic CSC, en-face OCT showed micro-RPE rips with Bruch’s membrane micro-rips. SD-OCT showed Bruch’s membrane micro-rips opening towards RPE with the fibrin in PED and there is a hyporeflective line underlying Bruch’s membrane (Figure 3).

Discussion
Central serous chorioretinopathy is characterized by a serous detachment of the neurosensory retina. Although the precise pathophysiology of CSC is unclear, a congested and hyperpermeable choriocapillaris and thickened choroid (pachychoroid) may be the primary source of serous fluid leakage. Additionally, it is hypothesized that due to secondary retinal pigment epithelium alterations, the outer blood-retinal barrier is dysfunctional, which subsequently leads to fluid passage into the subretinal space. CSC typically affects young adult men [4, 5].
In CSC, the macula is detached because of fluid leakage at the level of the retinal pigment epithelium. The fluid appears to originate from choroidal vascular hyperpermeability, but the etiology for the fluid is controversial. Choroidal venous overload provides a unifying concept and theory for an improved understanding of the pathophysiology and classification of a group of diseases to a greater extent than previous proposals [6].
Mechanisms of subretinal fluid (SRF) accumulation in CSC are still uncertain. Several theories have been proposed to explain fluid entry from the choroid toward the subretinal space: dilated and hyperpermeable choroidal vessels, changes in RPE cell polarity altering hydroionic pumping direction, multifocal rupture of the RPE barrier or active reverse flow by unknown triggering mechanisms [7]. Results of the current study indicate that the composition of SRF in CSC may be associated with choroidal blood composition. CSC is part of the spectrum of pachychoroid diseases that include CSC, pachychoroid pigment epitheliopathy, pachychoroid neovasculopathy, and polypoidal choroidal vasculopathy [8].
Subretinal space is well known, it is a gap between external limiting membrane and RPE. RPE is separated from the photoreceptor outer segments by the subretinal space [9]. The observation of this study is that SRF of CSC accumulate in the sub-interdigitation zone space on SD-OCT. As the disease duration increases, HRDs appear in SRF and increase in number with duration, indicative of chronicity of SRF. The origin of these HRDs is not clear, but they could be photoreceptor outer segments shedding, activated microglia and macrophages, or concentrated fibrin or lipid compounds [10, 11].
The double-layer sign describes the shallow and irregular elevation of the retinal pigment epithelium from the underlying intact Bruch’s membrane visualized on SD-OCT. Sato et al. initially described DLS in as a tomographic feature of the branching vascular networking polypoidal vasculopathy. However, DLS was also first described in eyes with chronic CSC indicating that DLS per se may not be disease specific and that there may be other features in the DLS that may help differentiate the variants within the pachychoroid spectrum. A hyporeflective gap within the DLS favored the diagnosis of chronic CSC [12]. In this study, DLS was observed in acute and chronic CSC respectively, not only chronic CSC but also acute CSC. In the eyes with DLS, the space between the undulated RPE line and the straight Bruch’s membrane line appeared hyporeflective on SD-OCT. Yang et al. were the first to study DLS as an important OCT feature of both acute and chronic CSC and the DLS in chronic CSC was more prevalent [13].
Larger dimensions of DLS in pachychoroid neovasculopathy (PCN) and polypoidal choroidal vasculopathy (PCV) is suggestive of extensive neovascular tissue with/without leaked exudation, which secondarily leads to significant separation of RPE from the Bruch’s membrane leading to increased horizontal extent along with augmented RPE elevation leading to increased vertical extent. In contrast, the smaller extent of DLS in chronic CSC is indicative of less significant RPE-Bruch’s membrane complex impairment [12].
The current understanding of the pathogenesis of CSC emphasizes the role of the choroid. The primary role of the choroid is further supported by the Enhanced depth imaging optical coherence tomography (EDI-OCT) finding of a thickened choroid in both eyes of patients with CSC [14]. Hyperpermeable choroidal vessels are thought to produce increased tissue hydrostatic pressure, which promotes the formation of PED, overwhelms the barrier function of the RPE, and leads to areas of fluid accumulation between the retina and the RPE. The role of the RPE in CSC pathogenesis remains poorly understood. Some refer to the pinpoint areas of leakage seen in acute CSC as micro-rips or blowouts. Retinal pigment epithelial detachments (PEDs) are common in CSC and could also represent a form of RPE decompensation in response to high choroidal hydrostatic pressure [15].
Gass proposed that CSC was the consequence of choroidal vascular hyperpermeability. This hypothesis was confirmed by recent studies, which found evidence of hyperpermeability from the choriocapillaris on Indocyanine green (ICG) angiography. If there is sufficient hydrostatic pressure from the choroidal vasculature causing leakage from RPE and serous retinal detachment [16]. SD-OCT studies of the RPE in CSC have shown RPE defects in the location of a fluorescein leakage in some patients [17].
The precise pathophysiological event causing the exudative neurosensory detachment in CSC is not known but it is now a common belief that the primary pathology begins with disturbance of the choroidal circulation. Increased choroidal leakage, local hyperperfusion, and elevated hydrostatic pressure may lead to serious detachment of the RPE. A mechanical disruption of the RPE may cause the characteristic focal fluorescein leakage whereas the chronic pressure may induce RPE atrophy [18].
Hyperpermeable choroidal vessels are thought to produce increased tissue hydrostatic pressure, which promotes the formation of PEDs, overwhelms the barrier function of the RPE, and leads to areas of fluid accumulation between the retina and the RPE. Since areas of choroidal staining are usually contiguous with foci of RPE leakage on FA, the hypothesis of a mechanistic relationship between the two findings is reasonable. Not all areas of choroidal staining are associated with RPE leaks, suggesting that in some instances the RPE may be able to withstand the stresses posed by choroidal disease [15].
The phenomena of RPE micro rip or RPE blowout was explained by Goldstein et al. The work by Ayachit et al. is appreciated for the documentation of a rare finding. The explanations for the finding given by them, e.g., possible presence of an RPE micro rip or vigorous leakage from choriocapillaris is well taken and supported by literature. An RPE micro-rip may explain the entry of leaked ICG dye (in the PED) into subretinal space. Although we could not identify the RPE micro-rip in either of our cases, its presence is supported by the presence of large NSDs in both the cases [19, 20].
In this study, the unusual images of CSC were observed by OCT. The fluid erupts passing through RPE/Bruch’s membrane complex into sub-interdigitation zone space from the choroid, it is like a “whale blows” both acute and chronic CSC. It suggests that SRF originate from choroid passing through the defective RPE/Bruch’s membrane complex leading to the SRF formation. Interestingly, blowout sign frequently occurs at the site of DLS or PED indicating the existence of underlying prolonged abnormality of RPE. The finding of Bruch’s membrane micro-rips is of important significance, which, together with micro-RPE rips, is important in explaining the pathogenesis of CSC. It is possible that the abnormality of RPE lead to RPE/Bruch’s membrane complex micro-rips then the liquid pass into the sub-interdigitation zone space from choroid.
References
- Alireza Maleki, Zahra Nezamdust, Amirmasoud Salari, Seyed Sajad Ahmadi, Hamideh Sabbaghi, et al. (2018) The Effect of Intravitreal Bevacizumab on Central Serous Chorioretinopathy. Med Hypothesis Discov Innov Ophthalmol 7(4): 176-182.
- Thomas J van Rijssen, Elon HC van D, Suzanne Yzer, Kyoko Ohno M, Jan EE Keunen, et al. (2019) Central serous chorioretinopathy: Towards an evidence-based treatment guideline. Prog Retin Eye Res 73: 100770.
- Magdy Moussa, Mahmoud Leila, Hagar Khalid, Mohamed Lolah (2017) Detection of Silent Type I Choroidal Neovascular Membrane in Chronic Central Serous Chorioretinopathy Using En Face Swept-Source Optical Coherence Tomography Angiography. J Ophthalmol 2017: 6913980.
- Danial Mohabati, Thomas J van Rijssen, Elon Hc van Dijk, Gregorius Pm Luyten, Tom O Missotten, et al. (2018) Clinical characteristics and long-term visual outcome of severe phenotypes of chronic central serous chorioretinopathy. Clin Ophthalmol 12: 1061-1070.
- Ilaria Zucchiatti, Riccardo Sacconi, Maria Cristina Parravano, Eliana Costanzo, Lea Querques, et al. (2018) Eplerenone Versus Observation in the Treatment of Acute Central Serous Chorioretinopathy: A Retrospective Controlled Study. Ophthalmol Ther 7(1): 109-118.
- Richard F Spaide, Chui Ming GC, Hidetaka Matsumoto, Shoji Kishi, Camiel JF Boon, et al. (2022) Venous overload choroidopathy: A hypothetical framework for central serous chorioretinopathy and allied disorders. Prog Retin Eye Res 86: 100973.
- Laura Kowalczuk, Alexandre Matet, Marianne Dor, Nasim Bararpour, Alejandra Daruich, et al. (2018) Proteome and Metabolome of Subretinal Fluid in Central Serous Chorioretinopathy and Rhegmatogenous Retinal Detachment: A Pilot Case Study. Transl Vis Sci Technol 7(1): 3.
- Shoji Kishi, Hidetaka Matsumoto, Shozo Sonoda, Takashi Hiroe, Taiji Sakamoto, et al. (2018) Geographic filling delay of the choriocapillaris in the region of dilated asymmetric vortex veins in central serous chorioretinopathy. PLoS One 13(11): e0206646.
- Claire H Mitchell, David Reigada (2008) Purinergic signalling in the subretinal space: a role in the communication between the retina and the RPE. Purinergic Signal. 4(2): 101-107.
- Nicholson B, Noble J, Forooghian F, Meyerle C (2013) Central serous chorioretinopathy: Update on pathophysiology and treatment. Surv Ophthalmol 58(2): 103-126.
- Alejandra Daruich, Alexandre Matet, Ali Dirani, Elodie Bousquet, Min Zhao, et al. (2015) Central serous chorioretinopathy: Recent findings and new physiopathology hypothesis. Prog Retin Eye Res 48: 82-118.
- Jay Sheth, Giridhar Anantharaman, Shruti Chandra, Sobha Sivaprasad (2018) "Double-layer sign" on spectral domain optical coherence tomography in pachychoroid spectrum disease. Indian J Ophthalmol 66(12): 1796-1801.
- Yang L, Jonas JB, Wei W (2013) Optical coherence tomography-assisted enhanced depth imaging of central serous chorioretinopathy. Invest Ophthalmol Vis Sci 54(7): 4659-4665.
- Imamura Y, Fujiwara T, Margolis R, Spaide RF (2009) Enhanced depth imaging optical coherence tomography of the choroid in central serous chorioretinopathy. Retina 29(10): 1469-1473.
- Benjamin Nicholson, Jason Noble, Farzin Forooghian, Catherine Meyerle (2013) Central Serous Chorioretinopathy: Update on Pathophysiology and Treatment. Surv Ophthalmol 58(2): 103-26.
- Yumusak E, Gokcinar NB, Ornek K (2018) Choroidal thickness changes in non-treated acute and ranibizumab-treated chronic central serous chorioretinopathy. Medicine (Baltimore) 97(43): e12885.
- Hisataka Fujimoto, Fumi Gomi, Taku Wakabayashi, Miki Sawa, Motokazu Tsujikawa, et al. (2008) Morphologic changes in acute central serous chorioretinopathy evaluated by fourier-domain optical coherence tomography. Ophthalmology 115(9): 1494-1500.
- WM Chan, DSC Lam, TYY Lai, BSM Tam, DTL Liu, et al. (2003) Choroidal vascular remodelling in central serous chorioretinopathy after indocyanine green guided photodynamic therapy with verteporfin: a novel treatment at the primary disease level. Br J Ophthalmol 87(12): 1453-1458.
- Goldstein BG, Pavan PR (1987) 'Blow-outs’ in the retinal pigment epithelium. Br J Ophthalmol 71(9): 676-681. (1988) Erratum in: Br J Ophthalmol 72: 240.
- Apoorva Ayachit, Vinod Kumar, Nimmy Raj, Guruprasad Ayachit (2018) Smokestack leak on indocyanine green angiography in acute central serous chorioretinopathy. Indian J Ophthalmol 66(8): 1181-1182.






























